Is There Need for Pancreatic Enzyme Replacement Therapy in Patients with Exocrine Pancreatic Insufficiency When Using High-Caloric Liquid Diets? Orientating Studies on Praecaecal Digestibility in Pigs with Experimentally Induced Pancreatic Exocrine Insufficiency and Ileocaecal Fistula
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim of the Study
2.2. Materials and Methods
2.3. Animals
2.4. Liquid Test Diets—Commercial High-Calorific Drinks (HCDs) for Human Consumption
2.5. Test Design
2.6. Sampling
2.7. Sample Preparation and Analyses
2.8. Statistical Analyses
3. Results
3.1. Effect of PEI and PERT on pcDR of Fat
3.2. Effect of PEI and PERT on Apparent pcDR of Crude Protein
3.3. Effect of Liquid Drink on DR of Nutrients
4. Discussion
4.1. Critical Discussion of the Methods Used
4.2. Clinical Relevance of This Study
4.3. Consequences for Practical Recommendations for Nutritional Support for PEI Patients: Does the Labelled Nutrient Content Reflect the Real Nutrient Uptake?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Con | Control |
| DR | Disappearance rate |
| HCD | High-calorific drink |
| pcDR | Praecaecal disappearance rate |
| PEI | Pancreatic exocrine insufficiency |
| PERT | Pancreatic enzyme replacement therapy |
| PL | Pancreatic duct-ligated |
| PL + PERT | Pancreatic duct-ligated individual receiving pancreatic enzyme replacement therapy |
References
- Domínguez-Muñoz, J.E. Pancreatic exocrine insufficiency: Diagnosis and treatment. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S2), 12–16. [Google Scholar] [CrossRef]
- Dominguez-Muñoz, J.E.; Vujasinovic, M.; de la Iglesia, D.; Cahen, D.; Capurso, G.; Gubergrits, N.; Hegyi, P.; Hungin, P.; Ockenga, J.; Paiella, S.; et al. European guidelines for the diagnosis and treatment of pancreatic exocrine insufficiency: UEG, EPC, EDS, ESPEN, ESPGHAN, ESDO, and ESPCG evidence-based recommendations. United Eur. Gastroenterol. J. 2025, 13, 125–172. [Google Scholar] [CrossRef]
- Min, M.; Patel, B.D.; Han, S.; Bocelli, L.D.; Kheder, J.; Vaze, A.; Wassef, W. Exocrine Pancreatic Insufficiency and Malnutrition in Chronic Pancreatitis: Identification, Treatment, and Consequences. Pancreas 2018, 47, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Buchner, A.M.; Forsmark, C.E. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatic Insufficiency: Expert Review. Gastroenterology 2023, 165, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Rieke, J.G.; Almusaylim, K.; Kanchibhatla, A.; Blanchette, J.E.; Lewis, C. Exocrine Pancreatic Insufficiency Dosing Guidelines for Pancreatic Enzyme Replacement Therapy Vary Widely Across Disease Types. Dig. Dis. Sci. 2024, 69, 615–633. [Google Scholar] [CrossRef]
- de Rijk, F.E.M.; van Veldhuisen, C.L.; Besselink, M.G.; van Hooft, J.E.; van Santvoort, H.C.; van Geenen, E.J.; Hegyi, P.; Löhr, J.-M.; Dominguez-Munoz, J.E.; de Jonge, P.J.F.; et al. Diagnosis and treatment of exocrine pancreatic insufficiency in chronic pancreatitis: An international expert survey and case vignette study. Pancreatology 2022, 22, 457–465. [Google Scholar] [CrossRef]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS guideline on nutrition care for cystic fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef]
- Möβeler, A.; Tabeling, R.; Gregory, P.C.; Kamphues, J. Compensatory digestion of fat, protein and starch (rates and amounts) in the large intestine of minipigs in case of reduced precaecal digestion due to pancreatic duct ligation—A short review. Livest. Sci. 2007, 109, 50–52. [Google Scholar] [CrossRef]
- Mößeler, A.K. Variety of effects of exocrine pancreatic insufficiency—more than just steatorrhea! In New Findings from Studies Using the Animal Model of the Pancreatic Duct Ligated Pig and Subsequent Recommendations for Dietetic Measures in Patients; English Summary, Habilitation in German; Hanover Library of the University of Veterinary Medicine: Hannover, Germany, 2016; Available online: https://nbn-resolving.org/urn:nbn:de:gbv:95-h2785 (accessed on 3 June 2025).
- Mößeler, A.; Kamphues, J. Black-Box Gastrointestinal Tract—Needs and Prospects of Gaining Insights of Fate of Fat, Protein, and Starch in Case of Exocrine Pancreatic Insufficiency by Using Fistulated Pigs. Nutrients 2017, 9, 150. [Google Scholar] [CrossRef]
- Kramer, Nils. Investigations on the Precaecal Digestibility of Starch and Fat from Different Origin in Pancreatic Duct Ligated, Ileocaecally Fistulated Minipigs. Ph.D. Thesis, University of Veterinary Medicine Hanover, Hannover, Germany, 2010. Abstract auf Englisch bei. Available online: https://elib.tiho-hannover.de/servlets/MCRFileNodeServlet/etd_derivate_00001238/kramern_ss10.pdf (accessed on 3 June 2025).
- Schubert, D.C.; Mößeler, A.; Ahlfänger, B.; Langeheine, M.; Brehm, R.; Visscher, C.; El-Wahab, A.A.; Kamphues, J. Influences of exocrine pancreatic insufficiency on nutrient digestibility, growth parameters as well as anatomical and histological morphology of the intestine in a juvenile pig model. Front. Med. 2022, 9, 973589. [Google Scholar] [CrossRef]
- Redeker, C.; MoBeler, A.; Kamphues, J. Effects of high/low fat meals on prececal digestibility of protein and starch using recommended enzyme dosages (based on lipase and fat) of a multienzyme product—The pancreas duct ligated minipig as a model for exocrine pancreatic insufficiency. In Proceedings of the 18th Congress of the European Society of Veterinary and Comparative Nutrition (ESVCN), Utrecht, The Netherlands, 11–13 September 2014. [Google Scholar]
- Tabeling, R.; Gregory, P.; Kamphues, J. Studies on nutrient digestibilities (pre-caecal and total) in pancreatic duct-ligated pigs and the effects of enzyme substitution. J. Anim. Physiol. Anim. Nutr. 1999, 82, 251–263. [Google Scholar]
- Klassen, P.N.; Mazurak, V.C.; Baracos, V.; Martin, L.; Ghosh, S.; Kasnik, J.; Sawyer, M.B. Dose optimization of pancreatic enzyme replacement therapy is essential to mitigate muscle loss in patients with advanced pancreatic cancer and exocrine pancreatic insufficiency. Clin. Nutr. 2024, 43, 1900–1906. [Google Scholar] [CrossRef]
- Sikkens, E.C.M.; Cahen, D.L.; van Eijck, C.; Kuipers, E.J.; Bruno, M.J. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: A Dutch national survey. Pancreatology 2012, 12, 71–73. [Google Scholar] [CrossRef]
- Berry, A.J. Pancreatic enzyme replacement therapy during pancreatic insufficiency. Nutr. Clin. Pract. 2014, 29, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.E.; Hopper, A.D.; Leeds, J.S.; Roberts, K.J.; McGeeney, L.; Duggan, S.N.; Kumar, R. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021, 8, e000643. [Google Scholar] [CrossRef] [PubMed]
- Mösseler, A.; Loock, H.; Classen, J.; Gregory, P.; Kamphues, J. Endogenous nitrogen losses (praecaecal and total) in case of exocrine pancreatic insufficiency (induced by pancreatic duct ligation) in pigs—Used as a model for humans. Pancreatology 2013, 13, S24. [Google Scholar]
- Lindkvist, B.; Phillips, M.E.; Domínguez-Muñoz, J.E. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology 2015, 15, 589–597. [Google Scholar] [CrossRef]
- Airinei, G.; Gaudichon, C.; Bos, C.; Bon, C.; Kapel, N.; Bejou, B.; Raynaud, J.J.; Luengo, C.; Aparicio, T.; Levy, P.; et al. Postprandial protein metabolism but not a fecal test reveals protein malabsorption in patients with pancreatic exocrine insufficiency. Clin. Nutr. 2011, 30, 831–837. [Google Scholar] [CrossRef]
- Imondi, A.R.; Stradley, R.P.; Wolgemuth, R. Enzyme Replacement Therapy in the Pancreatic Duct Ligated Swine. Proc. Soc. Exp. Biol. Med. 1972, 141, 367–372. [Google Scholar] [CrossRef]
- Abello, J.; Pascaud, X.; Simoes-Nunes, C.; Cuber, J.C.; Junien, J.L.; Rozé, C. Total pancreatic insufficiency in pigs: A model to study intestinal enzymes and plasma levels of digestive hormones after pancreatic supplementation by a whole pancreas preparation. Pancreas 1989, 4, 556–564. [Google Scholar]
- Gregory, P.C.; Tabeling, R.; Kamphues, J. Growth and digestion in pancreatic duct ligated pigs. Effect of enzyme supplementation. In Biology of the Pancreas in Growing Animals; Pierzynowski, S.G., Zabielski, R., Eds.; Elsevier Science Health Science: St Louis, MO, USA, 1999; pp. 381–394. [Google Scholar]
- Ishihara, R.; Gregory, P.; Koch, H.F.; Kolleck, S.; Mößeler, A.; Kamphues, J.; Uchida, I. Comparison Study of Pharmacological Efficacy of Pancreatic Digestive Enzyme Replacement (LipaCreon Granules) and of Digestive Enzymes (Berizym Combination Granules and Excelase Combination Granules) on Digestibility of Fat, Protein and Starch in a Minipig Model of Pancreatic Exocrine Insufficiency (in vivo). J. New Rem. Clin. 2012, 61, 1044–1053. [Google Scholar]
- Gregory, P.C.; Hoffmann, K.; Kamphues, J.; Möeler, A. The pancreatic duct ligated (mini)pig as model for pancreatic exocrine insufficiency in man. Pancreas 2016, 45, 1213–1226. [Google Scholar] [CrossRef]
- Tabeling, R. Untersuchungen am Pankreasgangligierten Schwein zu Effekten Einer Enzympräparation auf die Nährstoffverdaulichkeit (praecaecal/in toto). Ph.D. Thesis, University of Veterinary Medicine Hanover, Hanover, Germany, 1998. [Google Scholar]
- Becker, C. Development of A Screening-Test to Assess Activity of Substituted Proteolytic and Amylolytic Enzymes by Using Pancreatic Duct Ligated Minipigs. Ph.D. Thesis, University of Veterinary Medicine Hanover, Hannover, Germany, 2005. Available online: http://elib.tiho-hannover.de/dissertations/beckerc_ws05.html (accessed on 3 June 2025).
- Classen, J. Development of A Novel Screening Test to Assess the Efficacy of Multi-Enzyme Products by Using pancreatic Duct Ligated, Ileo-Caecal Fistulated Minipigs. Doctoral Dissertation, University of Veterinary Medicine Hanover, Hannover, Germany, 2008. Available online: https://elib.tiho-hannover.de/servlets/MCRFileNodeServlet/etd_derivate_00001684/classenj_ss08.pdf (accessed on 3 June 2025).
- Naumann, C.; Bassler, R. Methoden der landwirtschaftlichen Forschungs- und Untersuchungsanstalt, Biochemische Untersuchung von Futtermitteln. In Methodenbuch III; VDLUFA: Darmstadt, Germany, 2012. [Google Scholar]
- Petry, H.; Rapp, W. Zur Problematik der Chromoxidbestimmung in Verdauungsversuchen. Z. Tierphysiol. Tierernähr. Futtermittelkunde 1970, 27, 181–189. [Google Scholar] [CrossRef]
- Hauenschild, A.; Ewald, N.; Klauke, T.; Liebchen, A.; Bretzel, R.G.; Kloer, H.; Hardt, P.D. Effect of liquid pancreatic enzymes on the assimilation of fat in different liquid formula diets. J. Parenter. Enteral Nutr. 2008, 32, 98–100. [Google Scholar] [CrossRef]
- Sans, M.D.; Crozier, S.J.; Vogel, N.L.; D’Alecy, L.G.; Williams, J.A. Dietary Protein and Amino Acid Deficiency Inhibit Pancreatic Digestive Enzyme mRNA Translation by Multiple Mechanisms. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 99–115. [Google Scholar] [CrossRef]
- Mößeler, A.; Kramer, N.; Loock, H.; Kalla, K.; Classen, J.; Gregory, P.C.; Kamphues, J. New findings regarding praecaecal fat digestion in pancreatic duct ligated pigs. In Proceedings of the 13th Congress of the European Society of Veterinary and Comparative Nutrition (ESVCN), Zürich, Switzerland, 6–8 September 2010; p. 45. [Google Scholar]
- Pierzynowski, S.G.; Wychowański, P.; Szczesny, W.; Galloto, R.; Zaworski, K.; Szkopek, D.; Woliński, J.; Donaldson, J.; Pierzynowska, K. Algorithm and ninhydrin method allow for measurement of the postprandial appearance of peptides in blood. Sci. Rep. 2025, 15, 19704. [Google Scholar] [CrossRef]

| HCD | Fat (Total) | Fat Saturated | Protein | kJ/kcal |
|---|---|---|---|---|
| A | 9.3 | 0.9 | 9.6 | 1010/240 |
| B | 9.35 | 0.83 | 10.2 | 1008/240 |
| C | 5.8 | 0.4 | 5.6 | 630/150 |
| D | 4.9 | 0.5 | 6.25 | 630/150 |
| HCD | Dry Matter Intake (g) | Fat Intake (g) | Protein Intake (g) |
|---|---|---|---|
| A | 414 | 75 | 81 |
| B | 422 | 77 | 85 |
| C | 260 | 47 | 44 |
| D | 273 | 42 | 50 |
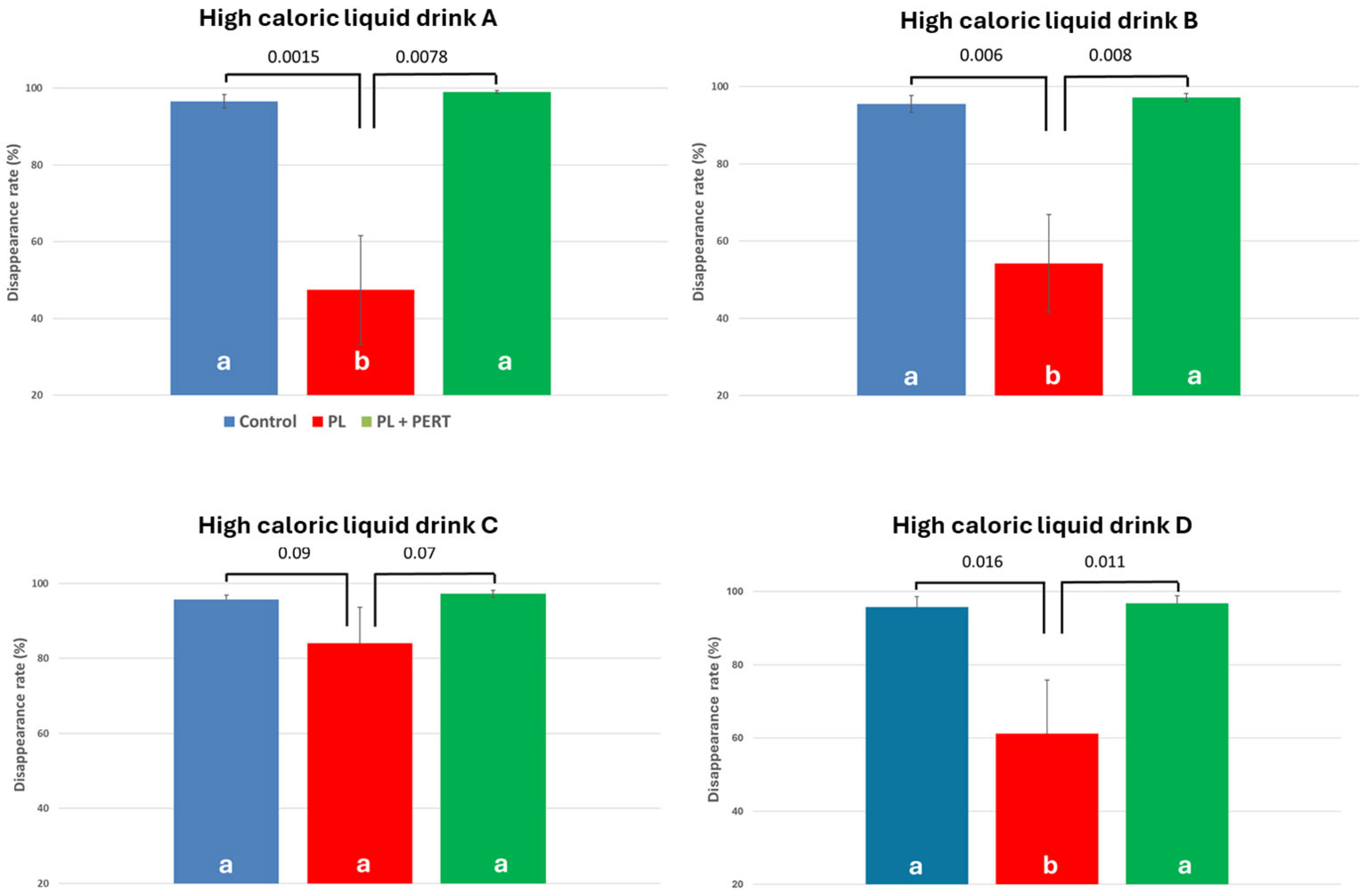
| HCD | CON | PL | PL + PERT |
|---|---|---|---|
| A | 96.6 ± 1.75 a | 47.4 ± 14.2 a | 99.0 ± 0.365 a |
| B | 95.5 ± 2.20 a | 54.3 ± 12.6 a | 97.2 ± 1.05 a |
| C | 95.7 ± 1.20 a | 84.0 ± 9.60 b | 97.3 ± 0.820 a |
| D | 96.4 ± 1.47 a | 52.8 ± 6.01 a | 96.6 ± 1.17 a |
| HCD | CON | PL | PL + PERT |
|---|---|---|---|
| A | 76.9 ± 4.88 | 30.2 ± 4.89 | 76.7 ± 4.26 |
| B | 77.1 ± 6.27 | 22.4 ± 3.53 | 79.8 ± 2.92 |
| C | 78.6 ± 8.43 | 33.5 ± 11.6 | 75.3 ± 2.31 |
| D | 70.2 ± 9.19 | 31.9 ± 9.93 | 78.8 ± 4.88 |
| HCD | Crude Fat (g/100 mL) | Amount of Digested Crude Fat (g/100 mL) | ||
|---|---|---|---|---|
| CON | PL | PERT | ||
| A | 9.30 (2) | 8.98 (1) | 4.41 (3) | 9.20 (1) |
| C | 9.35 (1) | 8.93 (2) | 5.07 (1) | 9.09 (2) |
| D | 5.80 (3) | 5.55 (3) | 4.87 (2) | 5.64 (3) |
| F | 4.90 (4) | 4.73 (4) | 2.59 (4) | 4.73 (4) |
| HCD | Fat Source | Emulsifier | Protein Source | Carbohydrate Source |
|---|---|---|---|---|
| A | rapeseed oil, sunflower oil | lecithin from soy | cow milk | glucose |
| B | rapeseed oil, sunflower oil, corn oil | lecithin from soy, E466 | cow milk | hydrolysed corn starch, saccharose |
| C | rapeseed oil, sunflower oil | E471, lecithin from soy | cow milk | maltodextrin, sugar |
| D | rapeseed oil, corn oil, sunflower oil | E471 | cow milk | glucose, saccharose |
| HCD | Crude Protein (g/100 mL) | Amount of Digested Crude Protein (g/100 mL) | ||
|---|---|---|---|---|
| CON | PL | PERT | ||
| A | 9.60 (2) | 7.38 (2) | 2.90 (1) | 7.36 (2) |
| B | 10.2 (1) | 7.86 (1) | 2.29 (2) | 8.14 (1) |
| C | 5.60 (4) | 4.40 (4) | 1.88 (4) | 4.22 (4) |
| D | 6.30 (3) | 4.42 (3) | 2.01 (3) | 4.96 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mößeler, A.K.; Liesegang, A.; Torgerson, P.; Kamphues, J. Is There Need for Pancreatic Enzyme Replacement Therapy in Patients with Exocrine Pancreatic Insufficiency When Using High-Caloric Liquid Diets? Orientating Studies on Praecaecal Digestibility in Pigs with Experimentally Induced Pancreatic Exocrine Insufficiency and Ileocaecal Fistula. Biomolecules 2025, 15, 1392. https://doi.org/10.3390/biom15101392
Mößeler AK, Liesegang A, Torgerson P, Kamphues J. Is There Need for Pancreatic Enzyme Replacement Therapy in Patients with Exocrine Pancreatic Insufficiency When Using High-Caloric Liquid Diets? Orientating Studies on Praecaecal Digestibility in Pigs with Experimentally Induced Pancreatic Exocrine Insufficiency and Ileocaecal Fistula. Biomolecules. 2025; 15(10):1392. https://doi.org/10.3390/biom15101392
Chicago/Turabian StyleMößeler, Anne Katrin, Annette Liesegang, Paul Torgerson, and Josef Kamphues. 2025. "Is There Need for Pancreatic Enzyme Replacement Therapy in Patients with Exocrine Pancreatic Insufficiency When Using High-Caloric Liquid Diets? Orientating Studies on Praecaecal Digestibility in Pigs with Experimentally Induced Pancreatic Exocrine Insufficiency and Ileocaecal Fistula" Biomolecules 15, no. 10: 1392. https://doi.org/10.3390/biom15101392
APA StyleMößeler, A. K., Liesegang, A., Torgerson, P., & Kamphues, J. (2025). Is There Need for Pancreatic Enzyme Replacement Therapy in Patients with Exocrine Pancreatic Insufficiency When Using High-Caloric Liquid Diets? Orientating Studies on Praecaecal Digestibility in Pigs with Experimentally Induced Pancreatic Exocrine Insufficiency and Ileocaecal Fistula. Biomolecules, 15(10), 1392. https://doi.org/10.3390/biom15101392







