PRDM16 Enhances Osteoblastogenic RUNX2 via Canonical WNT10b/β-CATENIN Pathway in Testosterone-Treated Hypogonadal Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Testosterone Therapy
2.3. Areal BMD
2.4. Gene Expression Studies
2.4.1. Peripheral Blood Mononuclear Cells
2.4.2. Relative Quantification
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical and Serum Biochemical Parameters at Baseline and After 6 Months of T Therapy
3.2. T Therapy Enhanced Gene Expression of PRDM16 and WNT10b—β-Catenin-RUNX2 Signaling Pathway in the PBMC
3.3. T Therapy Increased Protein Levels of PRDM16, Canonical Markers WNT-10b and β-Catenin and Osteoblastogenic Marker RUNX2
3.4. Correlation Studies
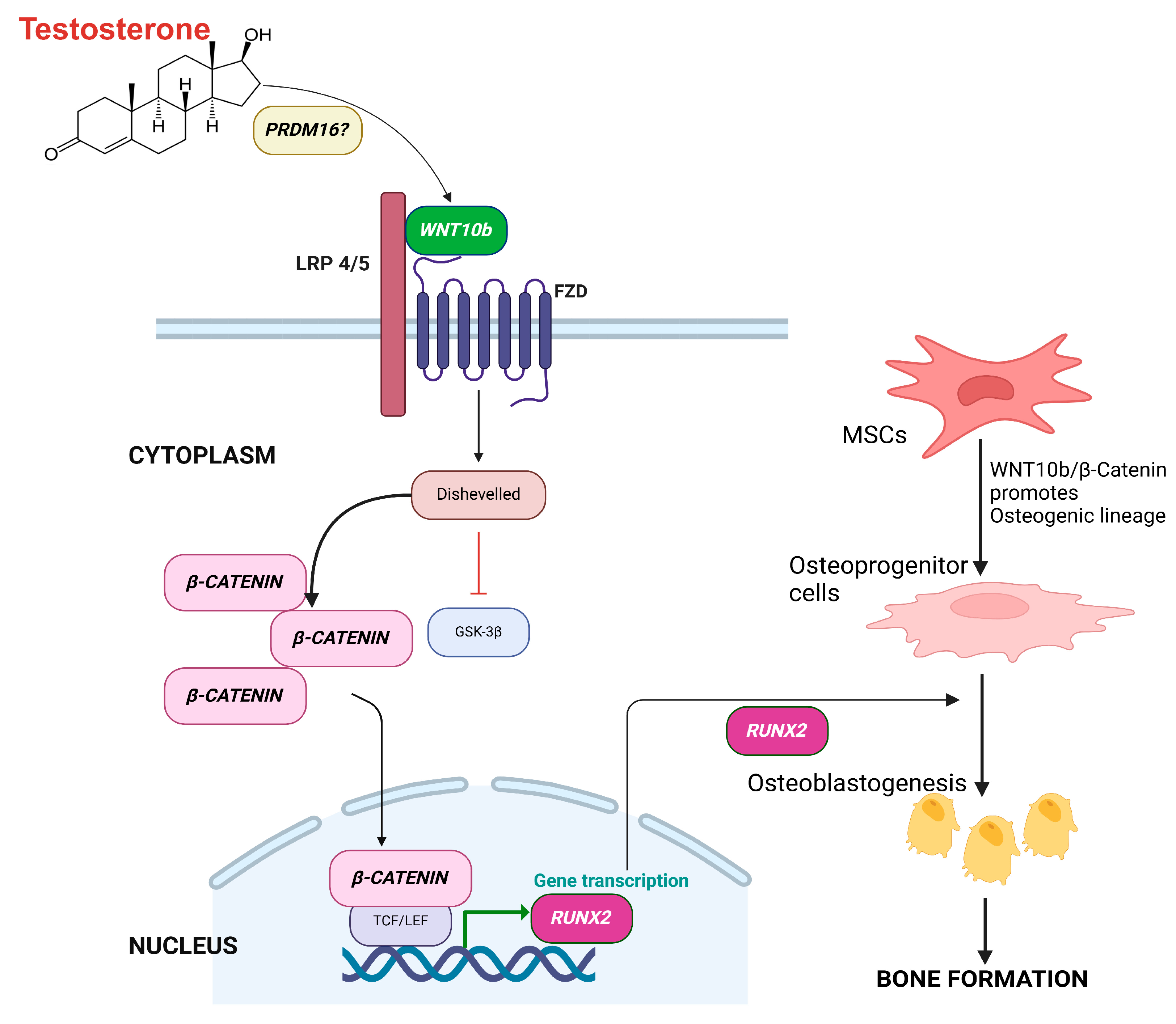
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer/Author’s Note
References
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef]
- Huhtaniemi, I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J. Androl. 2014, 16, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Klibanski, A.; Neer, R.M.; Greenspan, S.L.; Rosenthal, D.I.; Crowley, W.F., Jr. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann. Intern. Med. 1987, 106, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, S.L.; Neer, R.M.; Ridgway, E.C.; Klibanski, A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann. Intern. Med. 1986, 104, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.E.; Colleluori, G.; Robbins, D.; Dorin, R.; Shah, V.O.; Chen, R.; Jan, I.Z.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Bone and body composition response to testosterone therapy vary according to polymorphisms in the CYP19A1 gene. Endocrine 2019, 65, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Bauer, D.C.; Ellenberg, S.S.; Cauley, J.A.; Buhr, K.A.; Bhasin, S.; Miller, M.G.; Khan, N.S.; Li, X.; Nissen, S. Testosterone Treatment and Fractures in Men with Hypogonadism. N. Engl. J. Med. 2024, 390, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Dhindsa, S.; Green, K.; Abuaysheh, S.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Increase in Osteocalcin Following Testosterone Therapy in Men With Type 2 Diabetes and Subnormal Free Testosterone. J. Endocr. Soc. 2019, 3, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Ng Tang Fui, M.; Hoermann, R.; Bracken, K.; Handelsman, D.J.; Inder, W.J.; Stuckey, B.G.; Yeap, B.B.; Ghasem-Zadeh, A.; Robledo, K.P.; Jesudason, D.; et al. Effect of Testosterone Treatment on Bone Microarchitecture and Bone Mineral Density in Men: A 2-Year RCT. J. Clin. Endocrinol. Metab. 2021, 106, e3143–e3158. [Google Scholar] [CrossRef]
- Ng Tang Fui, M.; Hoermann, R.; Nolan, B.; Clarke, M.; Zajac, J.D.; Grossmann, M. Effect of testosterone treatment on bone remodelling markers and mineral density in obese dieting men in a randomized clinical trial. Sci. Rep. 2018, 8, 9099. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M. Androgens and bone growth: It’s location, location, location. Curr. Opin. Pharmacol. 2005, 5, 626–632. [Google Scholar] [CrossRef]
- Gao, K.; Wang, X.; Liu, Q.; Chen, W.; Wang, G.; Zhang, D.; Liu, L. Evaluation of osteoblast differentiation and function when cultured on mesoporous bioactive glass adsorbed with testosterone. J. Cell Biochem. 2018, 119, 5222–5232. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Colleluori, G.; Villareal, D.T.; Aguirre, L.; Chen, R.; Armamento-Villareal, R. A PRDM16-driven signal regulates body composition in testosterone-treated hypogonadal men. Front. Endocrinol. 2024, 15, 1426175. [Google Scholar] [CrossRef] [PubMed]
- Kaneda-Nakashima, K.; Igawa, K.; Suwanruengsri, M.; Naoyuki, F.; Ichikawa, T.; Funamoto, T.; Kurogi, S.; Sekimoto, T.; Yamashita, Y.; Chosa, E.; et al. Role of Mel1/Prdm16 in bone differentiation and morphology. Exp. Cell Res. 2022, 410, 112969. [Google Scholar] [CrossRef]
- Shull, L.C.; Sen, R.; Menzel, J.; Goyama, S.; Kurokawa, M.; Artinger, K.B. The conserved and divergent roles of Prdm3 and Prdm16 in zebrafish and mouse craniofacial development. Dev. Biol. 2020, 461, 132–144. [Google Scholar] [CrossRef]
- Stevens, J.R.; Miranda-Carboni, G.A.; Singer, M.A.; Brugger, S.M.; Lyons, K.M.; Lane, T.F. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J. Bone Miner. Res. 2010, 25, 2138–2147. [Google Scholar] [CrossRef]
- Shull, L.C.; Lencer, E.S.; Kim, H.M.; Goyama, S.; Kurokawa, M.; Costello, J.C.; Jones, K.; Artinger, K.B. PRDM paralogs antagonistically balance Wnt/β-catenin activity during craniofacial chondrocyte differentiation. Development 2022, 149, dev200082. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, S.A.; Taes, Y.; Fiers, T.; Toye, K.; Van Caenegem, E.; Roggen, I.; De Schepper, J.; Kaufman, J.M. Associations of sex steroids with bone maturation, bone mineral density, bone geometry, and body composition: A cross-sectional study in healthy male adolescents. J. Clin. Endocrinol. Metab. 2014, 99, E1272–E1282. [Google Scholar] [CrossRef] [PubMed]
- Deepika, F.N.; Ballato, E.; Colleluori, G.; Aguirre, L.; Chen, R.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Baseline Testosterone Predicts Body Composition and Metabolic Response to Testosterone Therapy. Front. Endocrinol. 2022, 13, 915309. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Kopperdahl, D.L.; Stephens-Shields, A.J.; Ellenberg, S.S.; Cauley, J.A.; Ensrud, K.E.; Lewis, C.E.; Barrett-Connor, E.; Schwartz, A.V.; Lee, D.C.; et al. Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. JAMA Intern. Med. 2017, 177, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.E.; Colleluori, G.; Fowler, K.E.; Jan, I.Z.; Villareal, K.; Qualls, C.; Robbins, D.; Villareal, D.T.; Armamento-Villareal, R. High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. Eur. J. Endocrinol. 2015, 173, 167–174. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Sesma, P.; Burrell, M.A. PRDM16: The interconvertible adipo-myocyte switch. Trends Cell Biol. 2009, 19, 141–146. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Wang, G.; Diao, Y.; Long, Y.; Fu, X.; Weng, M.; Zhou, L.; Sun, K.; Cheung, T.H.; Ip, N.Y.; et al. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev. Cell. 2017, 41, 382–391.e5. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S. Runx2/Cbfa1: A multifunctional regulator of bone formation. Curr. Pharm. Des. 2003, 9, 2677–2685. [Google Scholar] [CrossRef]
- Ding, H.L.; Clouthier, D.E.; Artinger, K.B. Redundant roles of PRDM family members in zebrafish craniofacial development. Dev. Dyn. 2013, 242, 67–79. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yang, Y.S.; Park, K.H.; Ge, X.; Xu, R.; Li, N.; Song, M.; Chun, H.; Bok, S.; Charles, J.F.; et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat. Commun. 2020, 11, 2289. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Sun, D.; Duan, Y.; Wen, P.; Dai, C.; Yang, J.; He, W. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 2016, 345, 206–217. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yang, L.; Zhou, J.; Tang, Y.; Zheng, L.; Qin, P. Runx2 alleviates high glucose-suppressed osteogenic differentiation via PI3K/AKT/GSK3β/β-catenin pathway. Cell Biol. Int. 2017, 41, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Finkelstein, J.S.; Schoenfeld, D.A.; Rosenthal, D.I.; Anderson, E.J.; Klibanski, A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J. Clin. Endocrinol. Metab. 1996, 81, 4358–4365. [Google Scholar] [PubMed]
- Wang, C.; Cunningham, G.; Dobs, A.; Iranmanesh, A.; Matsumoto, A.M.; Snyder, P.J.; Weber, T.; Berman, N.; Hull, L.; Swerdloff, R.S. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 2085–2098. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J., III; Atkinson, E.J.; O’Fallon, W.M.; Klee, G.G.; Riggs, B.L. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J. Clin. Endocrinol. Metab. 1998, 83, 2266–2274. [Google Scholar] [PubMed]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Colvard, D.S.; Eriksen, E.F.; Keeting, P.E.; Wilson, E.M.; Lubahn, D.B.; French, F.S.; Riggs, B.L.; Spelsberg, T.C. Identification of androgen receptors in normal human osteoblast-like cells. Proc. Natl. Acad. Sci. USA 1989, 86, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S.; Iranmanesh, A.; Dobs, A.; Snyder, P.J.; Cunningham, G.; Matsumoto, A.M.; Weber, T.; Berman, N.; Testosterone Gel Study Group. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin. Endocrinol. 2001, 54, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Khademian, N.; Mirzaei, A.; Hosseini, A.; Zare, L.; Nazem, S.; Babaheidarian, P.; Sheikhi, A.; Abdolvahabi, Z.; Ibrahimi, M.; Jamshidi, K.; et al. Expression pattern and clinical significance of β-catenin gene and protein in patients with primary malignant and benign bone tumors. Sci. Rep. 2022, 12, 9488. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, M.; Valenti, M.T.; Donatelli, L.; Zucal, C.; Dalle Carbonare, L. Runx-2 gene expression is associated with age-related changes of bone mineral density in the healthy young-adult population. J. Bone Miner. Metab. 2012, 30, 706–714. [Google Scholar] [CrossRef] [PubMed]
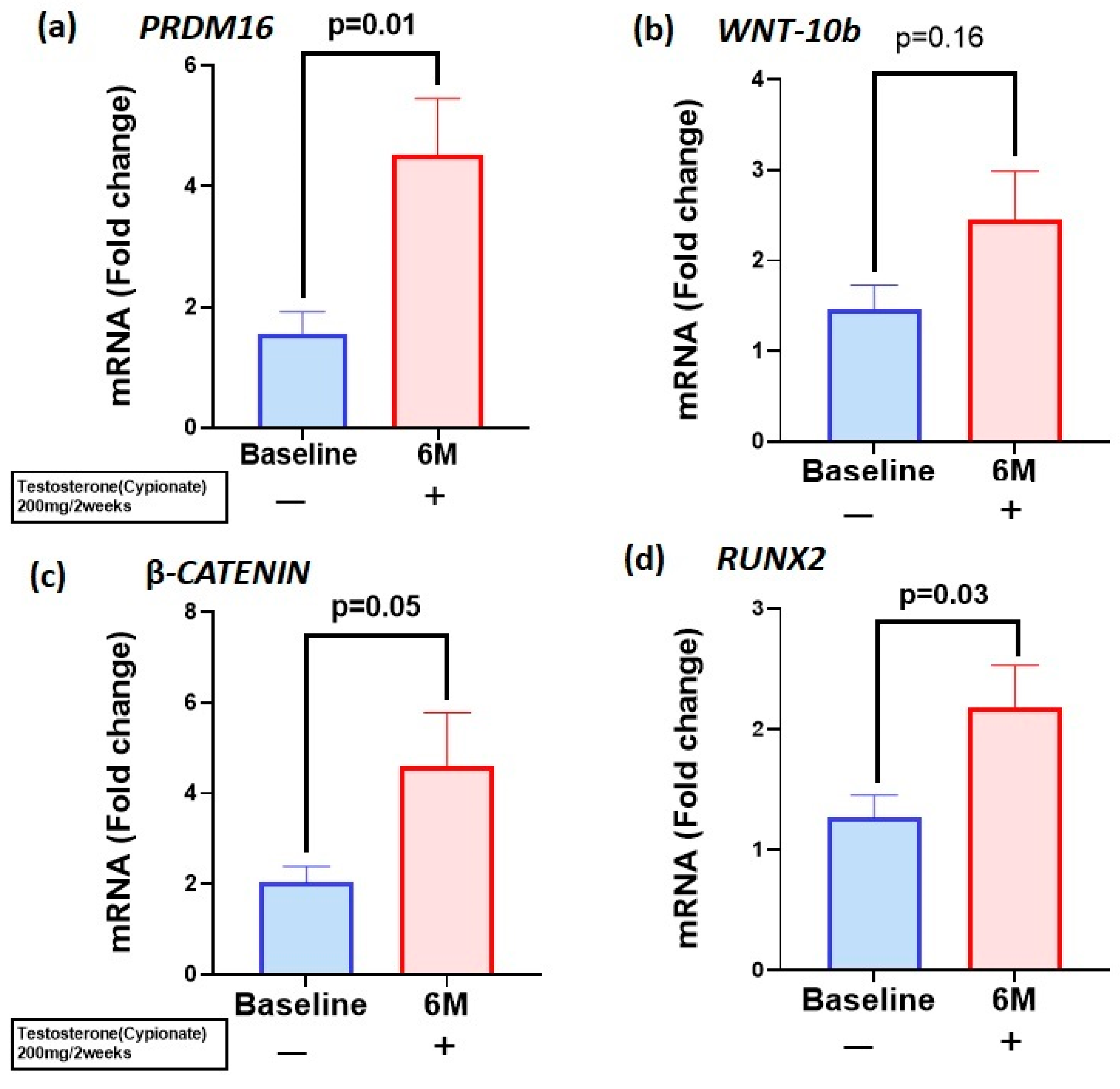

| Baseline | After 6 Months | p Value | |
|---|---|---|---|
| Age | 59.8 ± 8.5 | 60.1 ± 8.7 | 0.86 |
| BMI | 32.1 ± 5.1 | 32.2 ± 4.9 | 0.90 |
| Testosterone(T) | 258.6 ± 90.34 | 578.3 ± 241.7 | 0.001 |
| Estradiol | 15.69 ± 6.06 | 37.39 ± 21.2 | 0.001 |
| E/T | 0.70 ± 0.38 | 71.3 ± 40.1 | 0.001 |
| Osteocalcin (ng/mL) | 7.1 ± 5.4 | 4.9 ± 3.4 | <0.05 |
| CTX (ng/mL) | 0.34 ± 0.20 | 0.22 ± 0.12 | 0.005 |
| PRDM16 (ng/mL) | 0.30 ± 0.11 | 0.55 ± 0.72 | 0.04 |
| WNT-10b (ng/mL) | 0.74 ± 0.41 | 1.14 ± 0.58 | <0.01 |
| β-CATENIN (pg/mL) | 3164 ± 1170 | 3994 ± 1610 | <0.05 |
| RUNX2 (ng/mL) | 27.8 ± 33.8 | 67.7 ± 103.5 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathina, S.; Prado, M.; Fuenmayor Lopez, V.; Colleluori, G.; Aguirre, L.; Chen, R.; Villareal, D.T.; Armamento-Villareal, R. PRDM16 Enhances Osteoblastogenic RUNX2 via Canonical WNT10b/β-CATENIN Pathway in Testosterone-Treated Hypogonadal Men. Biomolecules 2025, 15, 79. https://doi.org/10.3390/biom15010079
Bathina S, Prado M, Fuenmayor Lopez V, Colleluori G, Aguirre L, Chen R, Villareal DT, Armamento-Villareal R. PRDM16 Enhances Osteoblastogenic RUNX2 via Canonical WNT10b/β-CATENIN Pathway in Testosterone-Treated Hypogonadal Men. Biomolecules. 2025; 15(1):79. https://doi.org/10.3390/biom15010079
Chicago/Turabian StyleBathina, Siresha, Mia Prado, Virginia Fuenmayor Lopez, Georgia Colleluori, Lina Aguirre, Rui Chen, Dennis T. Villareal, and Reina Armamento-Villareal. 2025. "PRDM16 Enhances Osteoblastogenic RUNX2 via Canonical WNT10b/β-CATENIN Pathway in Testosterone-Treated Hypogonadal Men" Biomolecules 15, no. 1: 79. https://doi.org/10.3390/biom15010079
APA StyleBathina, S., Prado, M., Fuenmayor Lopez, V., Colleluori, G., Aguirre, L., Chen, R., Villareal, D. T., & Armamento-Villareal, R. (2025). PRDM16 Enhances Osteoblastogenic RUNX2 via Canonical WNT10b/β-CATENIN Pathway in Testosterone-Treated Hypogonadal Men. Biomolecules, 15(1), 79. https://doi.org/10.3390/biom15010079





