Advancing CAR-T Therapy for Solid Tumors: From Barriers to Clinical Progress
Abstract
1. Introduction
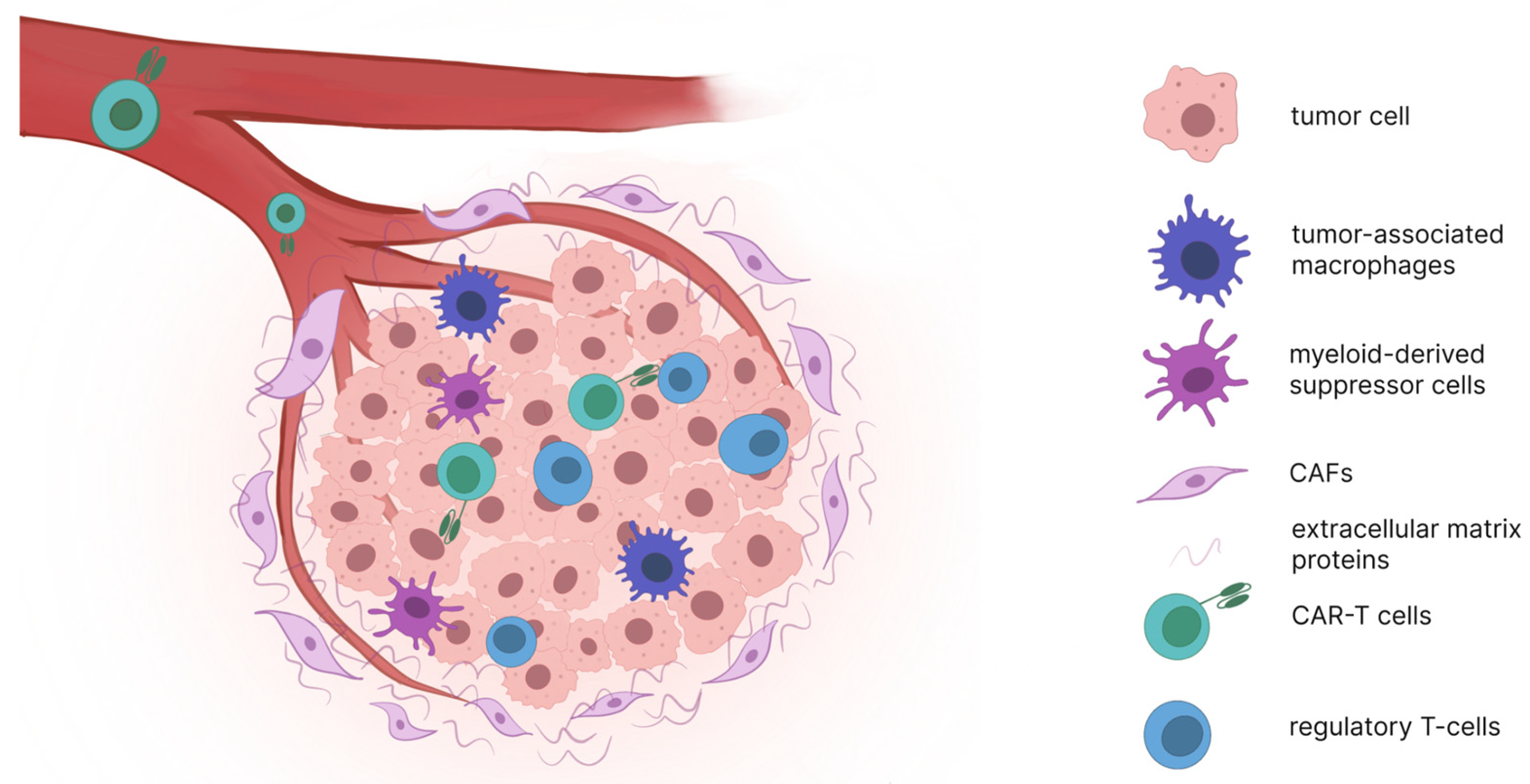
2. Barriers to Effective CAR-T Cell Therapy in Solid Tumors
3. Therapeutic Outcomes of 2nd and 3rd Generation CAR-T Cells in Solid Tumors
3.1. Off-Tumor Antigen-Specific Activation
3.2. Heterogeneity in Antigen Expression
3.3. Limited Persistence and Exhaustion
4. Results of Clinical Trials of Next-Generation CAR-T Cells
4.1. Strategies Aimed at Increasing Persistence, Infiltration, and Effector Functions
4.2. Blockade of Tumor Microenvironment Immunosuppression
4.3. Strategy Aimed at Overcoming Heterogeneity
4.4. Strategy Aimed at Overcoming Off-Tumor Antigen-Specific Activation
4.5. Local vs. Systemic Administration
4.6. Allogeneic (“Off-the-Shelf”) CAR-T Cell Therapies
| Tumor Type | Target Antigen | CAR Design (Conventional) | CAR Design (Next-Gen) | ORR (Conventional) | ORR (Next-Gen) | Key Engineering Mechanism Driving Improvement | References (2G → 4G) |
|---|---|---|---|---|---|---|---|
| Hepatocellular Carcinoma | GPC3 | 2G (CD3ζ + 4-1BB) | 4G (IL-15 secretion) | 0% (0/12) | 33.3% (4/12) | IL-15 enhances proliferation, cytolytic function, survival | [77] → [77] |
| Hepatocellular Carcinoma | GPC3 | 2G (CD3ζ + 4-1BB) | 4G (dnTGFβRII) | 0% (0/12) | 57% (14/24) | Dominant-negative TGFβRII blocks immunosuppression | [77] → [85] |
| Glioblastoma | EGFRvIII | 2G (CD3ζ + 4-1BB) | 4G (EGFR-BiTE secretion) | 0% (0/10) | 100% (3/3) | BiTE recruits endogenous T-cells against antigen escape | [42] → [93] |
| Neuroblastoma | GD2 | 2G (CD3ζ + CD28) | 3G + cytokine culture (IL-7/15) + iCasp9 | 27% (3/11 partial responses) | 63% (17/27) | Dual co-stimulation + cytokine conditioning + inducible caspase safety system | [103] → [72] |
| Colorectal Cancer | CEA | 2G (CD3ζ + CD28) | 4G (Hypoxia-activated) | 0% (0/10) | 25% (3/14) IP route | Hypoxia-switch restricts activation to tumor, reducing toxicity | [98] → [97] |
| Mesothelioma | Mesothelin | 2G (toxic, no efficacy reported) | 4G (PD-1 nanobody secretion) | — | 63.6% (7/11) | Local PD-1 blockade reverses TME immunosuppression | [49] → [89] |
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | Chimeric Antigen Receptor |
| ORR | Overall Response Rate |
| SD | Stable disease |
| PR | Partial response |
| DCR | Disease Control Rate |
References
- Mullard, A. FDA approves first tumour-infiltrating lymphocyte (TIL) therapy, bolstering hopes for cell therapies in solid cancers. Nat. Rev. Drug Discov. 2024, 23, 238. [Google Scholar] [CrossRef]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef]
- Qian, K.; Li, G.; Zhang, S.; Fu, W.; Li, T.; Zhao, J.; Lei, C.; Wang, Y.; Hu, S. CAR-T-cell products in solid tumors: Progress, challenges, and strategies. Interdiscip. Med. 2024, 2, e20230047. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; He, Z.; Li, L.; Liu, S.; Jiang, M.; Zhao, B.; Deng, M.; Wang, W.; Mi, X.; et al. Breakthrough of solid tumor treatment: CAR-NK immunotherapy. Cell Death Discov. 2024, 10, 40. [Google Scholar] [CrossRef]
- Chen, K.; Liu, M.L.; Wang, J.C.; Fang, S. CAR-macrophage versus CAR-T for solid tumors: The race between a rising star and a superstar. Biomol. Biomed. 2024, 24, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, Q. Clinical Progresses and Challenges of Bispecific Antibodies for the Treatment of Solid Tumors. Mol. Diagn. Ther. 2024, 28, 669–702. [Google Scholar] [CrossRef]
- Liguori, L.; Polcaro, G.; Nigro, A.; Conti, V.; Sellitto, C.; Perri, F.; Ottaiano, A.; Cascella, M.; Zeppa, P.; Caputo, A.; et al. Bispecific Antibodies: A Novel Approach for the Treatment of Solid Tumors. Pharmaceutics 2022, 14, 2442. [Google Scholar] [CrossRef]
- Hamieh, M.; Mansilla-Soto, J.; Rivière, I.; Sadelain, M. Programming CAR T Cell Tumor Recognition: Tuned Antigen Sensing and Logic Gating. Cancer Discov. 2023, 13, 829–843. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Huang, K.; Zeng, H.; Jing, J.; Wang, R.; Fang, S.; Chen, J.; Liu, X.; Huang, Z.; You, M.J.; et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 2021, 16, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Tousley, A.M.; Rotiroti, M.C.; Labanieh, L.; Rysavy, L.W.; Kim, W.-J.; Lareau, C.; Sotillo, E.; Weber, E.W.; Rietberg, S.P.; Dalton, G.N.; et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 2023, 615, 507–516. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef]
- Maher, J.; Brentjens, R.J.; Gunset, G.; Rivière, I.; Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 2002, 20, 70–75. [Google Scholar] [CrossRef]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef]
- Hawkins, E.R.; D’Souza, R.R.; Klampatsa, A. Armored CAR T-Cells: The Next Chapter in T-Cell Cancer Immunotherapy. Biologics 2021, 15, 95–105. [Google Scholar] [CrossRef]
- Tang, L.; Pan, S.; Wei, X.; Xu, X.; Wei, Q. Arming CAR-T cells with cytokines and more: Innovations in the fourth-generation CAR-T development. Mol. Ther. 2023, 31, 3146–3162. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert. Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Li, Z.; Zhou, J.; Wang, W. Current Status and Perspectives of Dual-Targeting Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Hematological Malignancies. Cancers 2022, 14, 3230. [Google Scholar] [CrossRef]
- Kloss, C.C.; Condomines, M.; Cartellieri, M.; Bachmann, M.; Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013, 31, 71–75. [Google Scholar] [CrossRef]
- Anderson, G.S.F.; Walker, I.; Roy, J.P.; Chapman, M.A. And-Gate CAR T-Cells to Improve Tumour Specificity and Targeting of Low-Expression Antigens in Multiple Myeloma. Blood 2023, 142, 751. [Google Scholar] [CrossRef]
- Patel, R.P.; Ghilardi, G.; Zhang, Y.; Chiang, Y.-H.; Xie, W.; Guruprasad, P.; Kim, K.H.; Chun, I.; Angelos, M.G.; Pajarillo, R.; et al. CD5 deletion enhances the antitumor activity of adoptive T cell therapies. Sci. Immunol. 2024, 9, eadn6509. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; López-Moyado, I.F.; Seo, H.; Lio, C.J.; Hempleman, L.J.; Sekiya, T.; Yoshimura, A.; Scott-Browne, J.P.; Rao, A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567, 530–534. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, A.J.; Yamazaki, T.; McCarty, K.; Fox, N.; Phillips, M.; Alice, A.; Blair, T.; Whiteford, M.; O’Brien, D.; Ahmad, R.; et al. TGFβ suppresses CD8(+) T cell expression of CXCR3 and tumor trafficking. Nat. Commun. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Sakemura, R.; Hefazi, M.; Siegler, E.L.; Cox, M.J.; Larson, D.P.; Hansen, M.J.; Manriquez Roman, C.; Schick, K.J.; Can, I.; Tapper, E.E.; et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood 2022, 139, 3708–3721. [Google Scholar] [CrossRef]
- Li, N.; Rodriguez, J.L.; Yin, Y.; Logun, M.T.; Zhang, L.; Yu, S.; Hicks, K.A.; Zhang, J.V.; Zhang, L.; Xie, C.; et al. Armored bicistronic CAR T cells with dominant-negative TGF-β receptor II to overcome resistance in glioblastoma. Mol. Ther. 2024, 32, 3522–3538. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef]
- Lanitis, E.; Irving, M.; Coukos, G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 2015, 33, 55–63. [Google Scholar] [CrossRef]
- Sterner, R.M.; Kenderian, S.S. Myeloid cell and cytokine interactions with chimeric antigen receptor-T-cell therapy: Implication for future therapies. Curr. Opin. Hematol. 2020, 27, 41–48. [Google Scholar] [CrossRef]
- Watson, M.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 2021, 591, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef]
- Ho, P.-C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.-C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef]
- Beavis, P.A.; Milenkovski, N.; Henderson, M.A.; John, L.B.; Allard, B.; Loi, S.; Kershaw, M.H.; Darcy, P.K.; Moynihan, K.D. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti–PD-1 Therapy in Melanoma. Cancer Immunol. Res. 2015, 3, 1090–1099. [Google Scholar] [CrossRef]
- Sorrentino, C.; Di Sanza, C.; D’Alessio, A.; Lucarini, V.; Sterpa, L.; Ferrone, A.; Caratelli, S.; Menna, G.; Di Rocco, A.; Ferrandina, G.; et al. Adenosine Deaminase Activity in Cancer Immunotherapy. Front. Immunol. 2021, 12, 678123. [Google Scholar]
- Sackstein, R.; Schatton, T.; Barthel, S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Investig. 2017, 97, 669–697. [Google Scholar] [CrossRef]
- Albelda, S.M. CAR T cell therapy for patients with solid tumours: Key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 2024, 21, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Textor, A.; Grunewald, L.; Anders, K.; Klaus, A.; Schwiebert, S.; Winkler, A.; Stecklum, M.; Rolff, J.; Henssen, A.G.; Höpken, U.E.; et al. CD28 Co-Stimulus Achieves Superior CAR T Cell Effector Function against Solid Tumors Than 4-1BB Co-Stimulus. Cancers 2021, 13, 1050. [Google Scholar] [CrossRef]
- Kantari-Mimoun, C.; Barrin, S.; Vimeux, L.; Haghiri, S.; Gervais, C.; Joaquina, S.; Mittelstaet, J.; Mockel-Tenbrinck, N.; Kinkhabwala, A.; Damotte, D.; et al. CAR T-cell Entry into Tumor Islets Is a Two-Step Process Dependent on IFNγ and ICAM-1. Cancer Immunol. Res. 2021, 9, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Wang, L.C.; Dolfi, D.V.; Wilson, C.B.; Ranganathan, R.; Sun, J.; Kapoor, V.; Scholler, J.; Puré, E.; Milone, M.C.; et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014, 20, 4262–4273. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.P.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: A phase 1 trial. Nat. Cancer 2024, 5, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.-y.; Chen, T.; Chang, L.-J.; et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol. Cancer 2023, 22, 3. [Google Scholar] [CrossRef]
- Gargett, T.; Truong, N.T.H.; Gardam, B.; Yu, W.; Ebert, L.M.; Johnson, A.; Yeo, E.C.F.; Wittwer, N.L.; Tapia Rico, G.; Logan, J.; et al. Safety and biological outcomes following a phase 1 trial of GD2-specific CAR-T cells in patients with GD2-positive metastatic melanoma and other solid cancers. J. Immunother. Cancer 2024, 12, e008659. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Haas, A.R.; Golden, R.J.; Litzky, L.A.; Engels, B.; Zhao, L.; Xu, F.; Taraszka, J.A.; Ramones, M.; Granda, B.; Chang, W.-J.; et al. Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T-cells. Mol. Ther. 2023, 31, 2309–2325. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Padfield, E.; Ellis, H.P.; Kurian, K.M. Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front. Oncol. 2015, 5, 5. [Google Scholar] [CrossRef]
- Hegde, M.; Navai, S.; DeRenzo, C.; Joseph, S.K.; Sanber, K.; Wu, M.; Gad, A.Z.; Janeway, K.A.; Campbell, M.; Mullikin, D.; et al. Autologous HER2-specific CAR T cells after lymphodepletion for advanced sarcoma: A phase 1 trial. Nat. Cancer 2024, 5, 880–894. [Google Scholar] [CrossRef]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell. Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Gong, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial final results. Nat. Med. 2024, 30, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, F.; Dusi, S.; Caligola, S.; Anselmi, C.; Petrova, V.; Rossi, B.; Angelini, G.; Erdeljan, M.; Wöll, S.; Schlitter, A.M.; et al. Expression of the membrane tetraspanin claudin 18 on cancer cells promotes T lymphocyte infiltration and antitumor immunity in pancreatic cancer. Immunity 2024, 57, 1378–1393.e1314. [Google Scholar] [CrossRef]
- Zhong, G.; Zhang, X.; Guo, Z.; Gao, Y.; Zhao, B.; Liu, X.; Chen, L.; Qiao, J.; Yu, C.; Wang, L.; et al. Complete remission of advanced pancreatic cancer induced by claudin18.2-targeted CAR-T cell therapy: A case report. Front. Immunol. 2024, 15, 1325860. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.T.; et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef]
- Monje, M.; Mahdi, J.; Majzner, R.; Yeom, K.W.; Schultz, L.M.; Richards, R.M.; Barsan, V.; Song, K.-W.; Kamens, J.; Baggott, C.; et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 2024, 632, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat. Med. 2018, 66, 572–579. [Google Scholar] [CrossRef]
- Mackensen, A.; Haanen, J.; Koenecke, C.; Alsdorf, W.; Wagner-Drouet, E.; Borchmann, P.; Heudobler, D.; Ferstl, B.; Klobuch, S.; Bokemeyer, C.; et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: The phase 1 BNT211-01 trial. Nat. Med. 2023, 29, 2844–2853. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Du, H.; Yang, X.; Fan, J.; Du, X. Claudin 6: Therapeutic prospects for tumours, and mechanisms of expression and regulation (Review). Mol. Med. Rep. 2021, 24, 677. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.; Li, Y.; Liu, K.; Zhao, Q.; Ouyang, L. Identification of Claudin-6 as a Molecular Biomarker in Pan-Cancer Through Multiple Omics Integrative Analysis. Front. Cell Dev. Biol. 2021, 9, 726656. [Google Scholar] [CrossRef]
- Daei Sorkhabi, A.; Mohamed Khosroshahi, L.; Sarkesh, A.; Mardi, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Baradaran, B. The current landscape of CAR T-cell therapy for solid tumors: Mechanisms, research progress, challenges, and counterstrategies. Front. Immunol. 2023, 14, 1113882. [Google Scholar] [CrossRef]
- Amorós-Pérez, B.; Rivas-Pardo, B.; Gómez del Moral, M.; Subiza, J.L.; Martínez-Naves, E. State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future? Cells 2024, 13, 725. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, L.; Yang, M.; Chen, X.; Liu, H.; Wang, Q.; Guo, M.; Luo, J. CAR-armored-cell therapy in solid tumor treatment. J. Transl. Med. 2024, 22, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mobark, N.; Hull, C.M.; Maher, J. Optimising CAR T therapy for the treatment of solid tumors. Expert. Rev. Anticancer. Ther. 2024, 24, 1–17. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Quintarelli, C.; Orlando, D.; Boffa, I.; Guercio, M.; Polito, V.A.; Petretto, A.; Lavarello, C.; Sinibaldi, M.; Weber, G.; Del Bufalo, F.; et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. OncoImmunology 2018, 7, e1433518. [Google Scholar] [CrossRef]
- Tumino, N.; Weber, G.; Besi, F.; Del Bufalo, F.; Bertaina, V.; Paci, P.; Quatrini, L.; Antonucci, L.; Sinibaldi, M.; Quintarelli, C.; et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CAR T-cells in patients with neuroblastoma. J. Hematol. Oncol. 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.S.M.; Dardalhon, V.; Devaud, C.; Taylor, N.; Darcy, P.K.; Kershaw, M.H. CAR T-cell therapy of solid tumors. Immunol. Cell Biol. 2017, 95, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Sasso, M.S.; Obenauf, A.C.; Fredriksen, T.; Lafontaine, L.; Bilocq, A.M.; Kirilovsky, A.; Tosolini, M.; et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl. Med. 2014, 6, 228ra37. [Google Scholar] [CrossRef]
- Steffin, D.; Ghatwai, N.; Montalbano, A.; Rathi, P.; Courtney, A.N.; Arnett, A.B.; Fleurence, J.; Sweidan, R.; Wang, T.; Zhang, H.; et al. Interleukin-15-armoured GPC3 CAR T cells for patients with solid cancers. Nature 2024, 632, 424–432. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, Y.-F.; Zhang, W.; Chen, X.; Xu, T.; Hu, S.; Liang, X.; Feng, M.; Yang, X.; Ho, M. Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells. Sci. Rep. 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Pawel, B.R.; Corao, D.A.; Venneti, S.; Russo, P.; Santi, M.; Sullivan, L.M. Immunohistochemical expression of glypican-3 in pediatric tumors: An analysis of 414 cases. Pediatr. Dev. Pathol. 2013, 16, 272–277. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef]
- Hu, J.F.; Wang, Z.W.; Liao, C.Y.; Chen, Z.W.; Kang, F.P.; Lin, C.F.; Lin, T.S.; Huang, L.; Tian, Y.F.; Chen, S. Induced expression of CCL19 promotes the anti-tumor ability of CAR-T cells by increasing their infiltration ability. Front. Immunol. 2022, 13, 958960. [Google Scholar] [CrossRef]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, Q.; Cao, W.; Wang, H.; Xu, X.; Huang, J.; Zou, A.; Zhu, J.; Wan, H.; Yao, Y.; et al. Phase I study of C-CAR031, a GPC3-specific TGFβRIIDN armored autologous CAR-T, in patients with advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2024, 42, 4019. [Google Scholar] [CrossRef]
- Hosseini, R.; Hosseinzadeh, N.; Asef-Kabiri, L.; Akbari, A.; Ghezelbash, B.; Sarvnaz, H.; Akbari, M.E. Small extracellular vesicle TGF-β in cancer progression and immune evasion. Cancer Gene Ther. 2023, 30, 1309–1322. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel Iii, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Li, L.; Sun, Y.; Lin, Z.; Rong, L.; Zhu, Z.; Song, Z.; Xue, H.; Duan, J.; et al. Abstract CT134: Non-viral mesothelin-targeted CAR-T cells armored with IFNg-induced secretion of PD-1 nanobody in treatment of malignant mesothelioma in phase I clinical trial. Cancer Res. 2023, 83, CT134. [Google Scholar] [CrossRef]
- Weller, M.; Roth, P.; Tabatabai, G.; Schittenhelm, J.; Wick, W. EGFR in Glioblastoma: From Biology to Therapy. Cancers 2021, 13, 5453. [Google Scholar] [CrossRef]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef]
- Choi, B.D.; Gedeon, P.C.; Herndon, J.E., 2nd; Archer, G.E.; Reap, E.A.; Sanchez-Perez, L.; Mitchell, D.A.; Bigner, D.D.; Sampson, J.H. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol. Res. 2013, 1, 163. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Kankanala, V.L.; Mukkamalla, S.K.R. Carcinoembryonic Antigen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhu, X.; Li, J.; Gao, Y.; Zhang, Y.; Tong, Z.; Fu, Q.; Bao, X.; Li, B.; et al. Phase I trial of hypoxia-responsive CEA CAR-T cell therapy in patients with heavily pretreated solid tumor via intraperitoneal or intravenous transfusion. J. Clin. Oncol. 2024, 42, 3514. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Li, W.; Xu, Y.; Shan, J.; Hong, J.; Zhao, Y.; Xu, H.; Ma, J.; Shen, J.; et al. Hypoxia-Responsive CAR-T Cells Exhibit Reduced Exhaustion and Enhanced Efficacy in Solid Tumors. Cancer Res. 2024, 84, 84–100. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017, 9, eaaj2013. [Google Scholar] [CrossRef] [PubMed]
- Baurain, J.-F.; Van den Eynde, M.; Derosa, L.; Marabelle, A.; Hollebecque, A.; Italiano, A.; Borg, C.; Taieb, J.; Guégan, J.-P.; Gomez-Roca, C.; et al. Phase 1 Trial of Allogeneic NKG2D-Based CAR T-Cell Therapy (CYAD-101) in Combination with FOLFOX Chemotherapy in Patients with Metastatic Colorectal Cancer: Final Results from the alloSHRINK Trial. J. Immunother. Cancer 2023, 11, 005123. [Google Scholar]
- Celyad Oncology. Clinical Study Report: NCT03692429—A Phase 1 Study of CYAD-101 in Combination with FOLFOX in mCRC. 2023. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03692429 (accessed on 15 May 2024).
- The Official ClinicalTrials.gov Registry Entry: U.S. National Library of Medicine. ALLO-316 in Advanced or Metastatic Clear Cell Renal Cell Carcinoma (alpha3). ClinicalTrials.gov Identifier: NCT05446447. Available online: https://clinicaltrials.gov/study/NCT05446447 (accessed on 29 September 2025).
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Feucht, J.; Sun, J.; Eyquem, J.; Ho, Y.-J.; Zhao, Z.; Leibold, J.; Dobrin, A.; Cabriolu, A.; Hamieh, M.; Sadelain, M. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019, 25, 82–88. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, S.; Zaritsky, Y.; Silonov, S.; Gavrilova, A.; Fonin, A. Advancing CAR-T Therapy for Solid Tumors: From Barriers to Clinical Progress. Biomolecules 2025, 15, 1407. https://doi.org/10.3390/biom15101407
Smirnov S, Zaritsky Y, Silonov S, Gavrilova A, Fonin A. Advancing CAR-T Therapy for Solid Tumors: From Barriers to Clinical Progress. Biomolecules. 2025; 15(10):1407. https://doi.org/10.3390/biom15101407
Chicago/Turabian StyleSmirnov, Sergei, Yuriy Zaritsky, Sergey Silonov, Anastasia Gavrilova, and Alexander Fonin. 2025. "Advancing CAR-T Therapy for Solid Tumors: From Barriers to Clinical Progress" Biomolecules 15, no. 10: 1407. https://doi.org/10.3390/biom15101407
APA StyleSmirnov, S., Zaritsky, Y., Silonov, S., Gavrilova, A., & Fonin, A. (2025). Advancing CAR-T Therapy for Solid Tumors: From Barriers to Clinical Progress. Biomolecules, 15(10), 1407. https://doi.org/10.3390/biom15101407







