Multi-Omics Alterations in Rat Kidneys upon Chronic Glyphosate Exposure
Abstract
1. Introduction
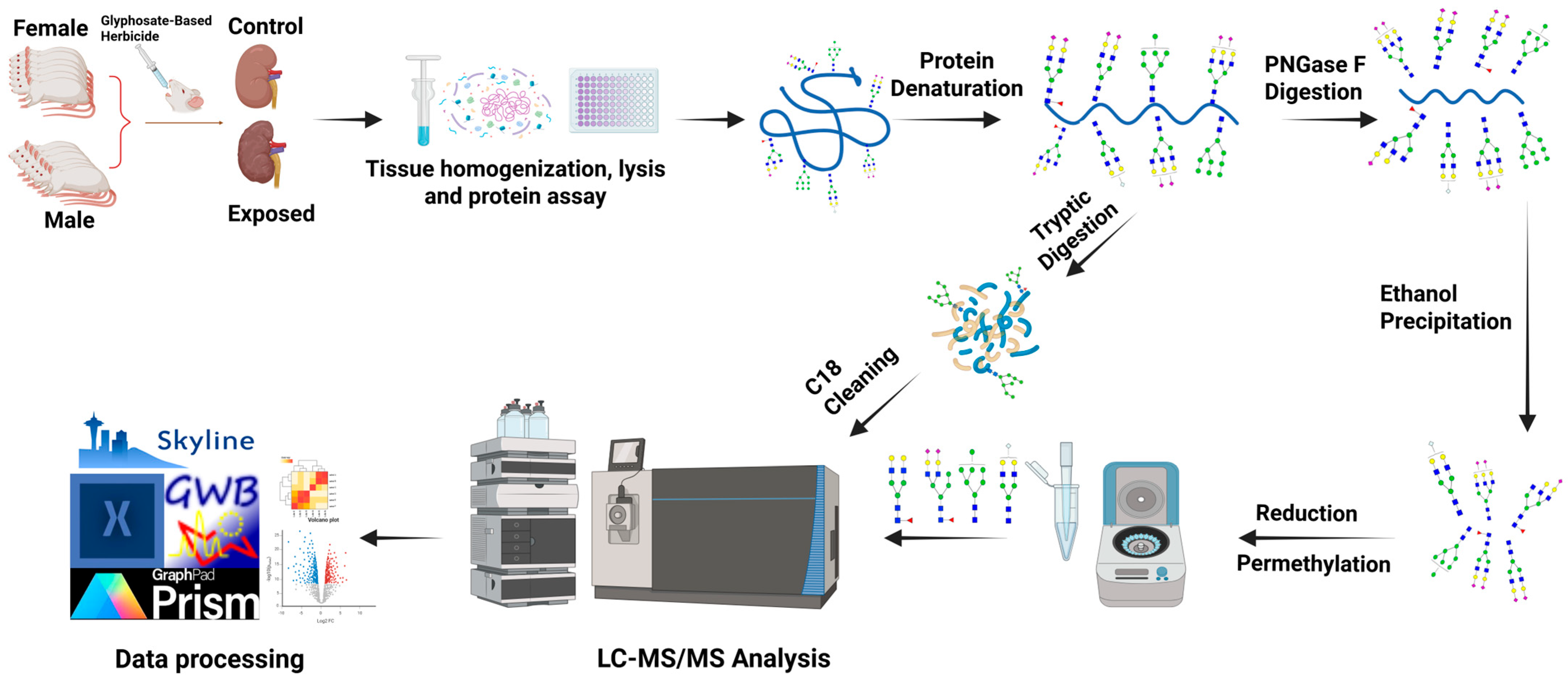
2. Materials and Methods
2.1. Materials and Reagent
2.2. Animal Study
2.3. Tissue Homogenization, Lysis, and Protein Extraction
2.4. N-Glycan Release, Purification, Reduction, and Permethylation
2.5. Tryptic Digestion
2.6. C18 Desalting
2.7. LC-MS/MS Glycomics Analysis
2.8. LC-MS/MS Proteomics Analysis
2.9. Data Analysis
3. Results
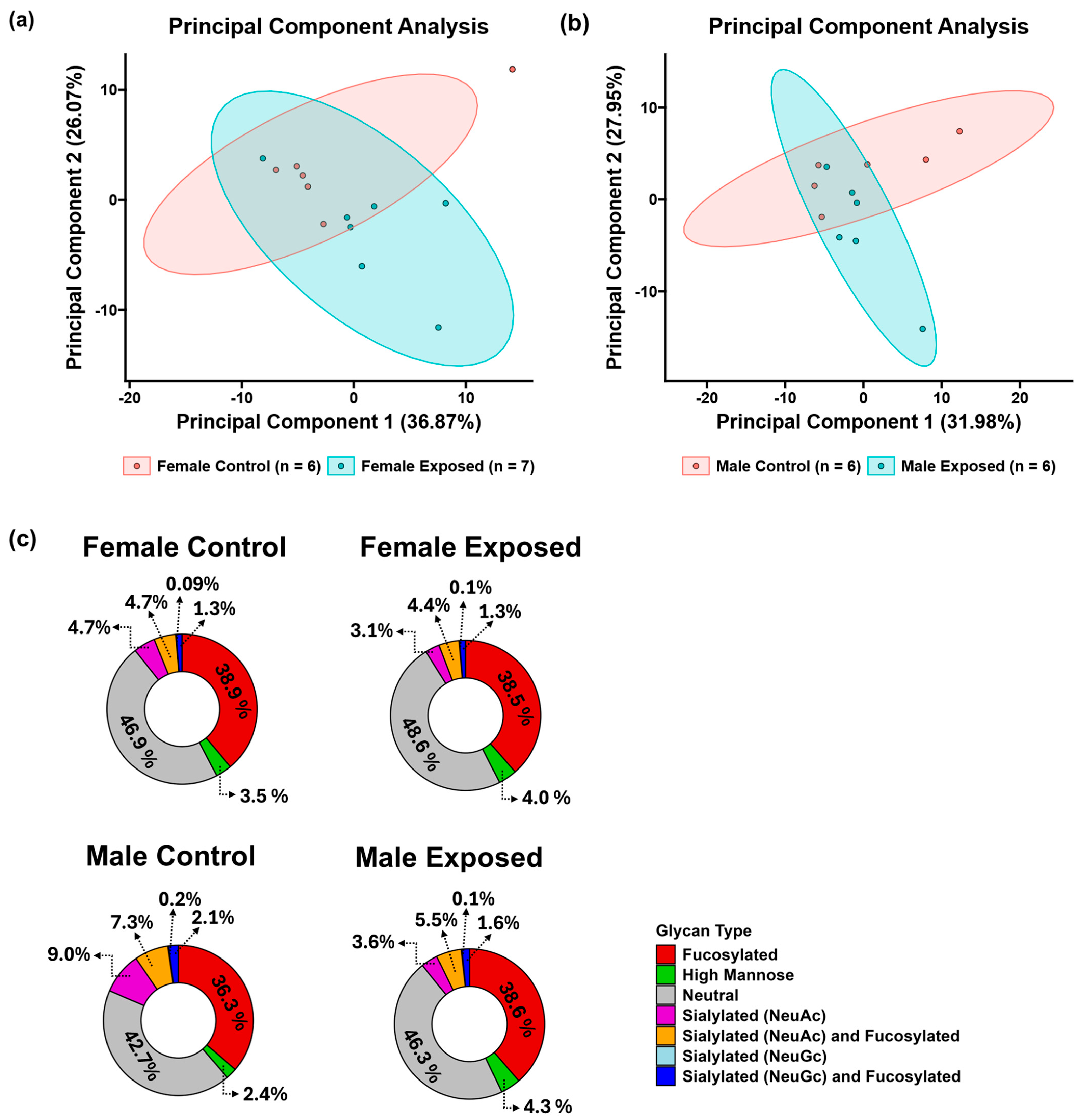
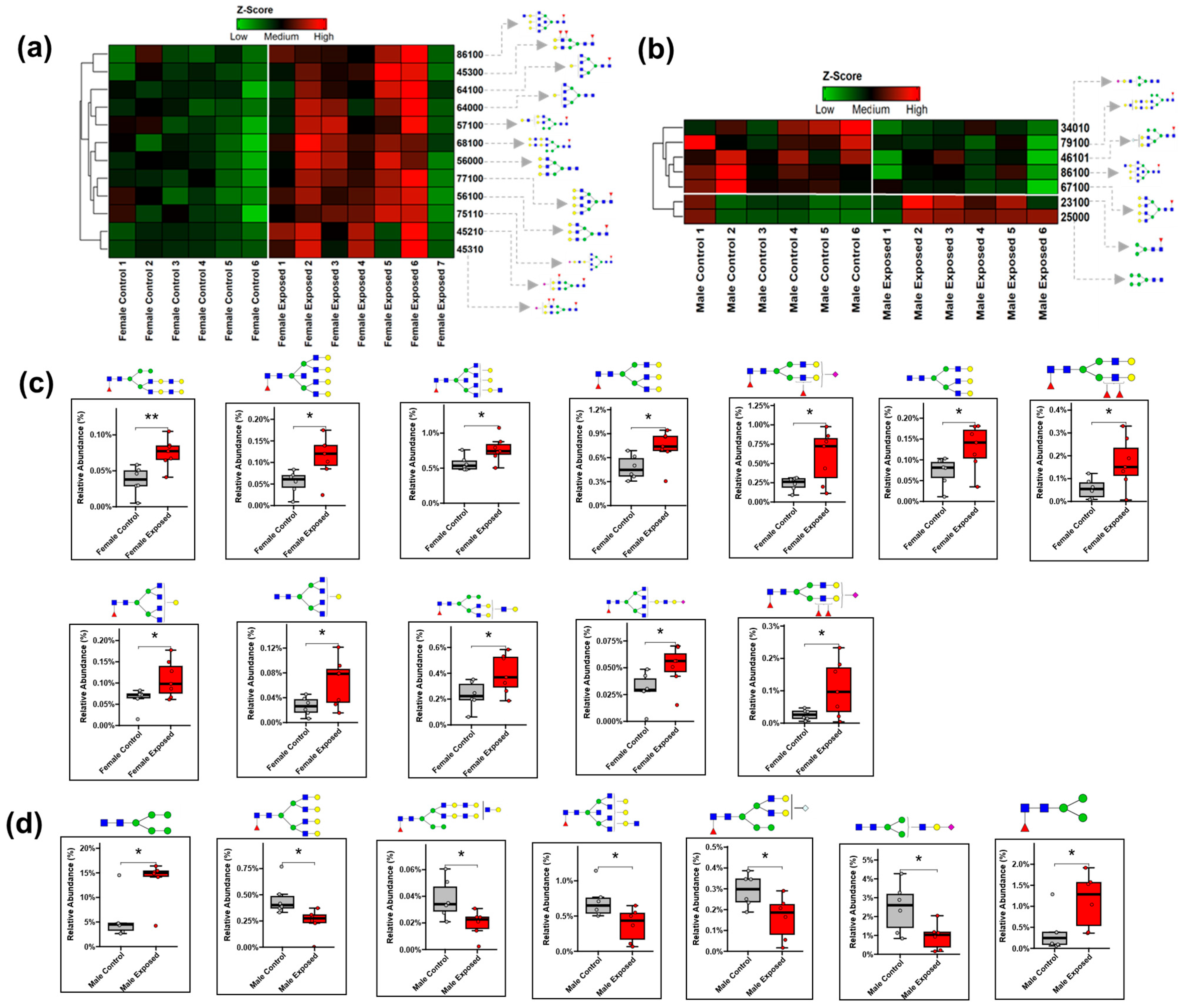
3.1. N-Glycomics Analysis
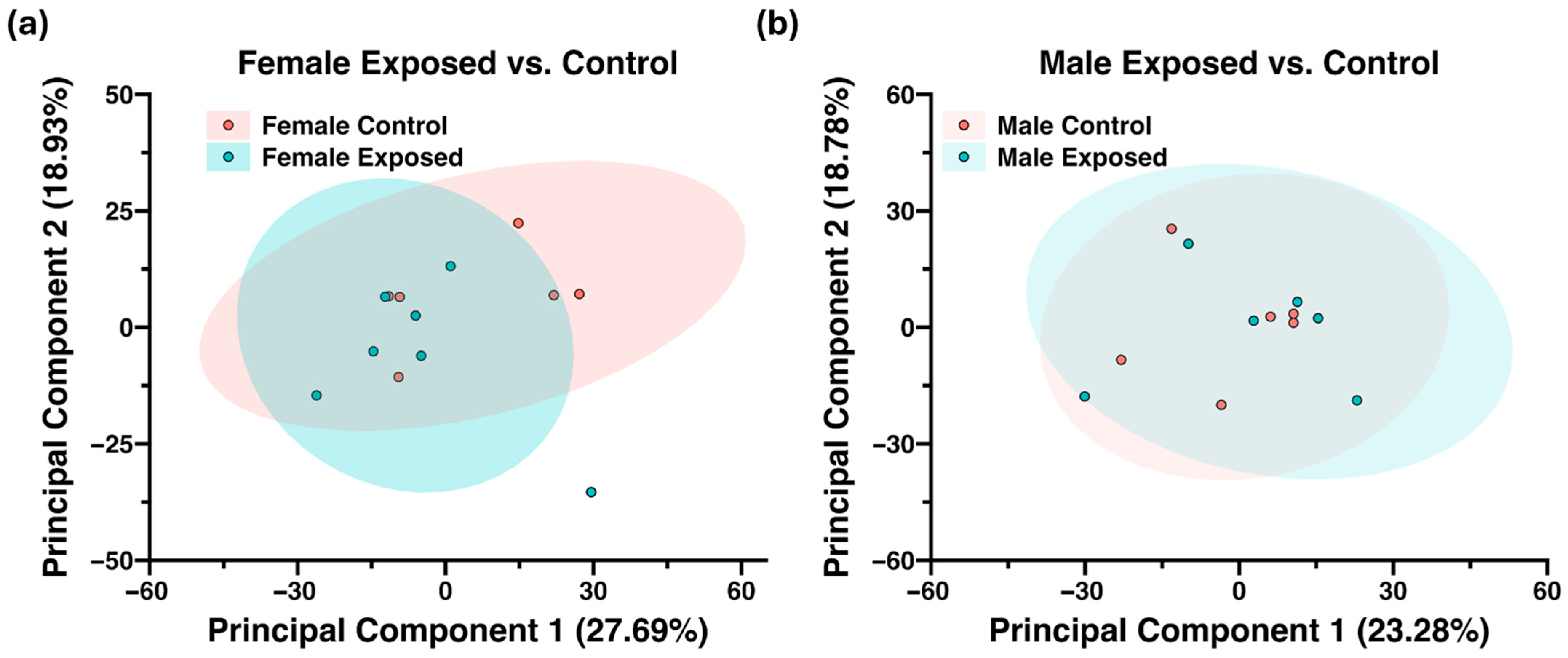
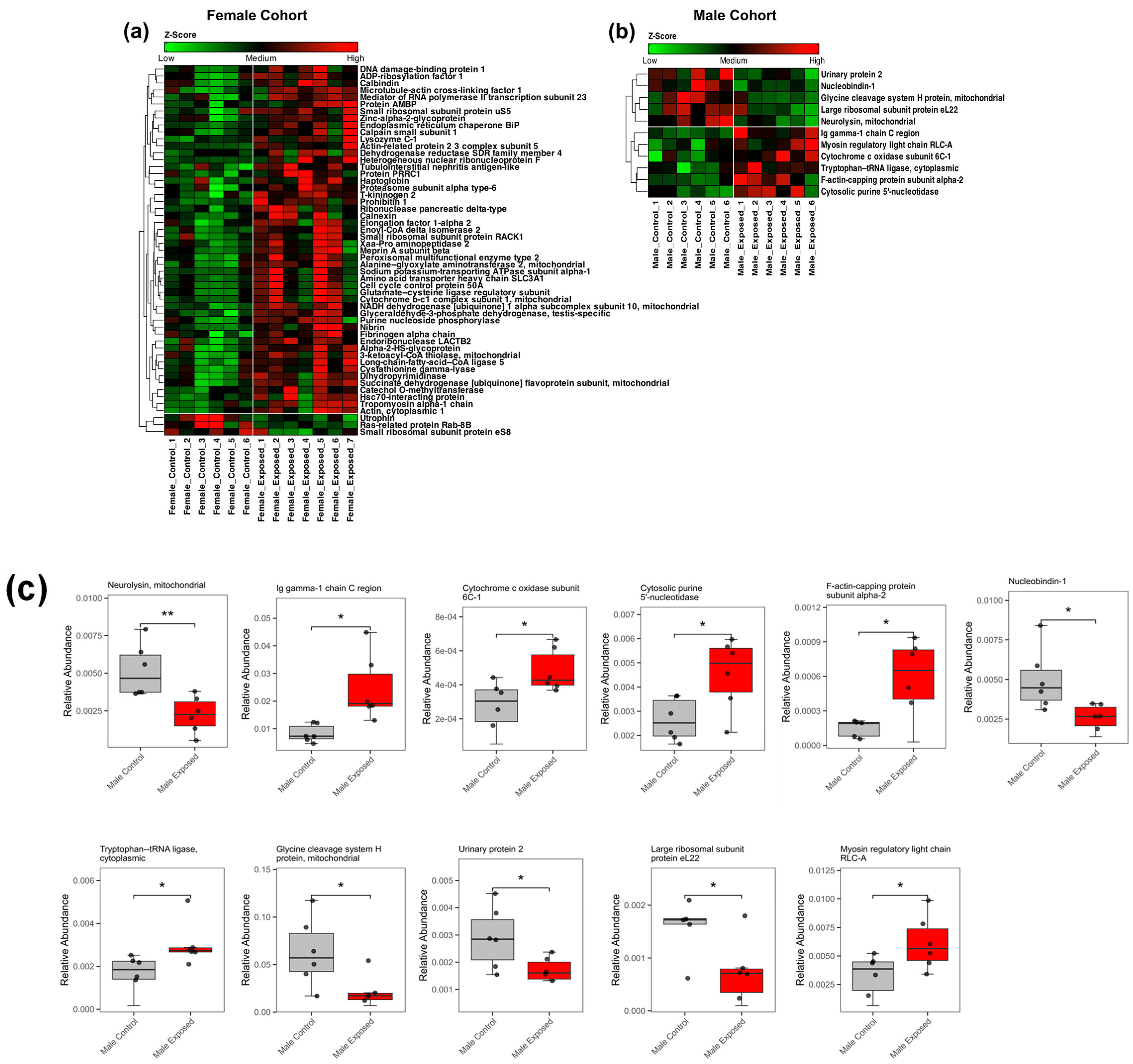
3.2. Proteomics Analysis
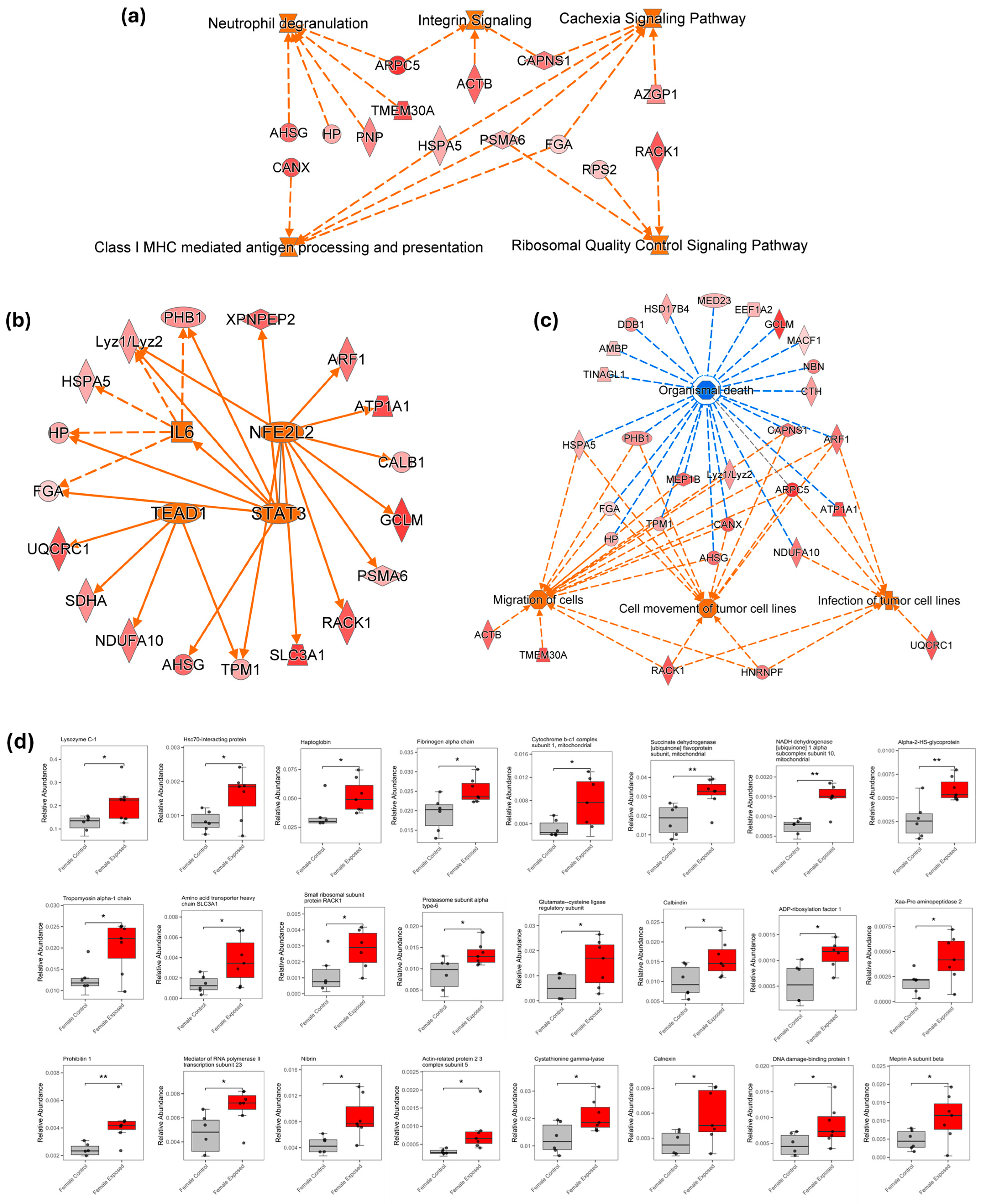
3.3. Ingenuity Pathway Analysis
4. Discussion
5. Perspective for Clinical Practice
6. Conclusions
Supplementary Materials
 , Gal (galactose)
, Gal (galactose)  , Man(mannose)
, Man(mannose)  , Fuc (fucose)
, Fuc (fucose)  , NeuAc (N-acetylneuraminic acid)
, NeuAc (N-acetylneuraminic acid)  , and NeuGc (N-glycolylneuraminic acid)
, and NeuGc (N-glycolylneuraminic acid)  . Figure S2: Representative MS and MS/MS identification process for the N-glycans. (a) Extracted ion chromatogram illustrating the core fucosylated N-glycan with the composition HexNAc4Hex3Fuc1 (4-3-1-0-0). Inset (b) shows the corresponding full MS spectra for the glycan. Inset (c) shows the tandem MS (MS/MS) spectrum of the HexNAc4Hex3Fuc1 structures, with the key fragment ions annotated adjacent to their respective peaks. N-glycan symbols are as in Figure S1. Figure S3: Two dimensional unsupervised principal component analysis (PCA) with a 95% confidence level (a), heatmap of the statistically significant N-glycan in the combined analysis of both cohorts. Total control (n = 12) vs. total GBH-exposed (n = 13) (b), and the boxplot of the two significant N-glycans in the combined analysis (c,d). N-glycan symbols are as in Figure S1. Figure S4: Two dimensional unsupervised principal component analysis (PCA) with a 95% confidence level (a), and heatmap (b) of the statistically significant proteins in the combined analysis of both cohorts. Total control (n = 12) vs. total GBH-exposed (n = 13). Figure S5: Summary of the implicated transcription regulators (a) and implicated diseases and functions (b) in the combined analysis of both female and male cohorts. (c) some of the differentially expressed proteins associated with diseases and functions (*—p value < 0.05; **—p value < 0.01). Table S1: The correlation coefficient (W) and pvalue from Shapiro–Wilk test confirming that the data is normally distributed. Table S2: The relative abundance of statistically significant N-glycans between the control and GBH-exposed group in the female cohort, their pvalues and AUC values. N-glycan symbols are as in Figure S1 (Note: Combined AUC value =1). Table S3: The relative abundance of statistically significant N-glycans between the control and GBH-Exposed group in the male cohort, their pvalues and AUC values. N-glycan symbols are as in Figure S1 (Note: Combined AUC value =1). Table S4: The relative abundance of statistically significant proteins in the female cohorts, their corresponding pvalues and AUC values. Table S5: The relative abundance of statistically significant proteins in the male cohorts, their corresponding p-values, and AUC values. The ARRIVE guidelines 2.0: Author Checklist.
. Figure S2: Representative MS and MS/MS identification process for the N-glycans. (a) Extracted ion chromatogram illustrating the core fucosylated N-glycan with the composition HexNAc4Hex3Fuc1 (4-3-1-0-0). Inset (b) shows the corresponding full MS spectra for the glycan. Inset (c) shows the tandem MS (MS/MS) spectrum of the HexNAc4Hex3Fuc1 structures, with the key fragment ions annotated adjacent to their respective peaks. N-glycan symbols are as in Figure S1. Figure S3: Two dimensional unsupervised principal component analysis (PCA) with a 95% confidence level (a), heatmap of the statistically significant N-glycan in the combined analysis of both cohorts. Total control (n = 12) vs. total GBH-exposed (n = 13) (b), and the boxplot of the two significant N-glycans in the combined analysis (c,d). N-glycan symbols are as in Figure S1. Figure S4: Two dimensional unsupervised principal component analysis (PCA) with a 95% confidence level (a), and heatmap (b) of the statistically significant proteins in the combined analysis of both cohorts. Total control (n = 12) vs. total GBH-exposed (n = 13). Figure S5: Summary of the implicated transcription regulators (a) and implicated diseases and functions (b) in the combined analysis of both female and male cohorts. (c) some of the differentially expressed proteins associated with diseases and functions (*—p value < 0.05; **—p value < 0.01). Table S1: The correlation coefficient (W) and pvalue from Shapiro–Wilk test confirming that the data is normally distributed. Table S2: The relative abundance of statistically significant N-glycans between the control and GBH-exposed group in the female cohort, their pvalues and AUC values. N-glycan symbols are as in Figure S1 (Note: Combined AUC value =1). Table S3: The relative abundance of statistically significant N-glycans between the control and GBH-Exposed group in the male cohort, their pvalues and AUC values. N-glycan symbols are as in Figure S1 (Note: Combined AUC value =1). Table S4: The relative abundance of statistically significant proteins in the female cohorts, their corresponding pvalues and AUC values. Table S5: The relative abundance of statistically significant proteins in the male cohorts, their corresponding p-values, and AUC values. The ARRIVE guidelines 2.0: Author Checklist.Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Davoren, M.J.; Schiestl, R.H. Glyphosate-based herbicides and cancer risk: A post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis 2018, 39, 1207–1215. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, M.; Gutierrez Reyes, C.D.; Chávez-Reyes, J.; Marichal-Cancino, B.A.; Solomon, J.; Fowowe, M.; Onigbinde, S.; Flores-Rodriguez, J.A.; Bhuiyan, M.M.A.A.; Mechref, Y. Serum N-Glycan Changes in Rats Chronically Exposed to Glyphosate-Based Herbicides. Biomolecules 2024, 14, 1077. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. glyphosate-based herbicides exposure: A review on their toxicity. J. Xenobiotics 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Roberts, D.M.; Buckley, N.A.; Mohamed, F.; Eddleston, M.; Goldstein, D.A.; Mehrsheikh, A.; Bleeke, M.S.; Dawson, A.H. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin. Toxicol. 2010, 48, 129–136. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, M.-L.; Deng, J.-F.; Yang, C.-C. The epidemiology of glyphosate-surfactant herbicide poisoning in Taiwan, 1986–2007: A poison center study. Clin. Toxicol. 2009, 47, 670–677. [Google Scholar] [CrossRef]
- Nagami, H.; Nishigaki, Y.; Matsushima, S.; Matsushita, T.; Asanuma, S.; Yajima, N.; Usuda, M.; Hirosawa, M. Hospital-based survey of pesticide poisoning in Japan, 1998–2002. Int. J. Occup. Environ. Health 2005, 11, 180–184. [Google Scholar] [CrossRef]
- Ospina, M.; Schütze, A.; Morales-Agudelo, P.; Vidal, M.; Wong, L.-Y.; Calafat, A.M. Exposure to glyphosate in the United States: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int. 2022, 170, 107620. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Zarn, J.A.; Dudler, V. Urine glyphosate level as a quantitative biomarker of oral exposure. Int. J. Hyg. Environ. Health 2020, 228, 113526. [Google Scholar] [CrossRef]
- Connolly, A.; Coggins, M.A.; Koch, H.M. Human biomonitoring of glyphosate exposures: State-of-the-art and future research challenges. Toxics 2020, 8, 60. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Liu, X.; Gao, M.; Li, X.; Wang, Y.; Chang, Y.; Zhang, X.; Huo, Z.; Zhang, L. Human serum lipidomics analysis revealed glyphosate may lead to lipid metabolism disorders and health risks. Environ. Int. 2023, 171, 107682. [Google Scholar] [CrossRef]
- Williams, G.M.; Aardema, M.; Acquavella, J.; Berry, S.C.; Brusick, D.; Burns, M.M.; de Camargo, J.L.V.; Garabrant, D.; Greim, H.A.; Kier, L.D.; et al. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit. Rev. Toxicol. 2016, 46, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Tarboush, N.A.; Almomani, D.H.; Khabour, O.F.; Azzam, M.I. Genotoxicity of Glyphosate on cultured human Lymphocytes. Int. J. Toxicol. 2022, 41, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Tessema, R.A.; Budnik, L.T.; Ádám, B. Comparative cyto-and genotoxicity assessment of glyphosate and glyphosate-based herbicides in human peripheral white blood cells. Environ. Res. 2019, 179, 108851. [Google Scholar] [CrossRef]
- Chávez-Reyes, J.; Gutiérrez-Reyes, C.D.; Hernández-Cuellar, E.; Marichal-Cancino, B.A. Neurotoxicity of glyphosate: Focus on molecular mechanisms probably associated with alterations in cognition and behavior. Environ. Toxicol. Pharmacol. 2024, 106, 104381. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.; Gutierrez-Reyes, C.D.; Chávez-Reyes, J.; Onigbinde, S.; Marichal-Cancino, B.A.; López-Lariz, C.H.; Beck, M.; Mechref, Y. Neuroglycome alterations of hippocampus and prefrontal cortex of juvenile rats chronically exposed to glyphosate-based herbicide. Front. Neurosci. 2024, 18, 1442772. [Google Scholar] [CrossRef]
- Daramola, O.; Gutierrez Reyes, C.D.; Chávez-Reyes, J.; Marichal-Cancino, B.A.; Nwaiwu, J.; Onigbinde, S.; Adeniyi, M.; Solomon, J.; Bhuiyan, M.M.A.A.; Mechref, Y. Metabolomic changes in rat serum after chronic exposure to glyphosate-based herbicide. Metabolites 2024, 14, 50. [Google Scholar] [CrossRef]
- Qi, L.; Zhu, J.; Qi, Y.; Chao, H.; Cheng, Y.; Yang, X.; Li, H.; Li, G.; Liu, J. Glyphosate Based-Herbicide Induced Nephrotoxicity through Oxidative Stress and the Imbalance of Osmotic Pressure in Mice. Biomed. Environ. Sci. 2024, 37, 1453–1457. [Google Scholar]
- Ingaramo, P.; Alarcón, R.; Muñoz-de-Toro, M.; Luque, E.H. Are glyphosate and glyphosate-based herbicides endocrine disruptors that alter female fertility? Mol. Cell. Endocrinol. 2020, 518, 110934. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Q.; Guo, J.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Oxidative Stress and Metabolism: A Mechanistic Insight for Glyphosate Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 617–639. [Google Scholar] [CrossRef]
- Mesnage, R.; Arno, M.; Costanzo, M.; Malatesta, M.; Séralini, G.-E.; Antoniou, M.N. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ. Health 2015, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.C.; Andreotti, G.; Ospina, M.; Parks, C.G.; Liu, D.; Shearer, J.J.; Rothman, N.; Silverman, D.T.; Sandler, D.P.; Calafat, A.M.; et al. Glyphosate exposure and urinary oxidative stress biomarkers in the Agricultural Health Study. JNCI J. Natl. Cancer Inst. 2023, 115, 394–404. [Google Scholar] [CrossRef]
- Jayasumana, C.; Paranagama, P.; Agampodi, S.; Wijewardane, C.; Gunatilake, S.; Siribaddana, S. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ. Health 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Vassalotti, J.A.; Centor, R.; Turner, B.J.; Greer, R.C.; Choi, M.; Sequist, T.D. Practical Approach to Detection and Management of Chronic Kidney Disease for the Primary Care Clinician. Am. J. Med. 2016, 129, 153–162.e157. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Alejo-Jacuinde, G.; Perez Sanchez, B.; Chavez Reyes, J.; Onigbinde, S.; Mogut, D.; Hernandez-Jasso, I.; Calderon-Vallejo, D.; Quintanar, J.L.; Mechref, Y. Multi omics applications in biological systems. Curr. Issues Mol. Biol. 2024, 46, 5777–5793. [Google Scholar] [CrossRef]
- Tsai, T.H.; Song, E.; Zhu, R.; Di Poto, C.; Wang, M.; Luo, Y.; Varghese, R.S.; Tadesse, M.G.; Ziada, D.H.; Desai, C.S. LC-MS/MS-based serum proteomics for identification of candidate biomarkers for hepatocellular carcinoma. Proteomics 2015, 15, 2369–2381. [Google Scholar] [CrossRef]
- Sanni, A.; Bennett, A.I.; Huang, Y.; Gidi, I.; Adeniyi, M.; Nwaiwu, J.; Kang, M.H.; Keyel, M.E.; Gao, C.; Reynolds, C.P. An Optimized Liquid Chromatography–Mass Spectrometry Method for Ganglioside Analysis in Cell Lines. Cells 2024, 13, 1640. [Google Scholar] [CrossRef]
- Nwaiwu, J.; Ibeh, S.; Reslan, M.A.; Bakkar, N.-M.Z.; Nasrallah, L.; Eid, A.H.; Mekhjian, S.; Sanni, A.; Haidar, M.A.; Goli, M. Western diet induces mild metabolic impairment and aggravates neuropathology in an experimental mouse model of traumatic brain injury. J. Neurorestoratol. 2024, 12, 100140. [Google Scholar] [CrossRef]
- Terrapon, N.; Henrissat, B.; Aoki-Kinoshita, K.F.; Surolia, A.; Stanley, P. A genomic view of glycobiology. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar] [CrossRef]
- Daramola, O.; Gautam, S.; Bennett, A.I.; Goli, M.; Guiterrez-Reyes, C.D.; Wang, J.; Mechref, Y. LC-MS/MS of Permethylated O-Glycans, Free Oligosaccharides, and Glycosphingolipid Glycans Using Mesoporous Graphitized Carbon Column. J. Sep. Sci. 2025, 48, e70187. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Cho, B.G.; Zhong, J.; Gautam, S.; Peng, W.; Williamson, S.D.; Banazadeh, A.; Torres-Ulloa, K.Y.; Mechref, Y. Advances in mass spectrometry-based glycomics. Electrophoresis 2018, 39, 3063–3081. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Onigbinde, S.; Peng, W.; Reddy, A.; Cho, B.G.; Goli, M.; Solomon, J.; Adeniyi, M.; Nwaiwu, J.; Fowowe, M.; Daramola, O.; et al. O-Glycome Profiling of Breast Cancer Cell Lines to Understand Breast Cancer Brain Metastasis. J. Proteome Res. 2024, 23, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Asadi Shehni, A.; Thoma, I.; McGurnaghan, S.J.; Blackbourn, L.A.K.; Wilkinson, H.; Collier, A.; Patrick, A.W.; Petrie, J.R.; McKeigue, P.M.; et al. Quantitative levels of serum N-glycans in type 1 diabetes and their association with kidney disease. Glycobiology 2020, 31, 613–623. [Google Scholar] [CrossRef]
- Guay, K.P.; Chou, W.-C.; Canniff, N.P.; Paul, K.B.; Hebert, D.N. N-glycan-dependent protein maturation and quality control in the ER. Nat. Rev. Mol. Cell Biol. 2025, 1–14. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, J.; Dong, X.; Banazadeh, A.; Huang, Y.; Hussien, A.; Mechref, Y. Clinical application of quantitative glycomics. Expert Rev. Proteom. 2018, 15, 1007–1031. [Google Scholar] [CrossRef]
- He, T. Implementation of Proteomics in Clinical Trials. Proteom.–Clin. Appl. 2019, 13, 1800198. [Google Scholar] [CrossRef]
- Sandilya, V.; El-Gameel, D.; Atashi, M.; Nguyen, T.; Fowowe, M.; Bhuiyan, M.M.A.A.; Daramola, O.; Nwaiwu, J.; Hamdy, N.A.; Ghanem, M.; et al. LC-MS/MS-Profiling of Human Serum Unveils Significant Increase in Neuroinflammation and Carcinogenesis Following Chronic Organophosphate Exposure. J. Proteome Res. 2025, 24, 1342–1355. [Google Scholar] [CrossRef]
- Patabandige, M.W.; Pfeifer, L.D.; Nguyen, H.T.; Desaire, H. Quantitative clinical glycomics strategies: A guide for selecting the best analysis approach. Mass Spectrom. Rev. 2022, 41, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, R.; Fan, C.; Chen, X. Diagnostic and prognostic utility of plasma thrombospondin-1 levels in traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2024, 50, 2229–2237. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Buonora, J.E.; Rhind, S.G.; Hutchison, M.G.; Baker, A.J.; Rizoli, S.B.; Diaz-Arrastia, R.; Mueller, G.P. Blood biomarkers in moderate-to-severe traumatic brain injury: Potential utility of a multi-marker approach in characterizing outcome. Front. Neurol. 2015, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.A.; Hightower, L.E. Stress proteins as molecular biomarkers for environmental toxicology. In Stress-Inducible Cellular Responses; Feige, U., Yahara, I., Morimoto, R.I., Polla, B.S., Eds.; Birkhäuser Basel: Basel, Switzerland, 1996; pp. 411–424. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bayne, K. Revised guide for the care and use of laboratory animals available. American Physiological Society. Physiologist 1996, 39, 199. [Google Scholar]
- Motulsky, H.J.; Michel, M.C. Commentary on the BJP’s new statistical reporting guidelines. Br. J. Pharmacol. 2018, 175, 3636–3637. [Google Scholar] [CrossRef]
- Pandey, A.; Dhabade, P.; Kumarasamy, A. Inflammatory Effects of Subacute Exposure of Roundup in Rat Liver and Adipose Tissue. Dose Response 2019, 17, 1559325819843380. [Google Scholar] [CrossRef]
- Kang, P.; Mechref, Y.; Novotny, M.V. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; McDowell, C.; West, C.; David, F.; Powers, T.W.; Nowling, T.; Bruner, E.; Mehta, A.S.; Angel, P.M.; Marlow, L.A.; et al. Defining the human kidney N-glycome in normal and cancer tissues using MALDI imaging mass spectrometry. J. Mass Spectrom. 2020, 55, e4490. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, J.; Yadav, S.P.S.; Molitoris, B.A.; Wagner, M.C.; Mechref, Y. Changes in the Expression of Renal Brush Border Membrane N-Glycome in Model Rats with Chronic Kidney Diseases. Biomolecules 2021, 11, 1677. [Google Scholar] [CrossRef]
- Kamel, M.F.; Nassar, M.; Elbendary, A.; Mohamed, A.G.A.; Abdullah, M.G.; Gomaa, H.R.A.; Awad, E.M.I.; Mahmoud, H.H.; Elfiki, M.A.; Abdalla, N.H.; et al. The potential use of urinary transferrin, urinary adiponectin, urinary Retinol Binding Protein, and serum zinc alpha 2 glycoprotein levels as novel biomarkers for early diagnosis of diabetic nephropathy: A case-control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102473. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Naik, B.I.; Xin, W.; Ma, J.Z.; Scalzo, D.C.; Thammishetti, S.; Thiele, R.H.; Zuo, Z.; Raphael, J. Haptoglobin 2-2 Phenotype Is Associated With Increased Acute Kidney Injury After Elective Cardiac Surgery in Patients With Diabetes Mellitus. J. Am. Heart Assoc. 2017, 6, e006565. [Google Scholar] [CrossRef]
- Lesseur, C.; Pirrotte, P.; Pathak, K.V.; Manservisi, F.; Mandrioli, D.; Belpoggi, F.; Panzacchi, S.; Li, Q.; Barrett, E.S.; Nguyen, R.H. Maternal urinary levels of glyphosate during pregnancy and anogenital distance in newborns in a US multicenter pregnancy cohort. Environ. Pollut. 2021, 280, 117002. [Google Scholar] [CrossRef]
- Antunes, A.M.; Rocha, T.L.; Pires, F.S.; de Freitas, M.A.; Leite, V.R.M.C.; Arana, S.; Moreira, P.C.; Sabóia-Morais, S.M.T. Gender-specific histopathological response in guppies exposed to glyphosate or its metabolite aminomethylphosphonic acid. J. Appl. Toxicol. 2017, 37, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, P.-O.; Breton, Y.; Léveillé, F.; Tessier, P.A.; Pelletier, M. The impact of the herbicide glyphosate and its metabolites AMPA and MPA on the metabolism and functions of human blood neutrophils and their sex-dependent effects on reactive oxygen species and CXCL8/IL-8 production. Environ. Res. 2024, 252, 118831. [Google Scholar] [CrossRef]
- Duan, C.; Wu, J.; Wang, Z.; Hou, X.; Han, C. Fucosylation in digestive inflammatory diseases and cancers: From mechanical studies to clinical translation. Genes Dis. 2025, 12, 101570. [Google Scholar] [CrossRef]
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; An, J.; Yi, S.; Tan, Z.; Wang, H.; Li, H.; Guan, X.; Liu, J.; Wang, Q. FUT8 and protein core fucosylation in tumours: From diagnosis to treatment. J. Cancer 2021, 12, 4109. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, J.; Gutierrez Reyes, C.D.; Lin, Y.; Tan, Z.; Wu, Z.; Zhang, J.; Cano, A.; Verschleisser, S.; Mechref, Y. Discovery of core-fucosylated glycopeptides as diagnostic biomarkers for early HCC in patients with NASH cirrhosis using LC-HCD-PRM-MS/MS. ACS Omega 2023, 8, 12467–12480. [Google Scholar] [CrossRef]
- Liu, A.; Wang, X.; Hu, X.; Deng, Y.; Wen, X.; Lin, B.; Zhou, M.; Wang, W.; Luo, Y.; Deng, J.; et al. Core fucosylation involvement in the paracrine regulation of proteinuria-induced renal interstitial fibrosis evaluated with the use of a microfluidic chip. Acta Biomater. 2022, 142, 99–112. [Google Scholar] [CrossRef]
- Alvarez, M.R.S.; Zhou, Q.; Tena, J.; Lebrilla, C.B.; Completo, G.C.; Heralde III, F.M.; Cabanatan, M.; Barzaga, M.T.; Tan-Liu, N.; Ladrera, G.I. N-Glycan and Glycopeptide Serum Biomarkers in Philippine Lung Cancer Patients Identified Using Liquid Chromatography–Tandem Mass Spectrometry. ACS Omega 2022, 7, 40230–40240. [Google Scholar] [CrossRef]
- Reyes, C.D.G.; Hakim, M.A.; Atashi, M.; Goli, M.; Gautam, S.; Wang, J.; Bennett, A.I.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS isomeric profiling of N-Glycans derived from low-abundant serum glycoproteins in mild cognitive impairment patients. Biomolecules 2022, 12, 1657. [Google Scholar] [CrossRef]
- Ščupáková, K.; Adelaja, O.T.; Balluff, B.; Ayyappan, V.; Tressler, C.M.; Jenkinson, N.M.; Claes, B.S.; Bowman, A.P.; Cimino-Mathews, A.M.; White, M.J.; et al. Clinical importance of high-mannose, fucosylated, and complex N-glycans in breast cancer metastasis. JCI Insight 2021, 6, e146945. [Google Scholar] [CrossRef] [PubMed]
- Malaker, S.A.; Quanico, J.; Raffo-Romero, A.; Kobeissy, F.; Aboulouard, S.; Tierny, D.; Bertozzi, C.R.; Fournier, I.; Salzet, M. On-tissue spatially resolved glycoproteomics guided by N-glycan imaging reveal global dysregulation of canine glioma glycoproteomic landscape. Cell Chem. Biol. 2022, 29, 30–42.e34. [Google Scholar] [CrossRef] [PubMed]
- Reider, B.; Jarvas, G.; Krenkova, J.; Guttman, A. Separation based characterization methods for the N-glycosylation analysis of prostate-specific antigen. J. Pharm. Biomed. Anal. 2021, 194, 113797. [Google Scholar] [CrossRef]
- Shah, P.; Wang, X.; Yang, W.; Toghi Eshghi, S.; Sun, S.; Hoti, N.; Chen, L.; Yang, S.; Pasay, J.; Rubin, A.; et al. Integrated Proteomic and Glycoproteomic Analyses of Prostate Cancer Cells Reveal Glycoprotein Alteration in Protein Abundance and Glycosylation*. Mol. Cell. Proteom. 2015, 14, 2753–2763. [Google Scholar] [CrossRef]
- Pearce, O.M.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Abbasi, A.; Hejazian, S.S.; Hekmatshoar, Y.; Ardalan, M.; Farnood, F.; Zununi Vahed, S. Combating chronic kidney disease-associated cachexia: A literature review of recent therapeutic approaches. BMC Nephrol. 2025, 26, 133. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.; Hardy, R.S.; Langen, R.C. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Simões e Silva, A.C.; Oliveira, E.A.; Cheung, W.W.; Mak, R.H. Redox Signaling in Chronic Kidney Disease-Associated Cachexia. Antioxidants 2023, 12, 945. [Google Scholar] [CrossRef]
- Maccio, A.; Madeddu, C.; Lai, E.; Scartozzi, M. Cancer cachexia and chronic inflammation: An unbreakable bond. Br. J. Cancer 2023, 128, 1609–1610. [Google Scholar] [CrossRef]
- Bock, F.; Li, S.; Pozzi, A.; Zent, R. Integrins in the kidney—Beyond the matrix. Nat. Rev. Nephrol. 2025, 21, 157–174. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. αv integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, Z.; Su, H.; Sun, K.; Liu, Q.; Zhang, Y.; Zhu, L.; Jiang, F.; Fan, Y.; Shou, S. Integrin subunit beta 6 is a potential diagnostic marker for acute kidney injury in patients with diabetic kidney disease: A single cell sequencing data analysis. Ren. Fail. 2024, 46, 2409348. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Zhang, Q.; Jin, B.; Lv, D.; Li, W.; Zhao, M.; Jiang, C.; Dai, C.; Liu, Z. Integrin β3 induction promotes tubular cell senescence and kidney fibrosis. Front. Cell Dev. Biol. 2021, 9, 733831. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Yao, R.Q.; Zhang, Z.C.; Zhu, S.Y.; Li, Y.X.; Ren, C.; Du, X.H.; Yao, Y.M. Eukaryotic ribosome quality control system: A potential therapeutic target for human diseases. Int. J. Biol. Sci. 2022, 18, 2497–2514. [Google Scholar] [CrossRef]
- Lu, B. Translational regulation by ribosome-associated quality control in neurodegenerative disease, cancer, and viral infection. Front. Cell Dev. Biol. 2022, 10, 970654. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Nishibata, Y.; Watanabe-Kusunoki, K.; Tomaru, U.; Ishizu, A. Neutrophils and NETs in kidney disease. Nat. Rev. Nephrol. 2025, 21, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Talal, S.; Mona, K.; Karem, A.; Yaniv, L.; Reut, H.-M.; Ariel, S.; Moran, A.-K.; Harel, E.; Campisi-Pinto, S.; Mahmoud, A.-A.; et al. Neutrophil degranulation and severely impaired extracellular trap formation at the basis of susceptibility to infections of hemodialysis patients. BMC Med. 2022, 20, 364. [Google Scholar] [CrossRef]
- Jia, H.; Yue, G.; Li, P.; Peng, R.; Jin, R.; Chen, Y.; Cao, H.; Yang, K.; Zhang, X.; Yi, X.; et al. Neutrophil extracellular traps license macrophage production of chemokines to facilitate CD8+ T cell infiltration in obstruction-induced renal fibrosis. Protein Cell 2025, 16, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Dersh, D.; Hollý, J.; Yewdell, J.W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 2021, 21, 116–128. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef]
- Jacobsen-Pereira, C.H.; Cardoso, C.C.; Gehlen, T.C.; Regina dos Santos, C.; Santos-Silva, M.C. Immune response of Brazilian farmers exposed to multiple pesticides. Ecotoxicol. Environ. Saf. 2020, 202, 110912. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, L.; Luo, W.; Yu, W.; Hu, X.; Guan, X.; Cai, Y.; Zou, C.; Yin, H.; Xu, Z. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 2019, 10, 848. [Google Scholar] [CrossRef]
- Ma, J.-h.; Qin, L.; Li, X. Role of STAT3 signaling pathway in breast cancer. Cell Commun. Signal. 2020, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Gujral, P.; Orozco-Alonso, E.; Saliba, J.; Yan, X.; Blank, V. The NFE2L2 (NRF2) transcription factor controls genes involved in the oxidative stress response and inflammation in myometrial cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2025, 1872, 119985. [Google Scholar] [CrossRef]
- Ju, Q.; Li, X.; Zhang, H.; Yan, S.; Li, Y.; Zhao, Y. NFE2L2 Is a Potential Prognostic Biomarker and Is Correlated with Immune Infiltration in Brain Lower Grade Glioma: A Pan-Cancer Analysis. Oxidative Med. Cell. Longev. 2020, 2020, 3580719. [Google Scholar] [CrossRef]
- Yu, M.-H.; Zhang, W. TEAD1 enhances proliferation via activating SP1 in colorectal cancer. Biomed. Pharmacother. 2016, 83, 496–501. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, L.; Li, X.; Zhu, X.; Zhang, Z.; Sun, X.; Xue, X.; Dai, C. Taz/Tead1 Promotes Alternative Macrophage Activation and Kidney Fibrosis via Transcriptional Upregulation of Smad3. J. Immunol. Res. 2024, 2024, 9512251. [Google Scholar] [CrossRef] [PubMed]
- Landin-Malt, A.; Benhaddou, A.; Zider, A.; Flagiello, D. An evolutionary, structural and functional overview of the mammalian TEAD1 and TEAD2 transcription factors. Gene 2016, 591, 292–303. [Google Scholar] [CrossRef]
- Hua, Y.-Q.; Zhang, K.; Sheng, J.; Ning, Z.-Y.; Li, Y.; Shi, W.-d.; Liu, L.-M. NUCB1 Suppresses Growth and Shows Additive Effects With Gemcitabine in Pancreatic Ductal Adenocarcinoma via the Unfolded Protein Response. Front. Cell Dev. Biol. 2021, 9, 641836. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, Q.; Gao, H.; Tian, J.; Che, G. Immunoglobulin heavy constant gamma 1 silencing decreases tonicity-responsive enhancer-binding protein expression to alleviate diabetic nephropathy. J. Diabetes Investig. 2024, 15, 572–583. [Google Scholar] [CrossRef]
- Mukherjee, D.; Bercz, L.S.; Torok, M.A.; Mace, T.A. Regulation of cellular immunity by activating transcription factor 4. Immunol. Lett. 2020, 228, 24–34. [Google Scholar] [CrossRef]
- Tang, H.; Kang, R.; Liu, J.; Tang, D. ATF4 in cellular stress, ferroptosis, and cancer. Arch. Toxicol. 2024, 98, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Liu, T.; Guo, T.; Tao, W.; Chen, X.; Chen, W.; Chen, L.; Xiao, Y. ATF4 promotes renal tubulointerstitial fibrosis by suppressing autophagy in diabetic nephropathy. Life Sci. 2021, 264, 118686. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zheng, G.; Zhu, A.; Cao, L.; Lu, J.; Wu, D.; Zhang, Z.; Fan, S.; Sun, C.; Hu, B. Epigenetic modification of miR-10a regulates renal damage by targeting CREB1 in type 2 diabetes mellitus. Toxicol. Appl. Pharmacol. 2016, 306, 134–143. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, J.; Huang, T.; Yu, Z.; Zhao, C.; Xu, Y.; Ma, B. Role of CREB1 dysregulation in calcium oxalate monohydrate crystals-induced tubular epithelial cell injury. Mol. Cell. Toxicol. 2024, 20, 939–948. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zou, X.; Shi, Y.; Liu, Q.; Huyan, T.; Su, J.; Wang, Q.; Zhang, F.; Li, X. FOXO1 inhibition prevents renal ischemia–reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br. J. Pharmacol. 2020, 177, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-S.; Noh, M.R.; Ha, L.; Kim, J.; Padanilam, B.J. Effect of Tissue-derived Angiotensinogen on Kidney Injury and Fibrosis in Obstructive Nephropathy. In Vivo 2024, 38, 2107. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Kang, A.-Y.; Jang, J.Y.; Kim, H.; Han, M.; Oh, K.-H.; Kim, S.H.; Noh, J.W.; Cheong, H.I.; Hwang, Y.-H. Increased urinary Angiotensinogen/Creatinine (AGT/Cr) ratio may be associated with reduced renal function in autosomal dominant polycystic kidney disease patients. BMC Nephrol. 2015, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, G.; Bertrand, M.J. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Sharif, P.M.; Jabbari, P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine 2020, 130, 155066. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, J.; Peng, W.; Yadav, S.P.S.; Molitoris, B.A.; Wagner, M.C.; Mechref, Y. Proteomics profiling of kidney brush border membrane from rats using LC-MS/MS analysis. Proteom.–Clin. Appl. 2023, 17, 2200063. [Google Scholar] [CrossRef]
- Pace, J.; Paladugu, P.; Das, B.; He, J.C.; Mallipattu, S.K. Targeting STAT3 signaling in kidney disease. Am. J. Physiol.-Ren. Physiol. 2019, 316, F1151–F1161. [Google Scholar] [CrossRef]
- Kshirsagar, A.V.; Zeitler, E.M.; Weaver, A.; Franceschini, N.; Engel, L.S. Environmental exposures and kidney disease. Kidney360 2022, 3, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Aoki-Kinoshita, K.F.; Ishihama, Y.; Okuda, S. GlycoPOST realizes FAIR principles for glycomics mass spectrometry data. Nucleic Acids Res. 2021, 49, D1523–D1528. [Google Scholar] [CrossRef] [PubMed]
 , Gal (galactose)
, Gal (galactose)  , Man (mannose)
, Man (mannose)  , Fuc (fucose)
, Fuc (fucose)  , NeuAc (N-acetylneuraminic acid)
, NeuAc (N-acetylneuraminic acid)  , and NeuGc (N-glycolylneuraminic acid)
, and NeuGc (N-glycolylneuraminic acid)  .
.
 , Gal (galactose)
, Gal (galactose)  , Man (mannose)
, Man (mannose)  , Fuc (fucose)
, Fuc (fucose)  , NeuAc (N-acetylneuraminic acid)
, NeuAc (N-acetylneuraminic acid)  , and NeuGc (N-glycolylneuraminic acid)
, and NeuGc (N-glycolylneuraminic acid)  .
.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwubueze, F.; Reyes, C.D.G.; Chávez-Reyes, J.; Solomon, J.; Sandilya, V.; Sahioun, S.; Marichal-Cancino, B.A.; Mechref, Y. Multi-Omics Alterations in Rat Kidneys upon Chronic Glyphosate Exposure. Biomolecules 2025, 15, 1399. https://doi.org/10.3390/biom15101399
Chukwubueze F, Reyes CDG, Chávez-Reyes J, Solomon J, Sandilya V, Sahioun S, Marichal-Cancino BA, Mechref Y. Multi-Omics Alterations in Rat Kidneys upon Chronic Glyphosate Exposure. Biomolecules. 2025; 15(10):1399. https://doi.org/10.3390/biom15101399
Chicago/Turabian StyleChukwubueze, Favour, Cristian D. Guiterrez Reyes, Jesús Chávez-Reyes, Joy Solomon, Vishal Sandilya, Sarah Sahioun, Bruno A. Marichal-Cancino, and Yehia Mechref. 2025. "Multi-Omics Alterations in Rat Kidneys upon Chronic Glyphosate Exposure" Biomolecules 15, no. 10: 1399. https://doi.org/10.3390/biom15101399
APA StyleChukwubueze, F., Reyes, C. D. G., Chávez-Reyes, J., Solomon, J., Sandilya, V., Sahioun, S., Marichal-Cancino, B. A., & Mechref, Y. (2025). Multi-Omics Alterations in Rat Kidneys upon Chronic Glyphosate Exposure. Biomolecules, 15(10), 1399. https://doi.org/10.3390/biom15101399







