TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses
Abstract
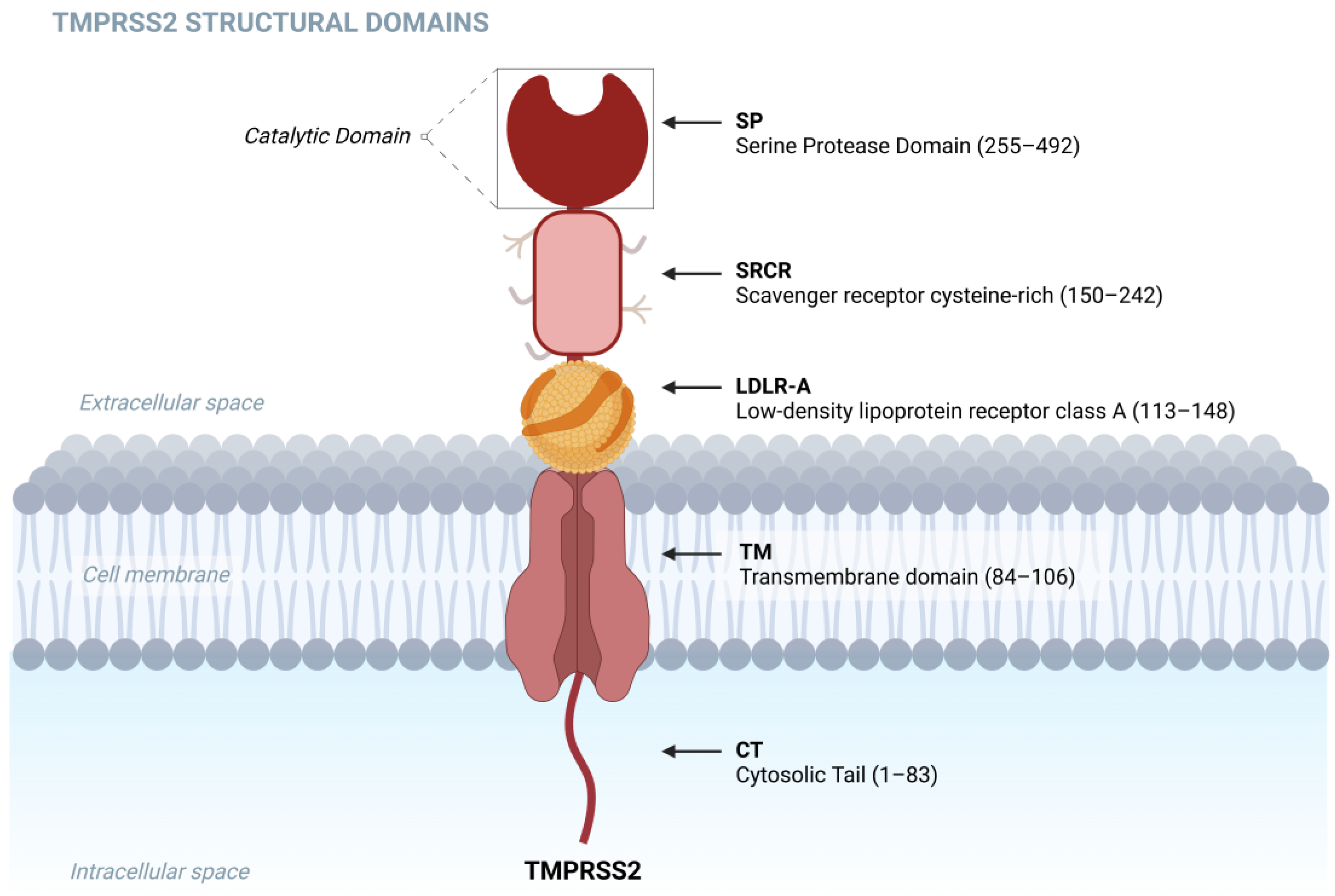
1. Introduction
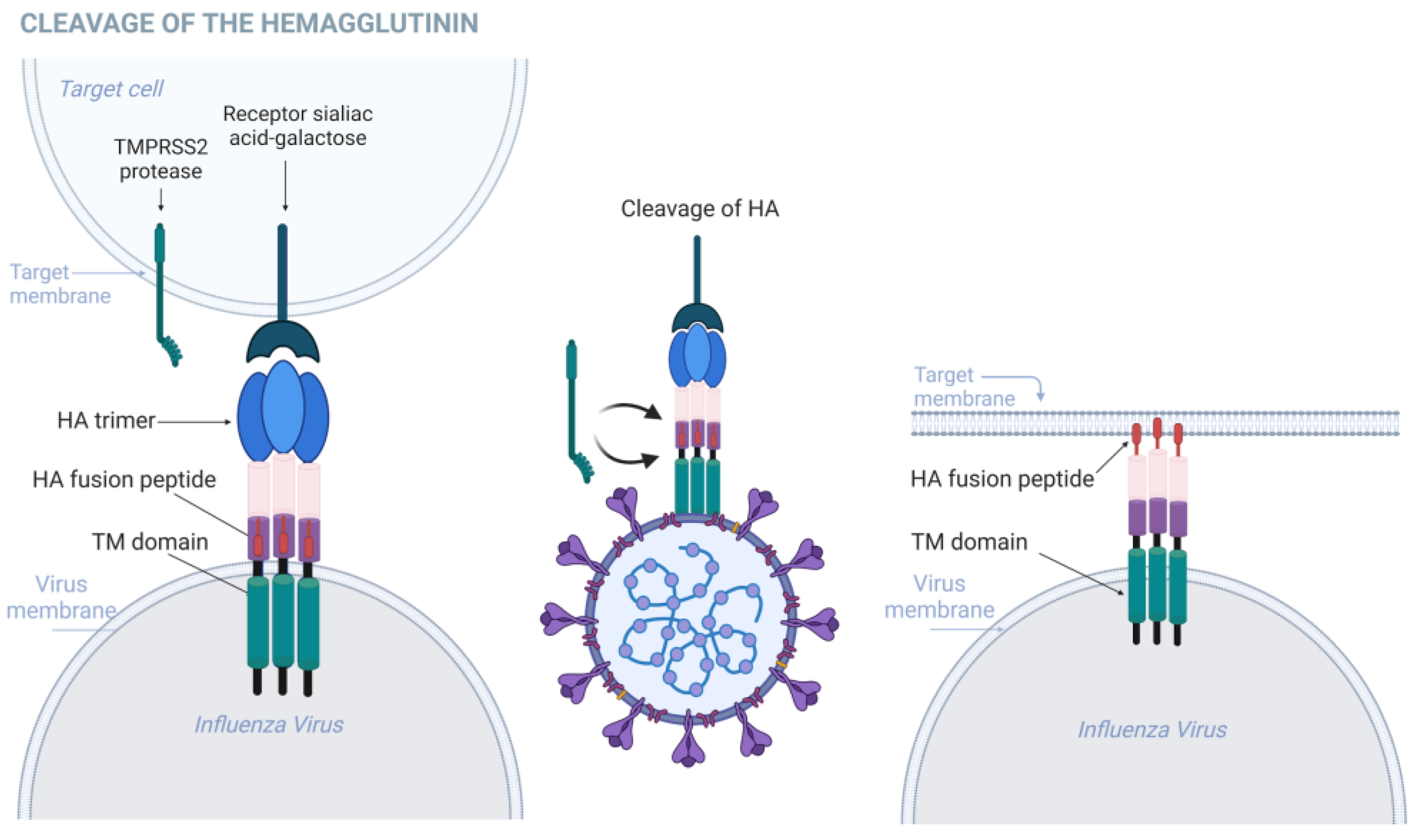
2. Role of TMPRSS2 in Influenza Infections
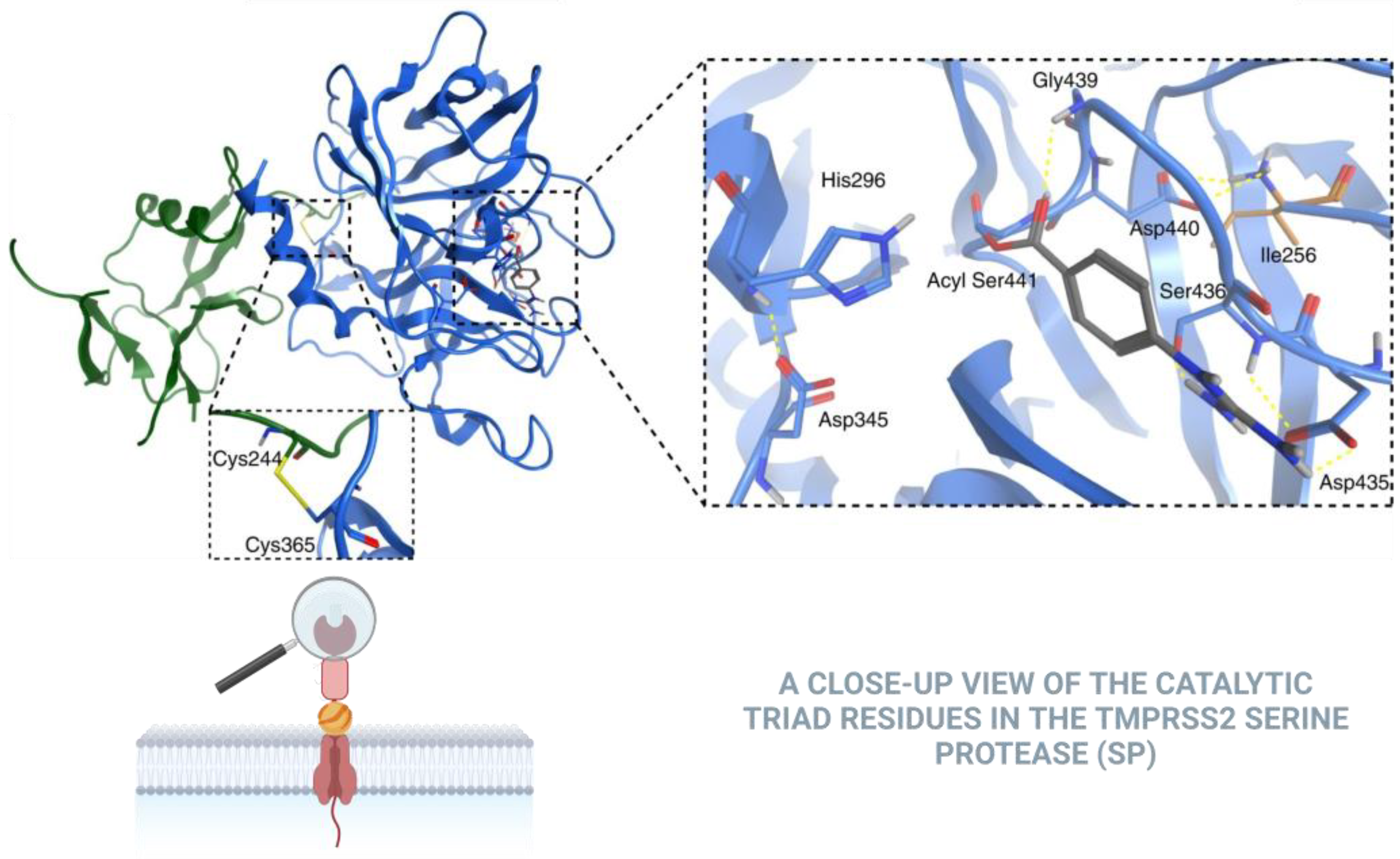
3. Role of TMPRSS2 in Coronavirus Infections
4. TMPRSS2 Polymorphisms and COVID-19
5. TMPRSS2 as a Potential Target for Treatment
6. Clinical Trials
7. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paoloni-Giacobino, A.; Chen, H.; Peitsch, M.C.; Rossier, C.; Antonarakis, S.E. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics 1997, 44, 309–320, Erratum in Genomics 2001, 77, 114. [Google Scholar] [CrossRef] [PubMed]
- Jacquinet, E.; Rao, N.V.; Rao, G.V.; Wang, Z.; Albertine, K.H.; Hoidal, J.R. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur. J. Biochem. 2001, 268, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999, 59, 4180–4184. [Google Scholar] [PubMed]
- Bugge, T.H.; Antalis, T.M.; Wu, Q. Type II transmembrane serine proteases. J. Biol. Chem. 2009, 284, 23177–23181. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, M.H.; Porvari, K.S.; Kellokumpu, S.; Kyllönen, A.P.; Vihko, P.T. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J. Pathol. 2001, 193, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.M.; True, L.; Hawley, S.; Matsumura, M.; Morrissey, C.; Vessella, R.; Nelson, P.S. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008, 215, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, M.-S.; Lucht, A.; Chou, F.-P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.-K.; Antalis, T.M.; Johnson, M.D.; et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–9718. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Hirsh, A.; Li, D.C.; Holloway, G.; Chao, J.; Boucher, R.C.; Gabriel, S.E. Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 2002, 277, 8338–8345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Faiq, M.A.; Pareek, V.; Raza, K.; Narayan, R.K.; Prasoon, P.; Kumar, P.; Kulandhasamy, M.; Kumari, C.; Kant, K.; et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med. Hypotheses 2020, 144, 110271. [Google Scholar] [CrossRef] [PubMed]
- Sure, F.; Bertog, M.; Afonso, S.; Diakov, A.; Rinke, R.; Madej, M.G.; Wittmann, S.; Gramberg, T.; Korbmacher, C.; Ilyaskin, A.V. Transmembrane serine protease 2 (TMPRSS2) proteolytically activates the epithelial sodium channel (ENaC) by cleaving the channel’s γ-subunit. J. Biol. Chem. 2022, 298, 102004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matarese, A.; Gambardella, J.; Sardu, C.; Santulli, G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Friebertshäuser, E.; Freuer, C.; Sielaff, F.; Schmidt, S.; Eickmann, M.; Uhlendorff, J.; Steinmetzer, T.; Klenk, H.D.; Garten, W. Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors. J. Virol. 2010, 84, 5605–5614. [Google Scholar] [CrossRef] [PubMed]
- Esumi, M.; Ishibashi, M.; Yamaguchi, H.; Nakajima, S.; Tai, Y.; Kikuta, S.; Sugitani, M.; Takayama, T.; Tahara, M.; Takeda, M.; et al. Transmembrane serine protease TMPRSS2 activates hepatitis C virus infection. Hepatology 2015, 61, 437–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melis, M.; Diaz, G.; Kleiner, D.E.; Zamboni, F.; Kabat, J.; Lai, J.; Mogavero, G.; Tice, A.; Engle, R.E.; Becker, S.; et al. Viral expression and molecular profiling in liver tissue versus microdissected hepatocytes in hepatitis B virus-associated hepatocellular carcinoma. J. Transl. Med. 2014, 12, 230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamamoto, Y.; Kawamura, M.; Uchida, H.; Hiramatsu, K.; Katori, C.; Asai, H.; Egawa, S.; Yoshida, K. Increased ACE2 and TMPRSS2 expression in ulcerative colitis. Pathol. Res. Pract. 2024, 254, 155108. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Nguyen, T.Q.; Tran, T.T.A. Genetic Susceptibility of ACE2 and TMPRSS2 in Six Common Cancers and Possible Impacts on COVID-19. Cancer Res. Treat. 2021, 53, 650–656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palese, P.; Buonaugurio, D.A. Influenza Viruses: Genome Structure, Transcription and Replication of Viral RNA; The Molecular Basis of Viral Replication. NATO ASI, Series; Bercoff, R.P., Ed.; Springer: Boston, MA, USA, 1987; Volume 136. [Google Scholar] [CrossRef]
- Treanor John, J.; Brian, R. Genes Involved in the Restriction of Replication of Avian Influenza A Viruses in Primates. In Virus Variability, Epidemiology and Control; Springer: Boston, MA, USA, 1990; pp. 159–176. [Google Scholar]
- Murakami, S.; Horimoto, T. Novel-type D-influenza virus. Uirusu 2017, 67, 161–170. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapoor, S.; Dhama, K. Insight into Influenza Viruses of Animals and Humans; Springer Nature: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, J.; Wang, L.; Ran, L.; Gao, G.F. Ecology and evolution of avian influenza viruses. Curr. Biol. 2024, 34, R716–R721. [Google Scholar] [CrossRef]
- Stone, H.; Notaras, A.; Chen, R.; Edgeworth, J.; Quigley, A. Highly Pathogenic Avian Influenza (H5N1) in Humans after the emergence of clade 2.3.4.4b in 2020. Glob. Biosecur. 2023, 5. [Google Scholar] [CrossRef]
- Mahase, E. Bird flu: First person with confirmed H5N2 infection dies. BMJ 2024, 385, q1260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fang, S.; Wang, T.; Li, J.; Cheng, X.; Zhao, C.; Wang, X.; Lv, X.; Wu, C.; Zhang, R.; et al. Applicability of a sensitive duplex real-time PCR assay for identifying B/Yamagata and B/Victoria lineages of influenza virus from clinical specimens. Appl. Microbiol. Biotechnol. 2012, 93, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Nakamura, K. The virological, epidemiological and clinical features of influenza A, B and C viruses. Nihon Rinsho 1997, 55, 2515–2520. (In Japanese) [Google Scholar] [PubMed]
- Klenk, H.D.; Rott, R.; Orlich, M.; Blödorn, J. Activation of influenza A viruses by trypsin treatment. Virology 1975, 68, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Sondra, G.; Lazarowitz Purnell, W. Choppin, Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 1975, 68, 440–454. [Google Scholar] [CrossRef]
- Böttcher-Friebertshäuser, E.; Garten, W.; Matrosovich, M.; Klenk, H.D. The hemagglutinin: A determinant of pathogenicity. Curr. Top. Microbiol. Immunol. 2014, 385, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Planitzer, S.D.; Wu, K.B.; Li, Z.; Niu, J.; Ivanovic, T. DNA-programmed surface display of receptors to dissect the functional interplay between receptor binding and membrane fusion of single influenza A virions. Biophys. J. 2024, 123, 303a. [Google Scholar] [CrossRef]
- Cross, K.J.; Langley, W.A.; Russell, R.J.; Skehel, J.J.; Steinhauer, D.A. Composition and functions of the influenza fusion peptide. Protein. Pept. Lett. 2009, 16, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bestle, D.; Heindl, M.R.; Limburg, H.; van Van Lam, T.; Pilgram, O.; Moulton, H.M.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, L.W.; Mao, H.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatesuer, B.; Bertram, S.; Mehnert, N.; Bahgat, M.M.; Nelson, P.S.; Pöhlmann, S.; Schughart, K. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. 2013, 9, e1003774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakai, K.; Ami, Y.; Tahara, M.; Kubota, T.; Anraku, M.; Abe, M.; Nakajima, N.; Sekizuka, T.; Shirato, K.; Suzaki, Y.; et al. The host protease TMPRSS2 plays a major role in In Vivo replication of emerging H7N9 and seasonal influenza viruses. J. Virol. 2014, 88, 5608–5616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambertz, R.L.O.; Gerhauser, I.; Nehlmeier, I.; Gärtner, S.; Winkler, M.; Leist, S.R.; Kollmus, H.; Pöhlmann, S.; Schughart, K. H2 influenza A virus is not pathogenic in Tmprss2 knock-out mice. Virol. J. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambertz, R.L.O.; Gerhauser, I.; Nehlmeier, I.; Gärtner, S.; Winkler, M.; Leist, S.R.; Kollmus, H.; Pöhlmann, S.; Schughart, K. Tmprss2 knock-out mice are resistant to H10 influenza A virus pathogenesis. J. Gen. Virol. 2019, 100, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D.A.; Moulton, H.M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J.; Schulte, L.; et al. TMPRSS2 Is the Major Activating Protease of Influenza A Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes. J. Virol. 2019, 93, e00649-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwon, T.; Artiaga, B.L.; McDowell, C.D.; Whitworth, K.M.; Wells, K.D.; Prather, R.S.; Delhon, G.; Cigan, M.; White, S.N.; Retallick, J.; et al. Gene editing of pigs to control influenza A virus infections. bioRxiv 2024, 13, 2387449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuzaki, Y.; Sugawara, K.; Furuse, Y.; Shimotai, Y.; Hongo, S.; Oshitani, H.; Mizuta, K.; Nishimura, H. Genetic Lineage and Reassortment of Influenza C Viruses Circulating between 1947 and 2014. J. Virol. 2016, 90, 8251–8265. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, J.; Hause, B.M.; Park, J.Y.; Sreenivasan, C.; Uprety, T.; Sheng, Z.; Wang, D.; Li, F. Emergence of new phylogenetic lineage of Influenza D virus with broad antigenicity in California, United States. Emerg. Microbes Infect. 2021, 10, 739–742. [Google Scholar] [CrossRef]
- Wang, M.; Veit, M. Hemagglutinin-esterase-fusion (HEF) protein of influenza C virus. Protein Cell 2016, 7, 28–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, K.; Hayashi, H.; Shimotai, Y.; Yamaya, M.; Hongo, S.; Kawakami, K.; Matsuzaki, Y.; Nishimura, H. TMPRSS2 Activates Hemagglutinin-Esterase Glycoprotein of Influenza C Virus. J. Virol. 2021, 95, e0129621-21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abe, M.; Tahara, M.; Sakai, K.; Yamaguchi, H.; Kanou, K.; Shirato, K.; Kawase, M.; Noda MKimura, H.; Matsuyama, S.; Fukuhara, H.; et al. TMPRSS2 Is an Activating Protease for Respiratory Parainfluenza Viruses. J. Virol. 2013, 87, 11930–11935. [Google Scholar] [CrossRef] [PubMed]
- Shirogane, Y.; Takeda, M.; Iwasaki, M.; Ishiguro, N.; Takeuchi, H.; Nakatsu, Y.; Tahara, M.; Kikuta, H.; Yanagi, Y. Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J. Virol. 2008, 82, 8942–8946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Straus, M.R.; Kinder, J.T.; Segall, M.; Dutch, R.E.; Whittaker, G.R. SPINT2 inhibits proteases involved in activation of both influenza viruses and metapneumoviruses. Virology 2020, 543, 43–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertram, S.; Dijkman, R.; Habjan, M.; Heurich, A.; Gierer, S.; Glowacka, I.; Welsch, K.; Winkler, M.; Schneider, H.; Hofmann-Winkler, H.; et al. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J. Virol. 2013, 87, 6150–6160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirato, K.; Kawase, M.; Matsuyama, S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 2013, 87, 12552–12561. [Google Scholar] [CrossRef]
- Ralph, A.; Tripp, R. Therapeutic considerations for Middle East respiratory syndrome coronavirus. J. Antivir. Antiretrovir. 2013, 5, 1–2. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Kotani, O.; Sato, H.; Sekimukai, H.; Fukushi, S.; Suzuki, T.; Sato, Y.; Takeda, M.; et al. Acute respiratory infection in human dipeptidyl peptidase 4-transgenic mice infected with Middle East respiratory syndrome coronavirus. J. Virol. 2019, 93, e01818. [Google Scholar] [CrossRef]
- Saunders, N.; Fernandez, I.; Planchais, C.; Michel, V.; Rajah, M.M.; Baquero Salazar, E.; Postal, J.; Porrot, F.; Guivel-Benhassine, F.; Blanc, C.; et al. TMPRSS2 is a functional receptor for human coronavirus HKU1. Nature 2023, 624, 207–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Ou, X.; Li, P.; Zan, F.; Tan, L.; Qian, Z. Identification of the critical residues of TMPRSS2 for entry and host range of human coronavirus HKU1. J. Virol. 2024, 98, e0158724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, Z.; Li, D.; Zhang, L. TMPRSS2 in microbial interactions: Insights from HKU1 and TcsH. PLoS Pathog. 2024, 20, e1012677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández, I.; Saunders, N.; Duquerroy, S.; Bolland, W.H.; Arbabian, A.; Baquero, E.; Blanc, C.; Lafaye, P.; Haouz, A.; Buchrieser, J.; et al. Structural basis of TMPRSS2 zymogen activation and recognition by the HKU1 seasonal coronavirus. Cell 2024, 187, 4246–4260.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koch, J.; Uckeley, Z.M.; Doldan, P.; Stanifer, M.; Boulant, S.; Lozach, P.Y. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 2021, 40, e107821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef] [PubMed]
- Shrimp, J.H.; Kales, S.C.; Sanderson, P.E.; Simeonov, A.; Shen, M.; Hall, M.D. An Enzymatic TMPRSS2 Assay for Assessment of Clinical Candidates and Discovery of Inhibitors as Potential Treatment of COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 997–1007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Du, C.; Hu, K.; Zhang, C.; Liu, M.; Wu, Q.; Dong, N. Transmembrane serine protease TMPRSS2 implicated in SARS-CoV-2 infection is autoactivated intracellularly and requires N-glycosylation for regulation. J. Biol. Chem. 2022, 298, 102643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [PubMed]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borisevich, S.S.; Zarubaev, V.V.; Shcherbakov, D.N.; Yarovaya, O.I.; Salakhutdinov, N.F. Molecular Modeling of Viral Type I Fusion Proteins: Inhibitors of Influenza Virus Hemagglutinin and the Spike Protein of Coronavirus. Viruses 2023, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Matsuyama, S.; Fukuhara, H.; Maenaka, K.; Kataoka, H.; Hashiguchi, T.; Takeda, M. The Physiological TMPRSS2 Inhibitor HAI-2 Alleviates SARS-CoV-2 Infection. J. Virol. 2021, 95, e00434-21. [Google Scholar] [CrossRef] [PubMed]
- Mykytyn, A.Z.; Breugem, T.I.; Geurts, M.H.; Beumer, J.; Schipper, D.; van Acker, R.; Doel, P.B.v.D.; van Royen, M.E.; Zhang, J.; Clevers, H.; et al. SARS-CoV-2 Omicron entry is type II transmembrane serine protease-mediated in human airway and intestinal organoid models. J. Virol. 2023, 97, e00851-23. [Google Scholar] [CrossRef]
- Prelli Bozzo, C.; Nchioua, R.; Volcic, M.; Koepke, L.; Krüger, J.; Schütz, D.; Heller, S.; Stürzel, C.M.; Kmiec, D.; Conzelmann, C.; et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition In Vitro. Nat. Commun. 2021, 12, 4584. [Google Scholar] [CrossRef]
- Mesner, D.; Reuschl, A.K.; Whelan, M.V.X.; Bronzovich, T.; Haider, T.; Thorne, L.G.; Ragazzini, R.; Bonfanti, P.; Towers, G.J.; Jolly, C. SARS-CoV-2 evolution influences GBP and IFITM sensitivity. Proc. Natl. Acad. Sci. USA 2023, 120, e2212577120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, H.; Winstone, H.; Jimenez-Guardeño, J.M.; Graham, C.; Doores, K.J.; Goujon, C.; Matthews, D.A.; Davidson, A.D.; Rihn, S.J.; Palmarini, M.; et al. TMPRSS2 promotes SARS-CoV-2 evasion from NCOA7-mediated restriction. PLoS Pathog. 2021, 17, e1009820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomris, I.; Bouwman, K.M.; Adolfs, Y.; Noack, D.; van der Woude, R.; Kerster, G.; Herfst, S.; Sanders, R.W.; van Gils, M.J.; Boons, G.J.; et al. Distinct spatial arrangements of ACE2 and TMPRSS2 expression in Syrian hamster lung lobes dictates SARS-CoV-2 infection patterns. PLoS Pathog. 2022, 18, e1010340. [Google Scholar] [CrossRef] [PubMed]
- Strobelt, R.; Adler, J.; Shaul, Y. The Transmembrane Protease Serine 2 (TMPRSS2) Non-Protease Domains Regulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike-Mediated Virus Entry. Viruses 2023, 15, 2124. [Google Scholar] [CrossRef]
- Kakee, S.; Kanai, K.; Tsuneki-Tokunaga, A.; Okuno, K.; Namba, N.; Tomita, K.; Chikumi, H.; Kageyama, S. Difference in TMPRSS2 usage by Delta and Omicron variants of SARS-CoV-2: Implication for a sudden increase among children. PLoS ONE 2024, 19, e0299445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. CITIID-NIHR BioResource COVID-19 Collaboration; Genotype to Phenotype Japan (G2P-Japan) Consortium; Ecuador-COVID19 Consortium, altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peña-Hernández, M.A.; Alfajaro, M.M.; Filler, R.B.; Moriyama, M.; Keeler, E.L.; Ranglin, Z.E.; Kong, Y.; Mao, T.; Menasche, B.L.; Mankowski, M.C.; et al. SARS-CoV-2-related bat viruses evade human intrinsic immunity but lack efficient transmission capacity. Nat. Microbiol. 2024, 9, 2038–2050. [Google Scholar] [CrossRef]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2 TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Narayan, R.K.; Kumari, C.; Faiq, M.A.; Kulandhasamy, M.; Kant, K.; Pareek, V. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med. Hypotheses 2020, 145, 110320. [Google Scholar] [CrossRef] [PubMed]
- Maccio, U.; Zinkernagel, A.S.; Shambat, S.M.; Zeng, X.; Cathomas, G.; Ruschitzka, F.; Schuepbach, R.A.; Moch, H.; Varga, Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 2021, 63, 103182. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Larabee, J.L.; Gao, L.; Shi, H.; Shao, B.; Hoover, C.M.; McDaniel, J.M.; Ho, Y.-C.; Silasi-Mansat, R.; Archer-Hartmann, S.A.; et al. L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus. J. Clin. Investig. 2021, 6, e148999. [Google Scholar] [CrossRef]
- Won, T.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Ma, Z.; Schneider, J.; Asady, B.; Goerlich, E.; Halushka, M.K.; Hays, A.G.; et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine 2022, 75, 103812. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lee, T.H.; Huang, R.T.D.; Guzy, R.; Schoettler, N.; Adegunsoye, A.; Mueller, J.; Husain, A.; Sperling, A.I.; Mutlu, G.M.; et al. SARS-CoV-2 infection is associated with reduced krüppel-like factor 2 in human lung autopsy. Am. J. Respir. Cell Mol. Biol. 2021, 65, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Uesaka, K. Genetic variations in the human severe acute respiratory syndrome coronavirus receptor ACE2 and serine protease TMPRSS2. J. Clin. Pathol. 2021, 74, 307–313. [Google Scholar] [CrossRef]
- Alves Feitosa, T. Association of TMPRSS2 Gene Polymorphisms with COVID-19 Severity and Mortality: A Case-Control Study with Computational Analyses. Front. Genet. 2022, 13, 801533. [Google Scholar]
- Posadas-Sánchez, R.; Fragoso, J.M.; Sánchez-Muñoz, F.; Rojas-Velasco, G.; Ramírez-Bello, J.; López-Reyes, A.; Martínez-Gómez, L.E.; Sierra-Fernández, C.; Rodríguez-Reyna, T.; Regino-Zamarripa, N.E.; et al. Association of the Transmembrane Serine Protease-2 (TMPRSS2) Polymorphisms with COVID-19. Viruses 2022, 14, 1976. [Google Scholar] [CrossRef]
- Pandey, R.K.; Srivastava, A.; Singh, P.P.; Chaubey, G. Genetic association of TMPRSS2 rs2070788 polymorphism with COVID-19 case fatality rate among Indian populations. Infect. Genet. Evol. 2022, 98, 105206. [Google Scholar] [CrossRef]
- Nawal, M.; Utba, M. Transmembrane Serine Protease-2 Gene Polymorphism and Expression in Iraqi COVID-19 Patients. J. Commun. Dis. 2023, 55, 75–82. [Google Scholar] [CrossRef]
- Saad, F.M.; Daif, A.; Sakr, M.; Ismail, H. Polymorphic Variations of ACE2 and TMPRSS2 Genes in Egyptian COVID-19 Patients: Correlation with Disease Severity and Sex-Related Gene Variations. Res. J. Appl. Biotechnol. 2024, 10, 15–26. [Google Scholar] [CrossRef]
- Putira Sacuena, E.R.; Lima, C.N.C.; Abreu, I.N.; da Silva, L.M.C.; Belleza, L.K.G.; Lemes, R.B.; de Araújo, G.S.; da Silva, H.P.; Vallinoto, A.C.R.; Guerreiro, J.F. Host genetics and the profile of COVID-19 in indigenous people from the Brazilian Amazon: A pilot study with variants of the ACE1, ACE2, and TMPRSS2 genes. Infect. Genet. Evol. 2024, 118, 105564. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Parkinson, N.; Peacock, T.P.; Pairo-Castineira, E.; Khanna, T.; Cobat, A.; Tenesa, A.; Sancho-Shimizu, V.; Casanova, J.-L.; Abel, L.; et al. A common TMPRSS2 variant has a protective effect against severe COVID-19. Curr. Res. Transl. Med. 2022, 70, 103333. [Google Scholar] [CrossRef] [PubMed]
- Bashar, N.A.S.; Gohar, N.M.A.; Tantawy, A.A.; Kamel, M.H.M. Evaluation of the relationship between TMPRSS2 p.(Val197Met) variant and COVID-19 susceptibility and severity. BMC Infect. Dis. 2024, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Elnagdy, M.H.; Magdy, A.; Eldars, W.; Elgamal, M.; El-Nagdy, A.H.; Salem, O.; Elmowafy, M.M.; Elborsh, O.A.; Elshafey, A.W.; Kesba, M.M.; et al. Genetic association of ACE2 and TMPRSS2 polymorphisms with COVID-19 severity; a single centre study from Egypt. Virol. J. 2024, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Kozik, V.A.; Karmanovskaya, S.A.; Shpagin, I.; Maksimova, S.V.; Lozhkina, N.G.; Maksimov, V.N. Assessment of the association of the rs12329760 polymorphism of the TMPRSS2 gene with acute coronary syndrome in patients with new coronavirus infection. Ateroscleroz 2024, 20, 35–41. [Google Scholar] [CrossRef]
- Qu, B.; Miskey, C.; Gömer, A.; Kleinert, R.D.V.; Ibanez, S.C.; Eberle, R.; Ebenig, A.; Postmus, D.; Nocke, M.K.; Herrmann, M.; et al. TMPRSS2-mediated SARS-CoV-2 uptake boosts innate immune activation, enhances cytopathology, and drives convergent virus evolution. Proc. Natl. Acad. Sci. USA 2024, 121, e2407437121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wettstein, L.; Immenschuh, P.; Weil, T.; Conzelmann, C.; Almeida-Hernández, Y.; Hoffmann, M.; Kempf, A.; Nehlmeier, I.; Lotke, R.; Petersen, M.; et al. Native and activated antithrombin inhibits TMPRSS2 activity and SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Redondo-Calvo, F.J.; Padín, J.F.; Muñoz-Rodríguez, J.R.; Serrano-Oviedo, L.; López-Juárez, P.; Porras Leal, M.L.; González Gasca, F.J.; Rodríguez Martínez, M.; Pérez Serrano, R.; Sánchez Cadena, A.; et al. Aprotinin treatment against SARS-CoV-2: A randomized phase III study to evaluate the safety and efficacy of a pan-protease inhibitor for moderate COVID-19. Eur. J. Clin. Investig. 2022, 52, e13776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tachoua, W.; Kabrine, M.; Mushtaq, M.; Selmi, A.; Ul-Haq, Z. Highlights in TMPRSS2 inhibition mechanism with guanidine derivatives approved drugs for COVID-19 treatment. J. Biomol. Struct. Dyn. 2023, 41, 12908–12922. [Google Scholar] [CrossRef]
- Moumbock, A.F.A.; Tran, H.T.T.; Lamy, E.; Günther, S. BC-11 is a covalent TMPRSS2 fragment inhibitor that impedes SARS-CoV-2 host cell entry. Arch. Pharm. 2023, 356, e2200371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, T.; Baig, M.H.; Khan, M.I.; Alotaibi, S.S.; Alorabi, M.; Dong, J.-J. Computational screening of camostat and related compounds against human TMPRSS2: A potential treatment of COVID-19. Saudi Pharm. J. 2022, 30, 217–224. [Google Scholar] [CrossRef]
- Xu, Y.M.; Inacio, M.C.; Liu, M.X.; Gunatilaka, A.A.L. Discovery of diminazene as a dual inhibitor of SARS-CoV-2 human host proteases TMPRSS2 and furin using cell-based assays. Curr. Res. Chem. Biol. 2022, 2, 100023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, S.H.; Lee, H.Y.; Ki, Y.J.; Kim, S.H.; Lim, K.J.; Jung, K.T. Gabexate mesilate ameliorates the neuropathic pain in a rat model by inhibition of proinflammatory cytokines and nitric oxide pathway via suppression of nuclear factor-κB. Korean J. Pain. 2020, 33, 30–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gamba, D.; Van Eijk, N.; Lányi, K.; Monostory, K.; Steinmetzer, T.; Marosi, A.; Rácz, A.; Bajusz, D.; Kruhl, D.; Böttcher-Friebertshäuser, E.; et al. PK/PD investigation of antiviral host matriptase/TMPRSS2 inhibitors in cell models. Sci. Rep. 2024, 14, 16621. [Google Scholar] [CrossRef]
- van Eijk, N.; Schmacke, L.C.; Steinmetzer, T.; Pilgram, O.; Poór, M.; Pászti-Gere, E. In vitro testing of host-targeting small molecule antiviral matriptase/TMPRSS2 inhibitors in 2D and 3D cell-based assays. Biomed. Pharmacother. 2023, 168, 115761. [Google Scholar] [CrossRef]
- Sharanya, C.S.; Wilbee, D.S.; Sathi, S.N.; Natarajan, K. Computational screening combined with well-tempered metadynamics simulations identifies potential TMPRSS2 inhibitors. Sci. Rep. 2024, 14, 16197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340–348. [Google Scholar] [CrossRef]
- Seccia, T.M.; Shagjaa, T.; Morpurgo, M.; Caroccia, B.; Sanga, V.; Faoro, S.; Venturini, F.; Iadicicco, G.; Lococo, S.; Mazzitelli, M.; et al. RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. J. Clin. Med. 2023, 12, 6618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Wang, K.; Sun, H.; Wu, S.; Wang, H.; Shi, Y.; Li, X.; Yan, H.; Yang, G.; Wu, M.; et al. Omicsynin B4 potently blocks coronavirus infection by inhibiting host proteases cathepsin L and TMPRSS2. Antivir. Res. 2023, 214, 105606. [Google Scholar] [CrossRef] [PubMed]
- Hempel, T.; Elez, K.; Krüger, N.; Raich, L.; Shrimp, J.H.; Danov, O.; Jonigk, D.; Braun, A.; Shen, M.; Hall, M.D.; et al. Synergistic inhibition of SARS-CoV-2 cell entry by otamixaban and covalent protease inhibitors: Pre-clinical assessment of pharmacological and molecular properties. Chem. Sci. 2021, 12, 12600–12609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wettstein, L.; Weil, T.; Conzelmann, C.; Müller, J.A.; Groß, R.; Hirschenberger, M.; Seidel, A.; Klute, S.; Zech, F.; Prelli Bozzo, C.; et al. Alpha-1 antitrypsin inhibits TMPRSS2 protease activity and SARS-CoV-2 infection. Nat. Commun. 2021, 12, 1726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gibo, J.; Ito, T.; Kawabe, K.; Hisano, T.; Inoue, M.; Fujimori, N.; Oono, T.; Arita, Y.; Nawata, H. Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity. Lab. Investig. 2005, 85, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.; Spichler-Moffarah, A.; Søgaard, O.S.; Esserman, D.; Dziura, J.; Danzig, L.; Chaurasia, R.; Patra, K.P.; Salovey, A.; Nunez, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, U.; Mubariz, M.; Khlidj, Y.; Nasir, M.M.; Ramadan, S.; Saeed, F.; Muhammad, A.; Abuelazm, M. Safety and Efficacy of Camostat Mesylate for Covid-19: A systematic review and Meta-analysis of Randomized controlled trials. BMC Infect. Dis. 2024, 24, 709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Mitre, M.P.; Morpeth, S.C.; Venkatesh, B.; Hills, T.E.; Davis, J.; Mahar, R.K.; McPhee, G.; Jones, M.; Totterdell, J.; Tong, S.Y.; et al. TMPRSS2 inhibitors for the treatment of COVID-19 in adults: A systematic review and meta-analysis of randomized clinical trials of nafamostat and camostat mesylate. Clin. Microbiol. Infect. 2024, 30, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, C.; Vasilakaki, S.; Gerogianni, V.-E.; Kokotos, G. The discovery and development of transmembrane serine protease 2 (TMPRSS2) inhibitors as candidate drugs for the treatment of COVID-19. Expert Opin. Drug Discov. 2022, 17, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, T.; Koshikawa, S.; Ota, K.; Kazama, M.; Mimura, N.; Hirasawa, Y. Nafamostat Mesilate: A regional anticoagulant for hemodialysis in patients at high risk for bleeding. Nephron 1993, 64, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P. Nafamostat Mesylate in Moderate to Severe COVID-19 Pneumonia: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase IIb Clinical Trial; Código NCT04352400; ClinicalTrials.gov: Bethesda, MD, USA, 2020. Available online: https://clinicaltrials.gov/study/NCT04352400 (accessed on 6 November 2024).
- Li, K.; Meyerholz, D.K.; Bartlett, J.A.; McCray, P.B. The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19. MBio 2021, 12, e0097021. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-J.; Song, Q.; Lv, F.-Q.; Wang, S.; Wang, Y.-R.; Luo, Y.-K.; Mei, X.-G.; Tang, J. A novel thermosensitive in-situ gel of gabexate mesilate for treatment of traumatic pancreatitis: An experimental study. J. Huazhong Univ. Sci. Technol. 2015, 35, 707–711. [Google Scholar] [CrossRef]
- Pérez-Vargas, J.; Lemieux, G.; Thompson, C.A.H.; Désilets, A.; Ennis, S.; Gao, G.; Gordon, D.G.; Schulz, A.L.; Niikura, M.; Nabi, I.R.; et al. Nanomolar anti-SARS-CoV-2 Omicron activity of the host-directed TMPRSS2 inhibitor N-0385 and synergistic action with direct-acting antivirals. Antivir. Res. 2024, 225, 105869. [Google Scholar] [CrossRef] [PubMed]
- Oduro-Kwateng, E.; Solimn, M.E.S. Unveiling therapeutic frontiers: DON/DRP-104 as innovative Plasma kallikrein inhibitors against carcinoma-associated hereditary angioedema shocks—A comprehensive molecular dynamics exploration. Cell Biochem. Biophys. 2024, 82, 1159–1177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, M.; Gao, R.; Liu, Z.; Liu, F.; Du, S.; Song, Y.; He, J. Ginsenosides, potential TMPRSS2 inhibitors, a trade-off between the therapeutic combination for anti-PD-1 immunotherapy and the treatment of COVID-19 infection of LUAD patients. Front. Pharmacol. 2023, 14, 1085509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breining, P.; Frølund, A.L.; Højen, J.F.; Gunst, J.D.; Staerke, N.B.; Saedder, E.; Cases-Thomas, M.; Little, P.; Nielsen, L.P.; Søgaard, O.S.; et al. Camostat mesylate against SARS-CoV-2 and COVID-19—Rationale, dosing and safety. Basic Clin. Pharmacol. Toxicol. 2021, 128, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Shinoda, M.; Nishizaki, Y.; Shiraki, K.; Hirai, Y.; Kichikawa, Y.; Tsushima, K.; Shinkai, M.; Komura, N.; Yoshida, K.; et al. A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study). BMC Med. 2022, 20, 342, Erratum in BMC Med. 2022, 20, 478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.J.; Kim, S.; Kim, H.; Gil, D.; Han, H.J.; Thimmulappa, R.K.; Choi, J.H.; Kim, J.H. Reciprocal enhancement of SARS-CoV-2 and influenza virus replication in human pluripotent stem cell-derived lung organoids. Emerg. Microbes Infect. 2023, 12, 2211685. [Google Scholar] [CrossRef] [PubMed]

| Variant (SNP ID) | Genotype Allele | Associated Health Condition | Association with COVID-19 Severity | Ref. |
|---|---|---|---|---|
| rs12329760 (p.Val197Met) | T/C | COVID-19 Severity | T allele linked to increased severity | [89,90] |
| rs17854725 | A/G | Aggressive COVID-19 | G allele linked to severe COVID-19 | [89] |
| rs2070788 | G/G | Increased TMPRSS2 Expression in Lungs | GG genotype associated with higher mortality in elderly COVID-19 patients | [91] |
| rs462574 | T/C | COVID-19 Severity | Significant association with severity | [90] |
| rs123297605 | C/T | Asymptomatic or mild cases, collective immunity | Lower frequency of risk alleles, and lower mortality | [94] |
| Compound | Inhibition (Type) | Approval State | Ref. |
|---|---|---|---|
| Antithrombin | Irreversible | Phase II | [100] |
| Aprotinin | Competitive | Phase III clinical trial | [101] |
| Argatroban | Competitive | Phase IV | [102] |
| BC-11 | Irreversible | Preclinical Phase | [103] |
| Camostat Mesylate | Irreversible | Phase III COVID-19, 2021 | [104] |
| Diminazene | Competitive | In vitro assay | [105] |
| Famotidine | Competitive | Phase IV | [102] |
| Gabexate | Competitive | Preclinical Phase | [62,106] |
| Guanadrel | Competitive | In silico simulation | [102] |
| Guanethidine | Competitive | In silico simulation | [102] |
| MI-485, MI-472, MI-1900, MI-1903, and MI-1904 | Competitive | In vitro assay | [107,108] |
| MI-463 | Not determined | In vitro assay | [108] |
| Narigin | Competitive | In silico simulation | [109] |
| N-0385 | Irreversible | Animal model | [110] |
| Nafamostat | Irreversible | Phase III COVID-19, 2020 | [111] |
| Neohesperidin | Competitive | In silico simulation | [109] |
| Omicsynin B4 | Irreversible | In vitro assay | [112] |
| Otamixaban | Competitive | Preclinical Phase | [113] |
| Rhoifolin | Competitive | In silico simulation | [109] |
| Vicenin-2 | Competitive | In silico simulation | [109] |
| α1-antitrypsin | Irreversible | In vitro assay | [114] |
| NCT Number | Status | Study Title | Conditions | Interventions |
|---|---|---|---|---|
| NCT04652765 | Terminated | Camostat With Bicalutamide for COVID-19 | COVID-19 | Drugs: Camostat Mesilate, Bicalutamide |
| NCT04608266 | Terminated | CAMOVID: Evaluation of Efficacy and Safety of Camostat Mesylate for the Treatment of SARS-CoV-2 Infection | COVID-19 | Drug: Camostat Mesylate |
| NCT04406389 | Terminated | Anticoagulation in Critically Ill Patients With COVID-19 (The IMPACT Trial) | Drugs: Enoxaparin sodiu, Fondapariniux | |
| NCT00589472 | Completed | Androgen Deprivation Therapy and Vorinostat Followed by Radical Prostatectomy in Treating Patients With Localized Prostate Cancer | Prostate Adenocarcinoma | Drugs: Bicalutamide, Goserelin Acetate |
| NCT04681430 | Completed | Reconvalescent Plasma/Camostat Mesylate Early in SARS-CoV-2 Q-PCR (COVID-19) Positive High-risk Individuals | COVID-19 | Biological: Convalescent plasma Drug: Camostat Mesylate |
| NCT04729491 | Completed | EAT-DUTA AndroCoV Trial | COVID-19 | Dutasteride 0.5 mg, Drug: Azithromycin, Nitazoxanide |
| NCT01075308 | Completed | SB939 in Treating Patients With Recurrent or Metastatic Prostate Cancer | Prostate Cancer | Drug: HDAC inhibitor SB939 |
| NCT04516850 | Completed | HSD3B1 Gene Polymorphisms With Outcomes in SARS-CoV-2 Infected Patients | COVID-19 | Genetic: Expression of receptors and activating proteases, Genetic: Polymorphism of the HSD3B1 |
| NCT01858441 | Completed | Pharmacogenetic Study in Castration-resistant Prostate Cancer Patients Treated With Abiraterone Acetate | Pharmacogenetic Study | Drug: Abiraterone Acetate |
| NCT01653925 | Active, not recruiting | Molecular Mechanisms of Dutasteride and Dietary Interventions to Prevent Prostate Cancer and Reduce Its Progression | Prostatic Neoplasms, Low Grade Prostate Cancer | Other: Dietary intervention first, Drug: (Dutasteride) intervention first |
| NCT04470544 | Recruiting | Camostat Mesilate Treating Patients With Hospitalized Patients With COVID-19 | Severe Acute Respiratory Syndrome | Drug: Camostat Mesilate, Other: Standard of Care |
| NCT04352400 | Recruiting | Efficacy of Nafamostat in COVID-19 Patients (RACONA Study) | COVID-19 | Drug: Nafamostat Mesilate |
| NCT05797597 | Recruiting | Long-term Aspirin Therapy as a Predictor of Decreased Susceptibility to SARS-CoV-2 Infection in Aspirin-Exacerbated Respiratory Disease | AERD—Aspirin Exacerbated Respiratory Disease | Drug: Aspirin 300 Mg Oral Tablet |
| NCT04473053 | Recruiting | DEFINE—Evaluating Therapies for COVID-19 | COVID-19 | Drugs: Nafamostat Mesilate, TD139, Other: Standard care |
| NCT03903835 | Recruiting | ProBio: A Biomarker Driven Study in Patients With Metastatic Prostate Cancer | Metastatic Castration-resistant Prostate Cancer, Metastatic Hormone-Sensitive Prostate Cancer | Drugs: Enzalutamide Oral Capsule, Abiraterone Oral Tablet, Carboplatin |
| NCT04578236 | Unknown status | Efficacy of Aerosol Combination Therapy of 13 Cis Retinoic Acid and Captopril for Treating COVID-19 Patients Via Indirect Inhibition of Transmembrane Protease, Serine 2 (TMPRSS2) | COVID-19 | Combination Product: Aerosolized 13 cis retinoic acid plus Inhalation administration by nebulization captopril 25 mg, Drug: Standard treatment |
| NCT04340349 | Unknown status | Low-dose Hydroxychloroquine and Bromhexine: a Novel Regimen for COVID-19 Prophylaxis in Healthcare Professionals | COVID-19 | Drugs: Hydroxychloroquine Sulfate, Bromhexine 8 MG |
| NCT04385836 | Unknown status | Trial of Alpha One Antitrypsin Inhalation in Treating Patient With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) | COVID-19 | Drug: alpha one antitrypsin inhalation |
| NCT04639440 | Unknown status | Impact of Adipose Tissue in COVID-19 | COVID-19 | Other: Adipose tissue |
| NCT04355026 | Unknown status | Use of Bromhexine and Hydroxychloroquine for Treatment of COVID-19 Pneumonia | COVID-19 | Drugs: Bromhexine Oral Tablet and/or hydroxychloroquine tablet |
| NCT04321096 | Unknown status | The Impact of Camostat Mesilate on COVID-19 Infection | COVID-19 | Drug: Camostat Mesilate, |
| NCT04457609 | Unknown status | Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically-Ill COVID-19 Patients | COVID, Pulmonary Infection, SARS-CoV2 | Drugs: Oseltamivir, Azithromycin, Biological: Umbilical Cord Mesenchymal Stem Cells |
| NCT04577378 | Unknown status | Efficacy and Safety of Drug Combination Therapy of Isotretinoin and Some Antifungal Drugs as A Potential Aerosol Therapy for COVID-19 | COVID-19 | Drug: Isotretinoin (Aerosolized 13 cis retinoic acid) plus Aerosolized Itraconazole |
| NCT04854343 | Unknown status | SLPI for Prostate Cancer | Prostate Cancer, Prostatic Neoplasm | Diagnostic Test: Secretory leukocyte protease inhibitor (SLPI) in prostate cancer, Diagnostic Test: Determination of molecular alterations, Diagnostic Test: SLPI Healthy |
| NCT04527133 | Unknown status | An Open Non-comparative Study of the Efficacy and Safety of Aprotinin in Patients Hospitalized With COVID-19 | COVID-19 | Drug: Aprotinin |
| NCT04836806 | Withdrawn | Cetirizine and Famotidine for COVID-19 | COVID-19 | Drug: Cetirizine and Famotidine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros de Lima, G.; Nencioni, E.; Thimoteo, F.; Perea, C.; Pinto, R.F.A.; Sasaki, S.D. TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses. Biomolecules 2025, 15, 75. https://doi.org/10.3390/biom15010075
Barros de Lima G, Nencioni E, Thimoteo F, Perea C, Pinto RFA, Sasaki SD. TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses. Biomolecules. 2025; 15(1):75. https://doi.org/10.3390/biom15010075
Chicago/Turabian StyleBarros de Lima, Gilmara, Everton Nencioni, Fábio Thimoteo, Camila Perea, Rafaela Fuzaro Alves Pinto, and Sergio Daishi Sasaki. 2025. "TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses" Biomolecules 15, no. 1: 75. https://doi.org/10.3390/biom15010075
APA StyleBarros de Lima, G., Nencioni, E., Thimoteo, F., Perea, C., Pinto, R. F. A., & Sasaki, S. D. (2025). TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses. Biomolecules, 15(1), 75. https://doi.org/10.3390/biom15010075






