The Contribution of CD26-Negative Fibroblasts to Endometrial Scarring
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
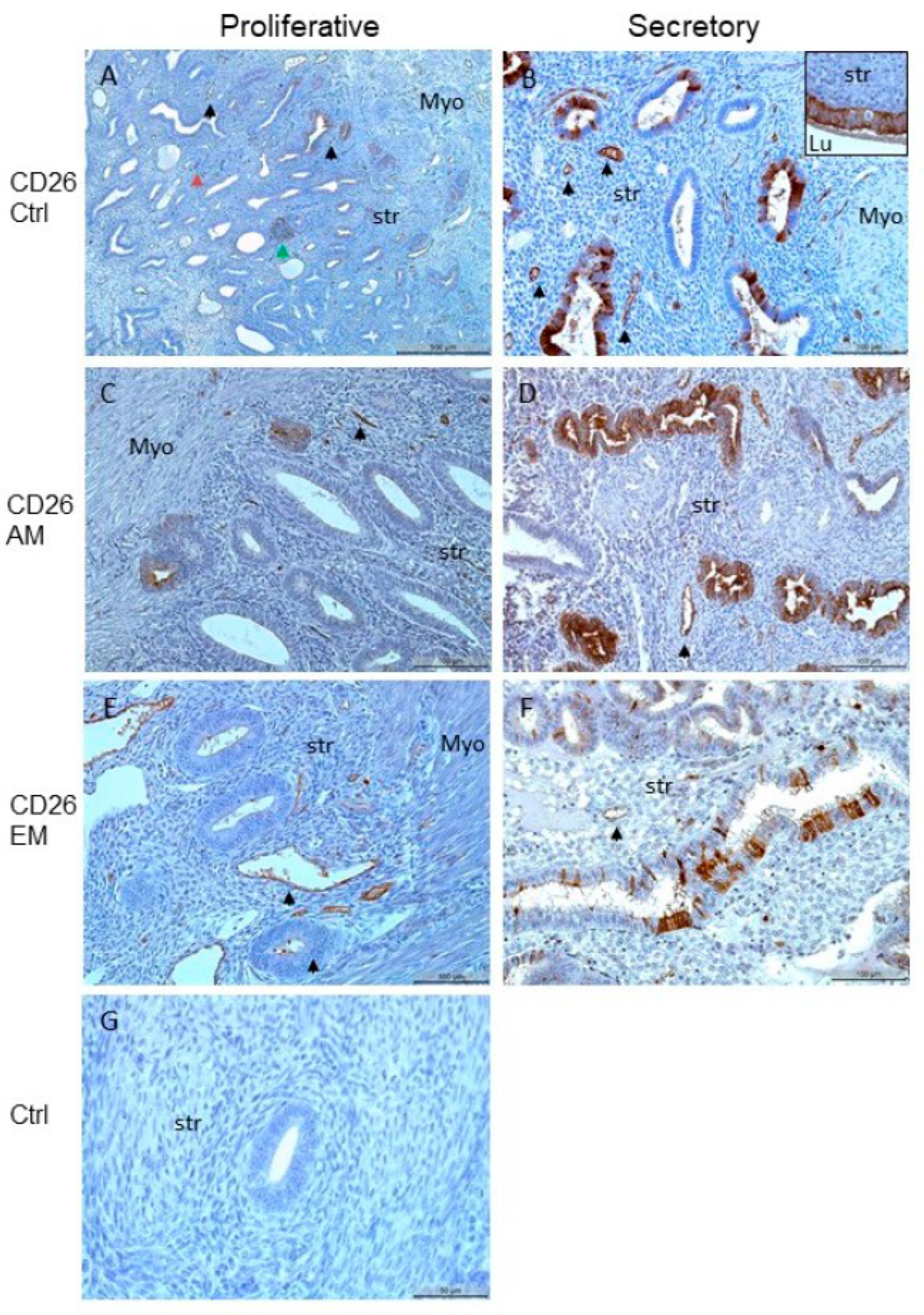
2.2. Immunohistochemical Analysis and Quantification
2.3. Isolation and Culture of Human Primary Endometrial Stromal Cells
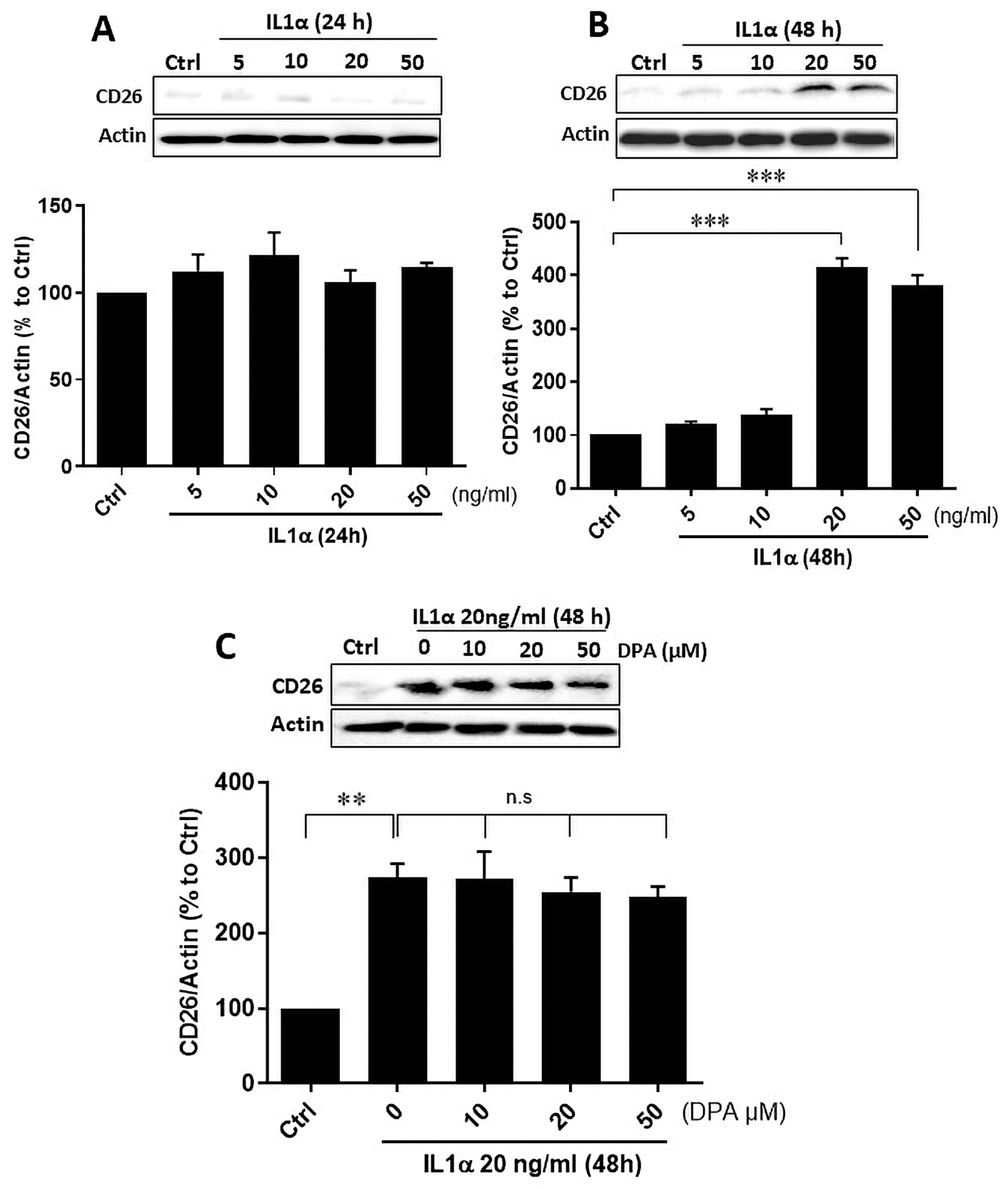
2.4. Recombinant Proteins, Inhibitors, and ELISAs
2.5. Cell Culture and Experimental Protocol
2.6. Collection of Supernatants of Cultured Cells
2.7. Preparation of Cell Lysates and Western Blot
2.8. ELISA for Pro-Collagen 1A1 and TGF-β3
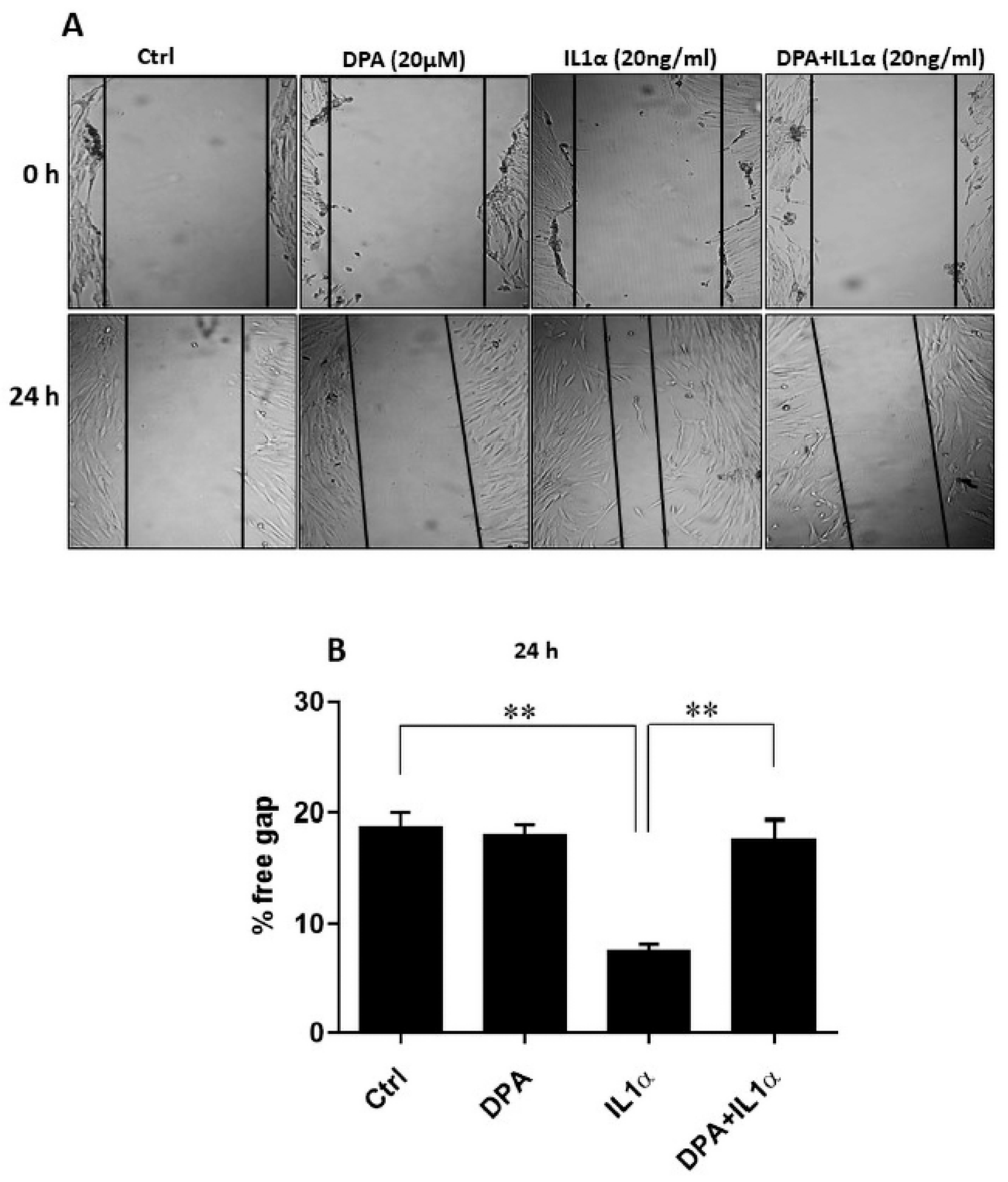
2.9. Wound Healing Assay
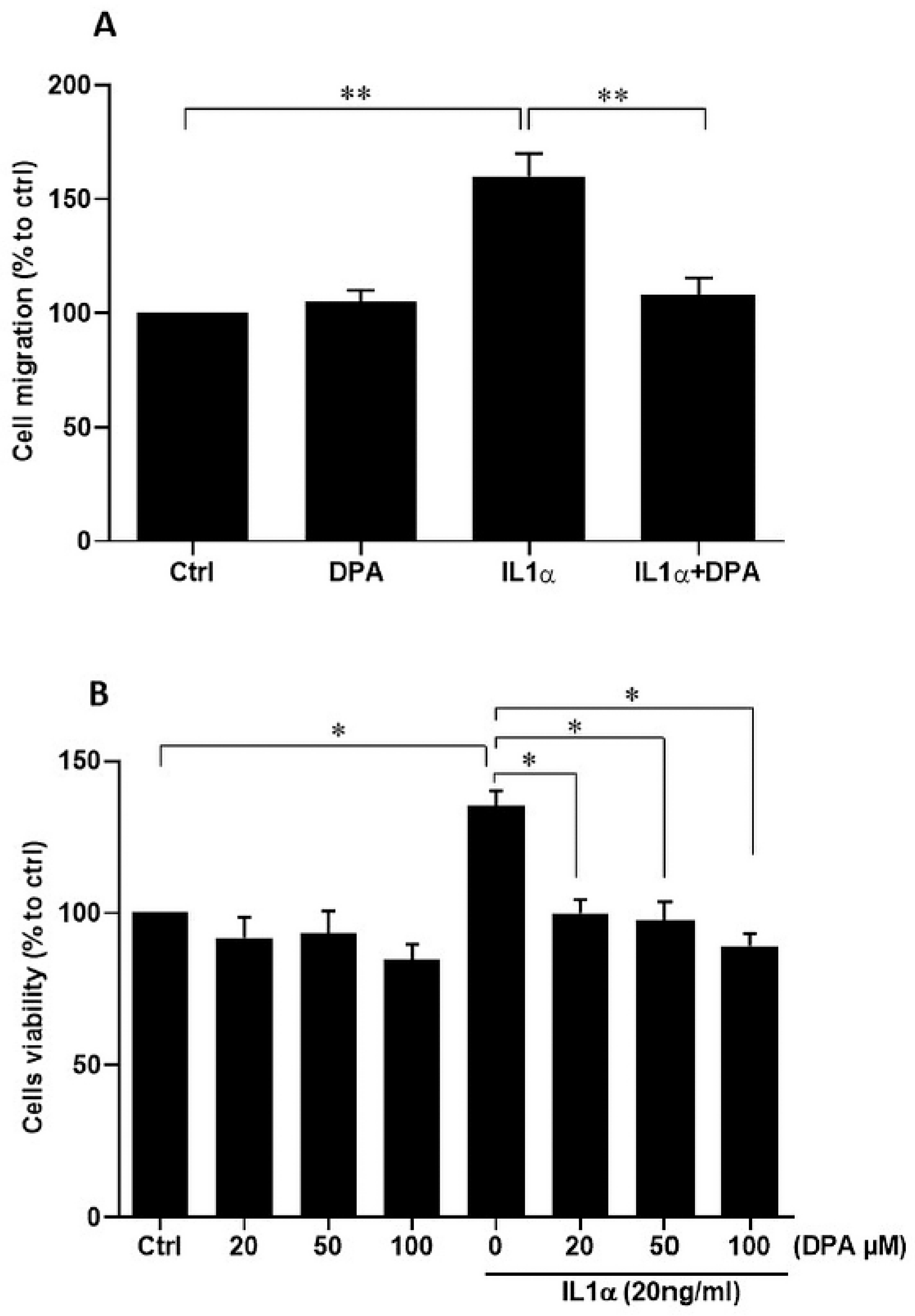
2.10. Cell Viability Assay
2.11. Cell Migration Assay
2.12. Statistical Analyses
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. A re–appraisal of the morphological changes within the endometrium during menstruation: A hysteroscopic, histological and scanning electron microscopic study. Hum. Reprod. 2009, 24, 1393–1401. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. Structural changes in endometrial basal glands during menstruation. BJOG 2010, 117, 1175–1185. [Google Scholar] [CrossRef]
- Murji, A.; Sanders, A.P.; Monteiro, I.; Haiderbhai, S.; Matelski, J.; Walsh, C.; Abbott, J.A.; Munro, M.G.; Maheux-Lacroix, S. Cesarean scar defects and abnormal uterine bleeding: A systematic review and meta-analysis. Fertil. Steril. 2022, 118, 758–766. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhao, G.; Jiang, P.; Song, M.; Ding, H.; Wang, Z.; Lv, H.; Hu, Y. CSF1–associated decrease in endometrial macrophages may contribute to Asherman’s syndrome. Am. J. Reprod. Immunol. 2020, 83, e13191. [Google Scholar] [CrossRef]
- Benor, A.; Gay, S.; DeCherney, A. An update on stem cell therapy for Asherman syndrome. J. Assist. Reprod. Genet. 2020, 37, 1511–1529. [Google Scholar] [CrossRef]
- Li, Z.; Bian, X.; Ma, Y.; Yang, Q.; Jia, W.; Liu, J.; Wang, F.; Liu, M.; Li, Y.X.; Shao, X.; et al. Uterine scarring leads to adverse pregnant consequences by impairing the endometrium response to steroids. Endocrinology 2020, 161, bqaa174. [Google Scholar] [CrossRef]
- Karpathiou, G.; Chauleur, C.; Dridi, M.; Baillard, P.; Corsini, T.; Dumollard, J.M.; Peoc’h, M. Histologic findings of uterine niches. Am. J. Clin. Pathol. 2020, 154, 645–655. [Google Scholar] [CrossRef]
- Raimondo, G.; Grifone, G.; Raimondo, D.; Seracchioli, R.; Scambia, G.; Masciullo, V. Hysteroscopic treatment of symptomatic cesarean–induced isthmocele: A prospective study. J. Minim. Invasive Gynecol. 2015, 22, 297–301. [Google Scholar] [CrossRef]
- Morris, H. Surgical pathology of the lower uterine segment caesarean section scar: Is the scar a source of clinical symptoms? Int. J. Gynecol. Pathol. 1995, 14, 16–20. [Google Scholar] [CrossRef]
- Kirkwood, P.M.; Shaw, I.W.; Saunders, P.T.K. Mechanisms of scarless repair at time of menstruation: Insights from mouse models. Front. Reprod. Health 2022, 3, 801843. [Google Scholar] [CrossRef]
- Evans, J.; Infusini, G.; McGovern, J.; Cuttle, L.; Webb, A.; Nebl, T.; Milla, L.; Kimble, R.; Kempf, M.; Andrews, C.J.; et al. Menstrual fluid factors facilitate tissue repair: Identification and functional action in endometrial and skin repair. FASEB J. 2019, 33, 584–605. [Google Scholar] [CrossRef]
- Jiang, D.; Rinkevich, Y. Scars or regeneration?–Dermal fibroblasts as drivers of divers skin wound responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative scar-free skin wound healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef]
- Ohm, B.; Moneke, I.; Jungraithmayr, W. Targeting cluster of differentiation 26/dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis. Br. J. Pharmacol. 2023, 180, 2846–2861. [Google Scholar] [CrossRef]
- Zhao, X.; Psarianos, P.; Ghoraie, L.S.; Yip, K.; Goldstein, D.; Gilbert, R.; Witterick, I.; Pang, H.; Hussain, A.; Lee, J.H.; et al. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat. Metab. 2019, 1, 147–157. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024, 30, 706–750. [Google Scholar] [CrossRef]
- Konrad, L.; Kortum, J.; Nabham, R.; Gronbach, J.; Dietze, R.; Oehmke, F.; Berkes, E.; Tinneberg, H.R. Composition of the stroma in the human endometrium and endometriosis. Reprod. Sci. 2018, 25, 1106–1115. [Google Scholar] [CrossRef]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horné, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial-mesenchymal transition in endometriosis—When does it happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef]
- Khin, E.E.; Kikkawa, F.; Ino, K.; Kajiyama, H.; Suzuki, T.; Shibata, K.; Tamakoshi, K.; Nagasaka, T.; Mizutani, S. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am. J. Obstet. Gynecol. 2003, 188, 670–676. [Google Scholar] [CrossRef]
- Aliagas, E.; Vidal, A.; Torrejón–Escribano, B.; del Rosario Taco, M.; Ponce, J.; Gómez de Aranda, I.; Sévigny, J.; Condom, E.; Martín-Satué, M. Ecto–nucleotidases distribution in human cyclic and postmenausic endometrium. Purinergic Signal 2013, 9, 227–237. [Google Scholar] [CrossRef]
- Imai, K.; Maeda, M.; Fujiwara, H.; Kariya, M.; Takakura, K.; Kanzaki, H.; Mori, T. Dipeptidyl peptidase IV as a differentiation marker of the human endometrial glandular cells. Hum. Reprod. 1992, 7, 1189–1194. [Google Scholar] [CrossRef]
- Trapero, C.; Vidal, A.; Fernández-Montolí, M.E.; Coroleu, B.; Tresserra, F.; Barri, P.; Gómez de Aranda, I.; Sévigny, J.; Ponce, J.; Matias-Guiu, X.; et al. Impaired expression of ectonucleotidases in ectopic and eutopic endometrial tissue is in favor of ATP accumulation in the tissue microenvironment in endometriosis. Int. J. Mol. Sci. 2019, 20, 5532. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Morison, N.B.; Salamonsen, L.A. Estrogen is not essential for full endometrial restoration after breakdown: Lessons from a mouse model. Endocrinology 2007, 10, 5105–5111. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Takahashi, T.; Mori, T.; Kawashima, S.; Takahashi, K.; Kobayashi, H. Hormonal modulation of prolyl endopeptidase and dipeptidyl peptidase IV activities in the mouse uteru and ovary. Acta Endocrinol. 1992, 127, 262–266. [Google Scholar]
- Nemoto, E.; Sugawara, S.; Takada, H.; Shoji, S.; Horiuch, H. Increase of CD26/dipeptidyl peptidase IV expression on human gingival fibroblasts upon stimulation with cytokines and bacterial components. Infect. Immun. 1999, 67, 6225–6233. [Google Scholar] [CrossRef]
- Konrad, L.; Hackethal, A.; Oehmke, F.; Berkes, E.; Engel, J.; Tinneberg, H.R. Localization of clusterin and clusterin receptors in the endometrium and clusterin levels in cervical mucus of endometriosis. Reprod. Sci. 2016, 23, 1371–1380. [Google Scholar] [CrossRef]
- Löffelmann, A.C.; Hoerscher, A.; Riaz, M.A.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. Claudin-10 expression is increased in endometriosis and adenomyosis and mislocalized in ectopic endometriosis. Diagnostics 2022, 12, 2848. [Google Scholar] [CrossRef]
- Mwaura, A.N.; Riaz, M.A.; Mecha, E.; Omwandho, C.O.A.; Scheiner-Bobis, G.; Meinhold-Heerlein, I.; Konrad, L. Activin A modulates betaglycan shedding via the ALK4-SMAD3-dependent pathway in endometriotic cells. Biomolecules 2022, 12, 1749. [Google Scholar] [CrossRef]
- Mwaura, A.N.; Riaz, M.A.; Mecha, E.; Omwandho, C.O.A.; Scheiner-Bobis, G.; Meinhold-Heerlein, I.; Konrad, L. Role of betaglycan in TGF-β signaling and wound healing in endometriotic epithelial cells and in endometriosis. Biology 2022, 11, 513. [Google Scholar] [CrossRef]
- Parry, D.; Allison, K. Is the future scarless?—Fibroblasts as targets for scarless wound healing: A narrative review. Scars Burns Heals. 2022, 8, 20595131221095348. [Google Scholar] [CrossRef]
- Niazmand, A.; Nedaeinia, R.; Vatandoost, N.; Jafarpour, S.; Safabakhsh, S.; Kolahdouz, M.; Ferns, G.A.; Salehi, R. The impacts of dipeptidyl-peptidase 4 (DPP-4) inhibitors on common female malignancies: A systematic review. Gene 2024, 927, 148659. [Google Scholar] [CrossRef]
- Worthen, C.A.; Cui, Y.; Orringer, J.S.; Johnson, T.M.; Voorhees, J.J.; Fisher, G.J. CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin. J. Investig. Dermatol. 2020, 140, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Michalak-Micka, K.; Klar, A.S.; Dasargyri, A.; Biedermann, T.; Reichmann, E.; Moehrlen, U. regeneration of human dermo-epidermal skin substitutes. Sci. Rep. 2022, 12, 1944. [Google Scholar] [CrossRef]
- Thielitz, A.; Vetter, R.W.; Schultze, B.; Wrenger, S.; Simeoni, L.; Ansorge, S.; Neubert, K.; Faust, J.; Lindenlaub, P.; Gollnick, H.P.M.; et al. Inhibitors of dipeptidyl peptidase IV-like activity mediate antifibrotic effects in normal and keloid-derived skin fibroblasts. J. Investig. Dermatol. 2008, 128, 855–866. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Zhu, M.; Qu, M.; Bogari, M.; Lin, L.; Aung, Z.M.; Chen, W.; Chen, X.; Chai, G.; et al. Expansion of CD26 positive fibroblast population promotes keloid progression. Exp. Cell Res. 2017, 356, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mamoun, L.S.; Rosenbloom, A.J.; Gasbeck, T.; Ziegler, M.; Widgerow, A.D. From glucose to gauze: A systematic review on the various wound healing properties of DPP-4 inhibitors beyond glycaemic control. Wound Repair Regen. 2025, 33, e70090. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.W.; Zhang, Z.Y.; Wu, J.J.; Yuan, Z.D.; Yuan, F.L.; Chen, J. Inhibiting dipeptidyl peptidase 4 positive fibroblasts using zinc sulfide cellulose nanofiber scaffolds to achieve scarless healing. Int. J. Biol. Macromol. 2024, 282 Pt 6, 137525. [Google Scholar] [CrossRef] [PubMed]
- Hudelist, G.; Lass, H.; Keckstein, J.; Walter, I.; Wieser, F.; Wenzl, R.; Mueller, R.; Czerwenka, K.; Kubista, E.; Singer, C.F. Interleukin 1α and tissue-lytic matrix metalloproteinase-1 are elevated in ectopic endometrium of patients with endometriosis. Hum. Reprod. 2005, 6, 1695–1701. [Google Scholar] [CrossRef]
- Kondera-Anasz, Z.; Sikora, J.; Mielczarek-Palacz, A.; Jońca, M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1sRII) and IL-1 receptor antagonist (IL-1Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 198–203. [Google Scholar] [CrossRef]
- Sahakian, V.; Anners, J.; Haskill, S.; Halme, J. Selective localization of interleukin-1 receptor antagonist in eutopic endometrium and endometriotic implants. Fertil. Steril. 1993, 60, 276–279. [Google Scholar] [CrossRef]
- McGuinness, C.; Wesley, U.V. Dipeptidyl peptidase IV(DPPIV), a candidate tumor suppressor gene in melanomas is silenced by promoter methylation. Front. Biosci. 2008, 13, 2435–2543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riaz, M.A.; Mecha, E.O.; Omwandho, C.O.A.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. The different gene expression profile in eutopic and ectopic endometrium sheds new light on the endometrial seed in endometriosis. Biomedicines 2024, 12, 1276. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fu, J.; Zhou, M.; Xiao, L.; Feng, X.; Chen, H.; Huang, W. Activated hippo/yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.C.P.; Heryet, A.R.; Bicknell, R. Immunohistochemical properties of the endothelial cells in the human uterus during the menstrual cycle. Hum. Reprod. 1993, 8, 1173–1178. [Google Scholar] [CrossRef]
- Lin, S.C.; Lee, H.C.; Hou, P.C.; Fu, J.L.; Wu, M.H.; Tsai, S.J. Targeting hypoxia–mediated YAP1 nuclear translocation ameliorates pathogenesis of endometriosis without compromising maternal fertility. J. Pathol. 2017, 242, 476–487. [Google Scholar] [CrossRef]

| Tissues | EM-P | EM-S | p Value |
|---|---|---|---|
| All samples (n) | 25 | 30 | |
| Age ± SD | 41.1 ± 8.0 | 40.3 ± 5.5 | n.s. |
| Adenomyosis | 13 | 12 | |
| Endometriosis | 14 | 16 | |
| Leiomyoma | 10 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.A.; Pecher, C.M.; Kary, F.L.; Maoga, J.B.; Dietze, R.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. The Contribution of CD26-Negative Fibroblasts to Endometrial Scarring. Biomolecules 2025, 15, 1433. https://doi.org/10.3390/biom15101433
Riaz MA, Pecher CM, Kary FL, Maoga JB, Dietze R, Zeppernick F, Meinhold-Heerlein I, Konrad L. The Contribution of CD26-Negative Fibroblasts to Endometrial Scarring. Biomolecules. 2025; 15(10):1433. https://doi.org/10.3390/biom15101433
Chicago/Turabian StyleRiaz, Muhammad Assad, Clara Marie Pecher, Franziska Louisa Kary, Jane Bosibori Maoga, Raimund Dietze, Felix Zeppernick, Ivo Meinhold-Heerlein, and Lutz Konrad. 2025. "The Contribution of CD26-Negative Fibroblasts to Endometrial Scarring" Biomolecules 15, no. 10: 1433. https://doi.org/10.3390/biom15101433
APA StyleRiaz, M. A., Pecher, C. M., Kary, F. L., Maoga, J. B., Dietze, R., Zeppernick, F., Meinhold-Heerlein, I., & Konrad, L. (2025). The Contribution of CD26-Negative Fibroblasts to Endometrial Scarring. Biomolecules, 15(10), 1433. https://doi.org/10.3390/biom15101433







