The Convergence of Biology and Material Science: Biomolecule-Driven Smart Drug Delivery Systems
Abstract
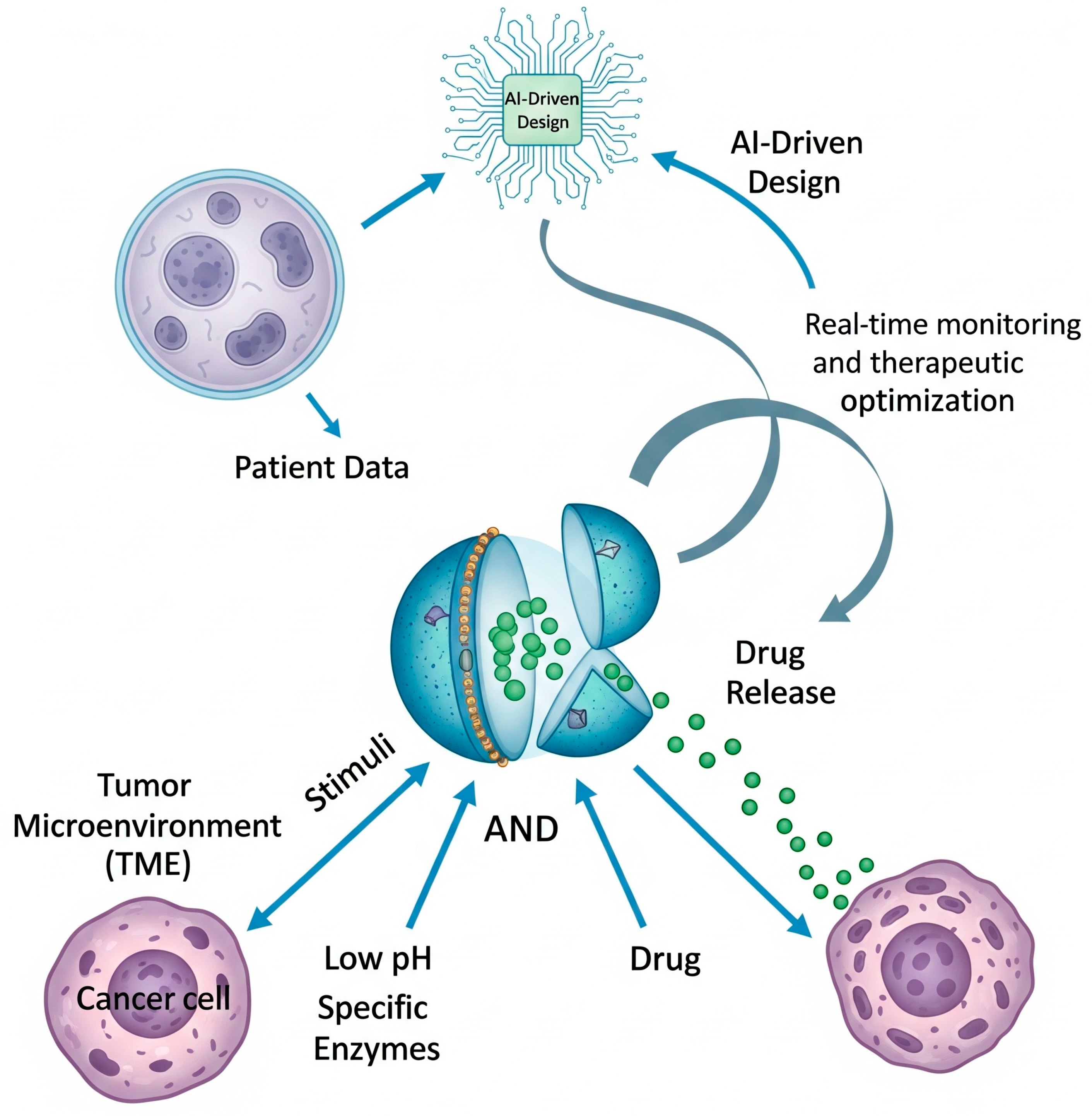
1. Introduction
2. Principles of Stimuli-Responsive Actuation
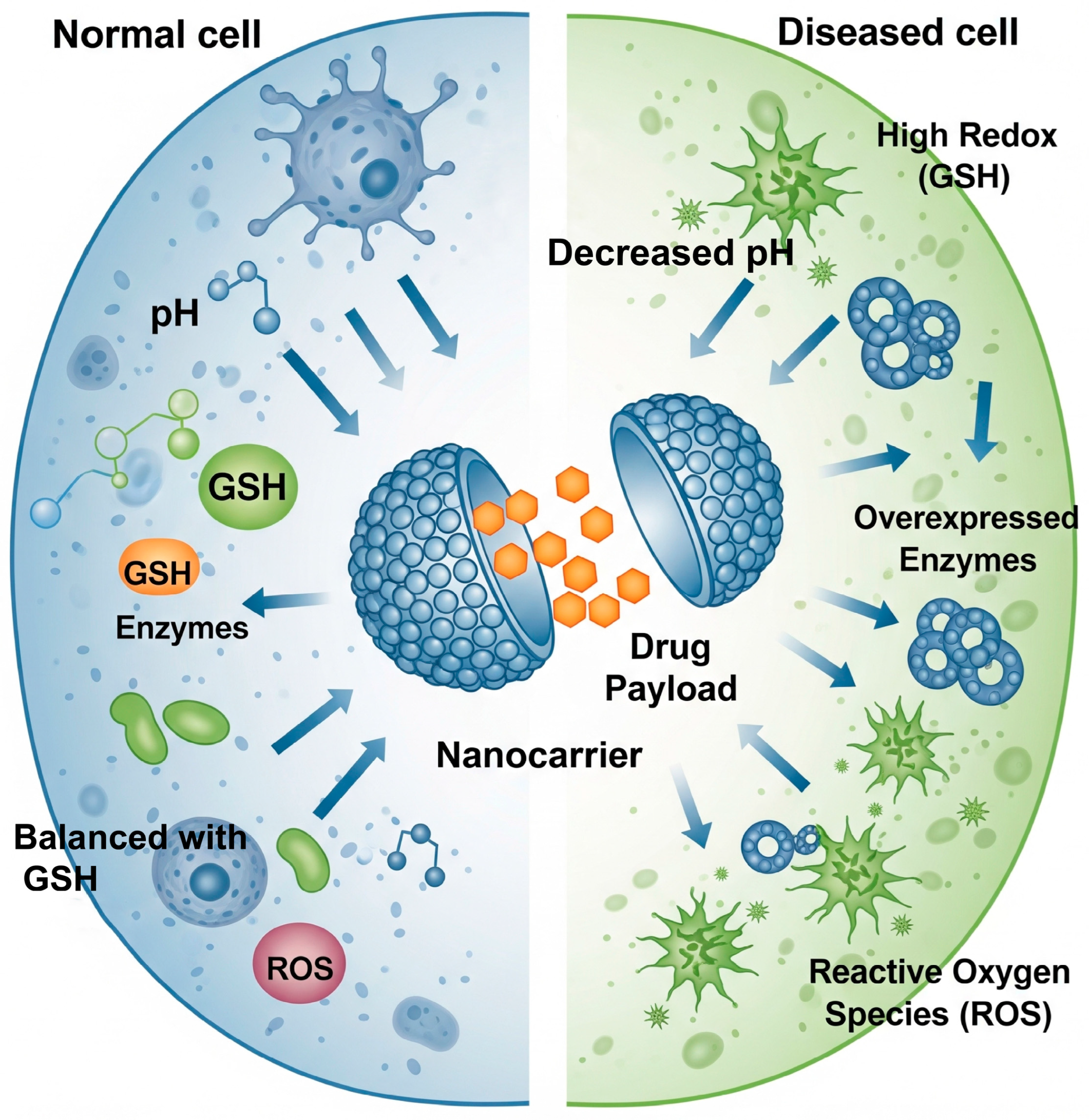
2.1. Exploiting Endogenous Cues: Responding to the Pathophysiological Milieu
2.2. Harnessing Exogenous Triggers: External Control for Spatiotemporal Precision
| Stimulus Type | Key Responsive Biomaterials/Moieties | Advantages | Limitations |
|---|---|---|---|
| pH [6,17,19] | Chitosan, poly (acrylic acid), histidine-rich peptides, DNA i-motifs, hydrazone/imine linkers. | Broad applicability (tumors, endosomes); autonomous activation. | Limited pH gradient between tumor/normal tissue; potential off-target activation in other acidic sites (inflammation). |
| Redox [16,22] | Disulfide cross-linkers, disulfide-conjugated drugs/polymers. | High specificity due to large intracellular/extracellular GSH gradient. | Primarily an intracellular trigger; less effective for extracellular release. |
| Enzymes [16,25] | Peptide sequences (MMP substrates), polysaccharides (hyaluronic acid), ester bonds. | Very high specificity and biological relevance; catalytic amplification. | Enzyme levels can be heterogeneous; potential for immunogenicity of peptide substrates. |
| ROS [28] | Bilirubin nanoparticles, polymers with thioether/thioketal linkers. | Targets oxidative stress characteristic of inflammation and cancer; can have dual therapeutic effect (drug delivery + ROS scavenging). | ROS levels can be transient and heterogeneous. |
| Temperature [35] | Elastin-like polypeptides (ELPs), poly(N-isopropylacrylamide) (PNIPAM). | Sharp, tunable response; can be triggered non-invasively with localized hyperthermia. | Requires external heating equipment; potential for damage to healthy tissue if heating is not precise. |
| Light [36,37] | Azobenzene, o-nitrobenzyl groups, gold nanoparticles, carbon nanotubes. | Unparalleled spatiotemporal control (on/off switching); non-invasive. | Limited tissue penetration depth, especially for UV/visible light; potential phototoxicity. |
| Magnetic Field [40] | Iron oxide nanoparticles (SPIONs) embedded in carriers. | Deep tissue penetration; enables dual imaging (MRI) and therapy. | Requires specialized equipment; potential for non-specific heating; guidance is limited to accessible sites. |
| Ultrasound [41] | Liposomes, micelles, nanoemulsions. | Non-invasive; deep tissue penetration; can enhance uptake via sonoporation. | Complex dose–response; potential for off-target tissue damage if not focused correctly. |
3. Biomolecular Architectures for Intelligent Drug Delivery
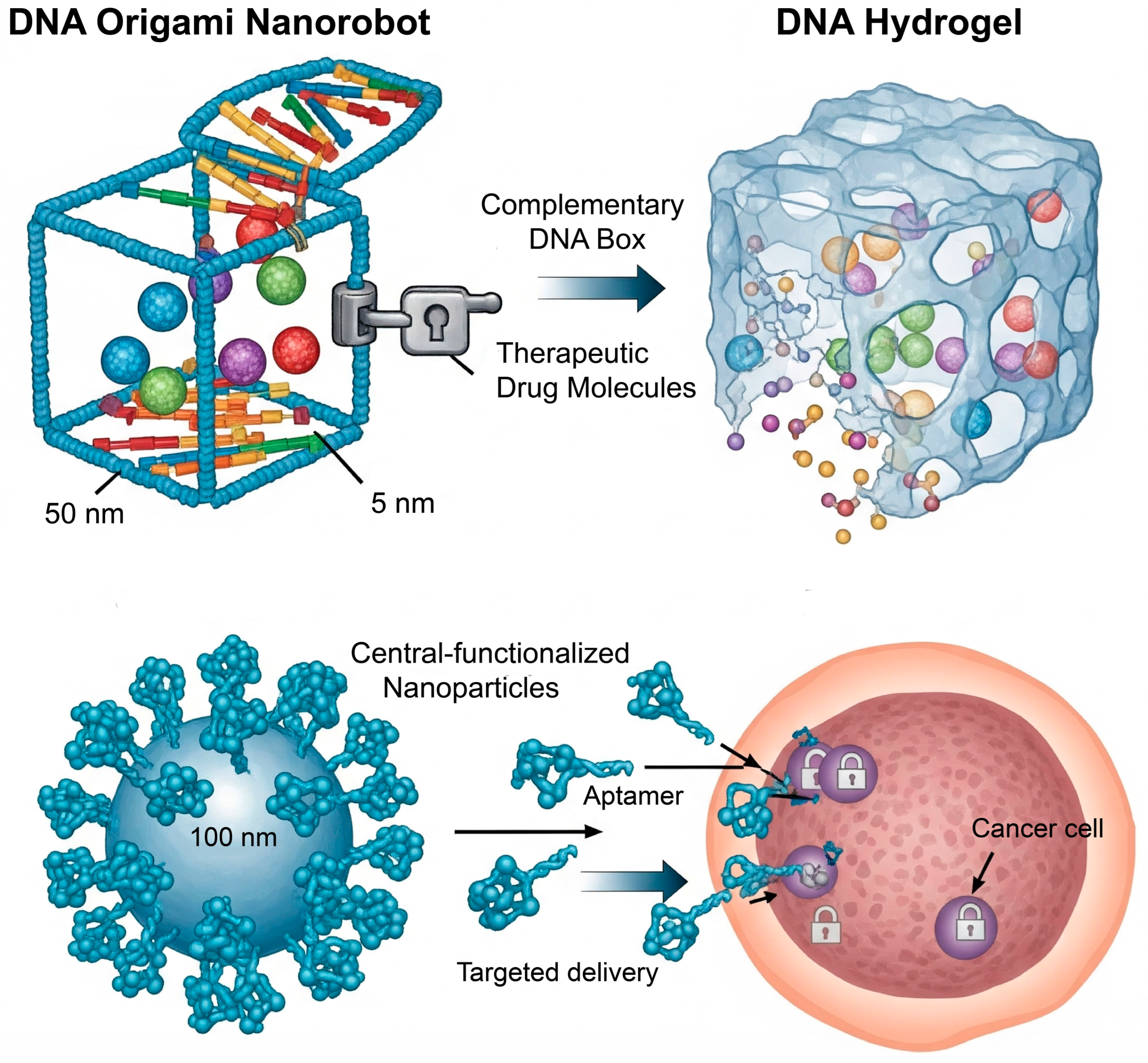
3.1. Nucleic Acid-Based Nanotechnology: Programmability at the Molecular Level
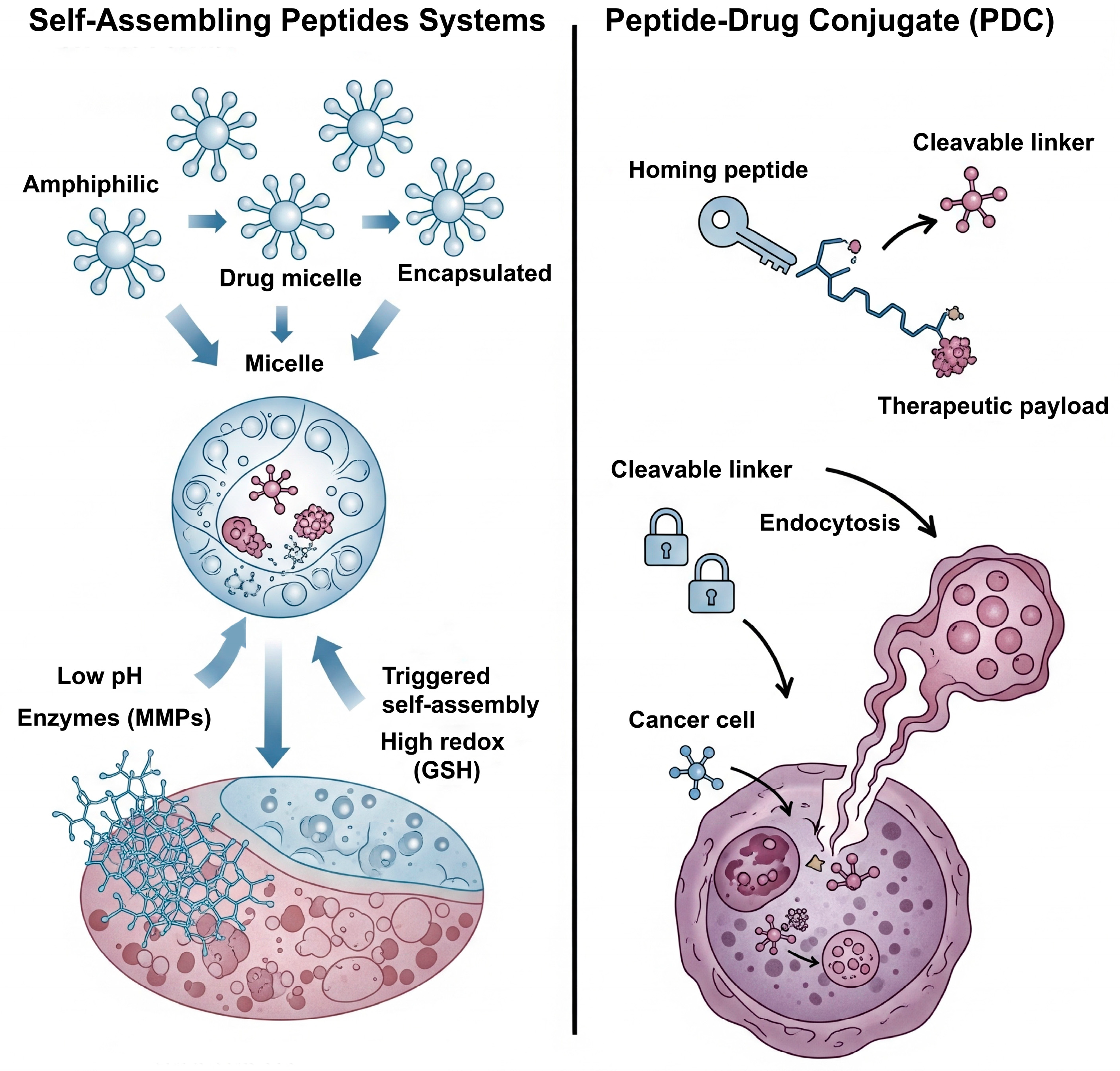
3.2. Peptide-Based Systems: Versatility in Structure and Function
3.3. Protein-Based Platforms: Leveraging Nature’s Workhorses
4. Therapeutic Frontiers and Applications
4.1. Oncology: Targeting the TME
4.2. Inflammatory and Autoimmune Diseases
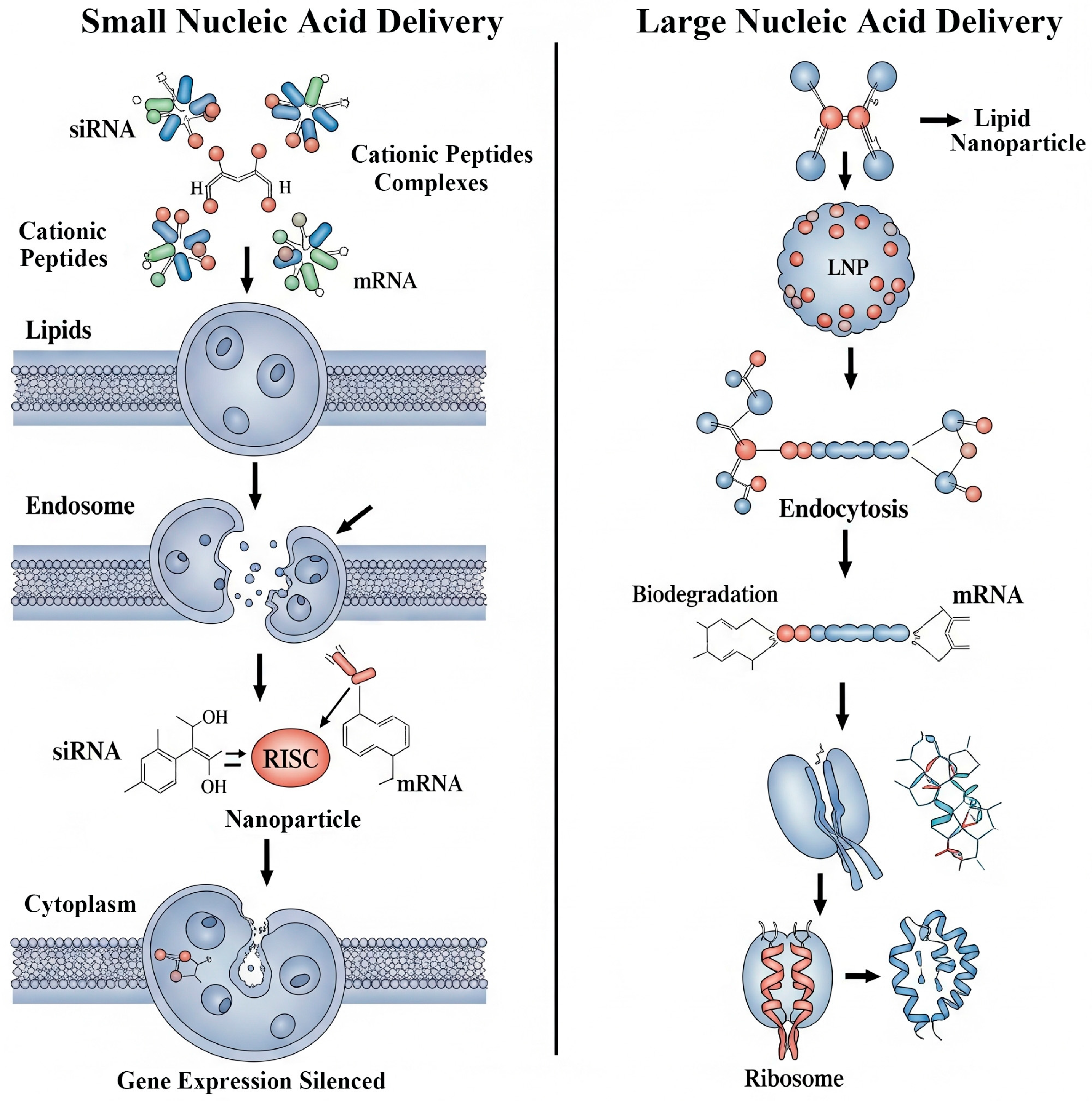
4.3. Gene Therapy: The Next Generation of Non-Viral Vectors
5. Translational Hurdles and Future Perspectives
5.1. The Bench-to-Bedside Chasm: Challenges in Clinical Translation
5.2. The Future of Smart Delivery: Towards Greater Complexity and Control
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savić, S.; Tamburić, S.; Savić, M.M. From conventional towards new—Natural surfactants in drug delivery systems design: Current status and perspectives. Expert Opin. Drug Deliv. 2010, 7, 353–369. [Google Scholar] [CrossRef]
- Aston, W.J.; Hope, D.E.; Nowak, A.K.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer 2017, 17, 684. [Google Scholar] [CrossRef]
- Corrie, P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2008, 36, 24–28. [Google Scholar] [CrossRef]
- Hami, Z. A brief review on advantages of nano-based drug delivery systems. Ann. Mil. Health Sci. Res. 2021, 19, e112274. [Google Scholar] [CrossRef]
- Traitel, T.; Goldbart, R.; Kost, J. Smart polymers for responsive drug-delivery systems. J. Biomater. Sci. Polym. Ed. 2008, 19, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Luo, C.; He, L.; Chen, F.; Fu, T.; Zhang, P.; Xiao, Z.; Liu, Y.; Tan, W. Stimulus-responsive nanomaterials containing logic gates for biomedical applications. Cell Rep. Phys. Sci. 2021, 2, 100350. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, L.; Hao, Z.; Liu, D. DNA-based nanostructures for RNA delivery. Med. Rev. 2024, 4, 207–224. [Google Scholar] [CrossRef]
- Abdollahzadeh, H.; Peeples, T.L.; Shahcheraghi, M. DNA nanotechnology in oligonucleotide drug delivery systems: Prospects for Bio-nanorobots in cancer treatment. Adv. Drug Deliv. Rev. 2025, 225, 115673. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Wu, J.-M.; Liu, Y.; Han, H.-Y.; Song, Z.-Y. Recent advances in endogenous and exogenous stimuli-responsive nanoplatforms for bacterial infection treatment. Biomed. Eng. Commun. 2023, 2, 2–23. [Google Scholar] [CrossRef]
- Guimarães, R.S.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of stimuli-responsive mesoporous organosilica nanocarriers for drug delivery. Pharmacol. Res. 2020, 155, 104742. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef] [PubMed]
- Vetrík, M.; Přádný, M.; Hrubý, M.; Michálek, J. Hydrazone-based hydrogel hydrolytically degradable in acidic environment. Polym. Degrad. Stab. 2011, 96, 756–759. [Google Scholar] [CrossRef]
- Lee, G.J.; Kim, T.I. pH-Responsive i-motif Conjugated Hyaluronic Acid/Polyethylenimine Complexes for Drug Delivery Systems. Pharmaceutics 2019, 11, 247. [Google Scholar] [CrossRef]
- Andreev, O.A.; Engelman, D.M.; Reshetnyak, Y.K. pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Mol. Membr. Biol. 2010, 27, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Yue, K.; Zhong, W. Mechanistic study of enhanced drug release in mixed pH-responsive peptide-loaded liposomes. J. Biomol. Struct. Dyn. 2025, 24, 1–15. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-manipulating nanocarriers for anticancer drug delivery: A systematic review. J. Nanobiotechnology 2024, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Khurana, R.K.; Garg, B.; Saini, S.; Kaur, R. Stimuli-Responsive Systems with Diverse Drug Delivery and Biomedical Applications: Recent Updates and Mechanistic Pathways. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 209–255. [Google Scholar] [CrossRef] [PubMed]
- Kistowski, M.; Dębski, J.; Karczmarski, J.; Paziewska, A.; Olędzki, J.; Mikula, M.; Ostrowski, J.; Dadlez, M. A strong neutrophil elastase proteolytic fingerprint marks the carcinoma tumor proteome. Mol. Cell. Proteom. 2017, 16, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Ross, B.P.; Liu, J.; Jambhrunkar, S.; Kleitz, F.; Qiao, S.Z. Enzyme-responsive controlled release of covalently bound prodrug from functional mesoporous silica nanospheres. Angew. Chem. Int. Ed. Engl. 2012, 51, 12486–12489. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Lee, C.C.; Lin, H.M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697. [Google Scholar] [CrossRef]
- de la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Repellin, M.; Guerin, H.; Catania, G.; Lollo, G. ROS-based nanomedicines for anti-inflammatory therapies. Redox Exp. Med. 2023, 2023, e230021. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 2662. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Xiao, C.; Zhuang, X.; Chen, X. Synthesis of a phenylboronic ester-linked PEG-lipid conjugate for ROS-responsive drug delivery. Polym. Chem. 2017, 8, 6209–6216. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Wei, C.; Xu, Y. Facile synthesis of ROS-responsive biodegradable main chain poly (carbonate-thioether) copolymers. Polym. Chem. 2018, 9, 904–911. [Google Scholar] [CrossRef]
- Pan, S.; Lan, Y.; Yang, Y.; Zhang, Y.; Yu, S.; Wang, T.; Zhu, Y.; Yang, S.; Yuan, X.; Gao, X.; et al. Metal-Polyphenol Network Coated Bilirubin Nanoparticles for the Alleviation of Periodontitis via Mild Photothermal Therapy and Immunomodulatory Therapy. ACS Nano 2025, 19, 23919–23944. [Google Scholar] [CrossRef]
- de Oliveira, T.E.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of stereochemistry and copolymerization on the LCST of PNIPAm. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef]
- Zhang, H.; Marmin, T.; Cuierrier, É.; Soldera, A.; Dory, Y.; Zhao, Y. A new comonomer design for enhancing the pH-triggered LCST shift of thermosensitive polymers. Polym. Chem. 2015, 6, 6644–6650. [Google Scholar] [CrossRef]
- Varanko, A.K.; Su, J.C.; Chilkoti, A. Elastin-like polypeptides for biomedical applications. Annu. Rev. Biomed. Eng. 2020, 22, 343–369. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Wang, W. Photopharmacology and photoresponsive drug delivery. Chem. Soc. Rev. 2025, 54, 5792–5835. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Sessa, L.; Panunzi, B.; Diana, R.; Piotto, S.; Concilio, S. Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels. Polymers 2024, 16, 1233. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Gold nanoparticles in photothermal cancer therapy: Current trends and outlook. Trends Sci. 2025, 22, 9433. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 10.1002/wnan.1449. [Google Scholar] [CrossRef]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M. Smart Nanomaterials for Biomedical Applications-A Review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jha, A.; Mishra, B. DNA-Based Nanostructured Platforms as Drug Delivery Systems. Chem. Bio Eng. 2024, 1, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhu, X.; Feng, C. DNA Nanodevice-Based Drug Delivery Systems. Biomolecules 2021, 11, 1855. [Google Scholar] [CrossRef]
- Yang, C.; Wu, X.; Liu, J.; Ding, B. Stimuli-responsive nucleic acid nanostructures for efficient drug delivery. Nanoscale 2022, 14, 17862–17870. [Google Scholar] [CrossRef]
- Linko, V.; Ora, A.; Kostiainen, M.A. DNA Nanostructures as Smart Drug-Delivery Vehicles and Molecular Devices. Trends Biotechnol. 2015, 33, 586–594. [Google Scholar] [CrossRef]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef]
- Heh, E.; Allen, J.; Ramirez, F.; Lovasz, D.; Fernandez, L.; Hogg, T.; Riva, H.; Holland, N.; Chacon, J. Peptide Drug Conjugates and Their Role in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 829. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, Y.; Jia, W.; Yu, Y.; Liu, C.; Yang, J.; Luan, Y.; Chen, J.; Wang, F. Advancements in SELEX Technology for Aptamers and Emerging Applications in Therapeutics and Drug Delivery. Biomolecules 2025, 15, 818. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, Y.; Song, W.; Nie, G.; Li, F. Functional integration of DNA and peptide-based supramolecular nanoassemblies for cancer therapy. Acc. Mater. Res. 2023, 4, 892–905. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, W.; Qiu, F. Self-assembling Peptides in Current Nanomedicine: Versatile Nanomaterials for Drug Delivery. Curr. Med. Chem. 2020, 27, 4855–4881. [Google Scholar] [CrossRef]
- Wilson, P. Synthesis and applications of protein/peptide-polymer conjugates. Macromol. Chem. Phys. 2017, 218, 1600595. [Google Scholar] [CrossRef]
- Otter, R.; Besenius, P. Supramolecular assembly of functional peptide–polymer conjugates. Org. Biomol. Chem. 2019, 17, 6719–6734. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Ishitsuka, T.; Harashima, H. Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J. Control. Release 2010, 147, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, S.; Yang, P.; Wang, P.; Lu, S.; Sheng, D.; Qian, K.; Cao, J.; Lu, W.; Zhang, Q. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J. Control. Release 2020, 320, 347–362. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Lau, E.Y.; Luo, J.; Yao, N.; Shi, C.; Meza, L.; Tseng, H.; Maeda, Y.; Kumaresan, P. The use of one-bead one-compound combinatorial library technology to discover high-affinity αvβ3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle. Mol. Cancer Ther. 2010, 9, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Lai, K.; Yan, J. Stimulus-Responsive DNA Hydrogel Biosensors for Food Safety Detection. Biosensors 2023, 13, 320. [Google Scholar] [CrossRef]
- Zhang, D.E.; He, T.; Shi, T.; Huang, K.; Peng, A. Trends in the research and development of peptide drug conjugates: Artificial intelligence aided design. Front. Pharmacol. 2025, 16, 1553853. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, Z.; Wu, X.; Wei, H.; Zhang, H.; Li, G.; Qian, Y.; Shahriari-Khalaji, M.; Hou, K.; Cao, R.; et al. Advances in Stimuli-Responsive Chitosan Hydrogels for Drug Delivery Systems. Macromol. Biosci. 2024, 24, e2300399. [Google Scholar] [CrossRef]
- Chau, Y.; Padera, R.F.; Dang, N.M.; Langer, R. Antitumor efficacy of a novel polymer–peptide–drug conjugate in human tumor xenograft models. Int. J. Cancer 2006, 118, 1519–1526. [Google Scholar] [CrossRef]
- Mukherjee, B.; Karmakar, S.D.; Hossain, C.M.; Bhattacharya, S. Peptides, proteins and peptide/protein-polymer conjugates as drug delivery system. Protein Pept. Lett. 2014, 21, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Nanoparticle albumin-bound paclitaxel (Abraxane®). In Albumin in Medicine: Pathological and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 101–119. [Google Scholar]
- Woldekidan, H.B.; Woldesemayat, A.A.; Adam, G.; Tafesse, M.; Thimiri Govinda Raj, D.B. Aptamer-Based Tumor-Targeted Diagnosis and Drug Delivery. Adv. Exp. Med. Biol. 2023, 1409, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Jamnongkan, W.; Thanan, R.; Techasen, A.; Namwat, N.; Loilome, W.; Intarawichian, P.; Titapun, A.; Yongvanit, P. Upregulation of transferrin receptor-1 induces cholangiocarcinoma progression via induction of labile iron pool. Tumour Biol. 2017, 39, 1010428317717655. [Google Scholar] [CrossRef]
- Al-Taie, A.; Özcan Bülbül, E. A paradigm use of monoclonal antibodies-conjugated nanoparticles in breast cancer treatment: Current status and potential approaches. J. Drug Target. 2024, 32, 45–56. [Google Scholar] [CrossRef]
- Frenz, T.; Grabski, E.; Durán, V.; Hozsa, C.; Stępczyńska, A.; Furch, M.; Gieseler, R.K.; Kalinke, U. Antigen presenting cell-selective drug delivery by glycan-decorated nanocarriers. Eur. J. Pharm. Biopharm. 2015, 95, 13–17. [Google Scholar] [CrossRef]
- Bidwell, G.L., 3rd. Novel Protein Therapeutics Created Using the Elastin-Like Polypeptide Platform. Physiology 2021, 36, 367–381. [Google Scholar] [CrossRef]
- Amruthwar, S.S.; Janorkar, A.V. Preparation and characterization of elastin-like polypeptide scaffolds for local delivery of antibiotics and proteins. J. Mater. Sci. Mater. Med. 2012, 23, 2903–2912. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. Applications of elastin-like polypeptides in drug delivery. J. Control. Release 2014, 190, 314–330. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Ruoslahti, E.; Meng, H. New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano. 2017, 11, 9567–9569. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Nan, Y.; Jia, L.; Sun, J.; Zhang, L.; Wang, Y. Hyaluronidase and pH Dual-Responsive Nanoparticles for Targeted Breast Cancer Stem Cells. Front. Oncol. 2021, 11, 760423. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix Metalloproteinase-sensitive Multistage Nanogels Promote Drug Transport in 3D Tumor Model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I.H. The role of multidrug resistance efflux pumps in cancer: Revisiting a JNCI publication exploring expression of the MDR1 (P-glycoprotein) gene. J. Natl. Cancer Inst. 2015, 107, djv222. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006, 7, 271–275. [Google Scholar] [CrossRef]
- Rana, A.; Adhikary, M.; Singh, P.K.; Das, B.C.; Bhatnagar, S. “Smart” drug delivery: A window to future of translational medicine. Front. Chem. 2022, 10, 1095598. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, T.J.; Zhang, L.; Deng, Q.Y.; Xia, Z.Y.; Chen, S.L.; Cheng, D.B.; Qiao, Z.Y.; Wang, H. Stimuli-responsive peptide-based nanodrug delivery systems for tumor therapy. Chem. Commun. 2025, 61, 7384–7407. [Google Scholar] [CrossRef]
- Kallepalli, B.; Garg, U.; Jain, N.; Nagpal, R.; Malhotra, S.; Tiwari, T.; Kaul, S.; Nagaich, U. Intelligent drug delivery: Pioneering stimuli-responsive systems to revolutionize disease management-an in-depth exploration. Curr. Drug Deliv. 2025, 22, 195–214. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Su, M.; Ran, Y.; Wang, Y.; Yin, Z. Reactive Oxygen Species–Responsive Celastrol-Loaded Bilirubin Nanoparticles for the Treatment of Rheumatoid Arthritis. AAPS J. 2021, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Abhang, A.; Kole, E.B.; Gadade, D.; Dusane, A.; Iyer, A.; Sharma, A.; Rout, S.K.; Gholap, A.D.; Naik, J.; et al. Peptide-Drug Conjugates as Next-Generation Therapeutics: Exploring the Potential and Clinical Progress. Bioengineering 2025, 12, 481. [Google Scholar] [CrossRef]
- Zhai, J.; Mantaj, J.; Vllasaliu, D. Ascorbyl Palmitate Hydrogel for Local, Intestinal Delivery of Macromolecules. Pharmaceutics 2018, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems-State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X. New protein-based smart materials. In Artificial Protein and Peptide Nanofibers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 415–436. [Google Scholar]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA versus miRNA as therapeutics for gene silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, W.; Sun, Y.; Huang, X.; Chu, R.; Wang, R.; Zhou, D.; Ye, S. Recent advances on drug delivery nanoplatforms for the treatment of autoimmune inflammatory diseases. Mater. Adv. 2022, 3, 7687–7708. [Google Scholar] [CrossRef]
- Zhang, S.; Ermann, J.; Succi, M.D.; Zhou, A.; Hamilton, M.J.; Cao, B.; Korzenik, J.R.; Glickman, J.N.; Vemula, P.K.; Glimcher, L.H. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 2015, 7, 300ra128. [Google Scholar] [CrossRef]
- Mittal, N.; Garg, V.; Bhadada, S.K.; Katare, O.P. Role of novel drug delivery systems in coronavirus disease-2019 (COVID-19): Time to act now. Curr. Drug Deliv. 2021, 18, 289–296. [Google Scholar] [CrossRef]
- Ren, S.; Wang, M.; Wang, C.; Wang, Y.; Sun, C.; Zeng, Z.; Cui, H.; Zhao, X. Application of Non-Viral Vectors in Drug Delivery and Gene Therapy. Polymers 2021, 13, 3307. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Uno, S.; Sugiura, K.; Sato, Y.; Fujioka, Y.; Ishida, A.; Ohba, Y.; Harashima, H.; Tokeshi, M. Development of Polymer-Lipid Hybrid Nanoparticles for Large-Sized Plasmid DNA Transfection. ACS Appl. Mater. Interfaces 2024, 16, 2110–2119. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; García-Hernandez, A.A.; Ramos, C.; Flores-Soto, E. Nanotechnology and Matrix Metalloproteinases in Cancer Diagnosis and Treatment. Front. Mol. Biosci. 2022, 9, 918789. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, M.; Bernardi, A.; Shah, N.; Papathanasiou, M.M. Emerging challenges and opportunities in pharmaceutical manufacturing and distribution. Processes 2021, 9, 457. [Google Scholar] [CrossRef]
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12, e2202688. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T. Smart drug delivery systems: Back to the future vs. clinical reality. Int. J. Pharm. 2013, 454, 527–529. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef] [PubMed]
- Panchpuri, M.; Painuli, R.; Kumar, C. Artificial intelligence in smart drug delivery systems: A step toward personalized medicine. RSC Pharm. 2025, 2, 882–914. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, C.; Luo, Y.; Chen, D. Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies. Pharmaceutics 2024, 16, 240. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Li, Y.; Wang, J.; He, X.; Zhang, J.; Qiao, Y.; Wu, H.; Zhu, L. Targeting and sensitizing MDR cancer by an MMP2 and pH dual-responsive ZnO-based nanomedicine. Cancer Nanotechnol. 2023, 14, 56. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef]
- Thangaraju, P.; Varthya, S.B. ISO 10993: Biological evaluation of medical devices. In Medical Device Guidelines and Regulations Handbook; Springer: Berlin/Heidelberg, Germany, 2022; pp. 163–187. [Google Scholar]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Paci, M.M.; Saha, T.; Djassemi, O.; Wu, S.; Chua, C.Y.X.; Wang, J.; Grattoni, A. Smart closed-loop drug delivery systems. Nat. Rev. Bioeng. 2025, 1–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Yu, X. The Convergence of Biology and Material Science: Biomolecule-Driven Smart Drug Delivery Systems. Biomolecules 2025, 15, 1383. https://doi.org/10.3390/biom15101383
Hou Y, Yu X. The Convergence of Biology and Material Science: Biomolecule-Driven Smart Drug Delivery Systems. Biomolecules. 2025; 15(10):1383. https://doi.org/10.3390/biom15101383
Chicago/Turabian StyleHou, Yaqin, and Xiaolei Yu. 2025. "The Convergence of Biology and Material Science: Biomolecule-Driven Smart Drug Delivery Systems" Biomolecules 15, no. 10: 1383. https://doi.org/10.3390/biom15101383
APA StyleHou, Y., & Yu, X. (2025). The Convergence of Biology and Material Science: Biomolecule-Driven Smart Drug Delivery Systems. Biomolecules, 15(10), 1383. https://doi.org/10.3390/biom15101383





