Visceral Adipose Tissue Inflammation and Vascular Complications in a Rat Model with Severe Dyslipidemia: Sex Differences and PAI-1 Tissue Involvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analytical Methods
2.3. Hematological and Coagulation Parameters
2.4. Relative mRNA Expression
2.5. Statistical Analysis
3. Results
3.1. Sex-Dependent Effects of Severe Dyslipidemia on Adiposity, Glucose, and Lipid Metabolism
3.2. Sex-Dependent Effects of Severe Dyslipidemia on Hematological and Coagulation Factors
3.3. Sex-Dependent Effects of Severe Dyslipidemia on Systemic Inflammation
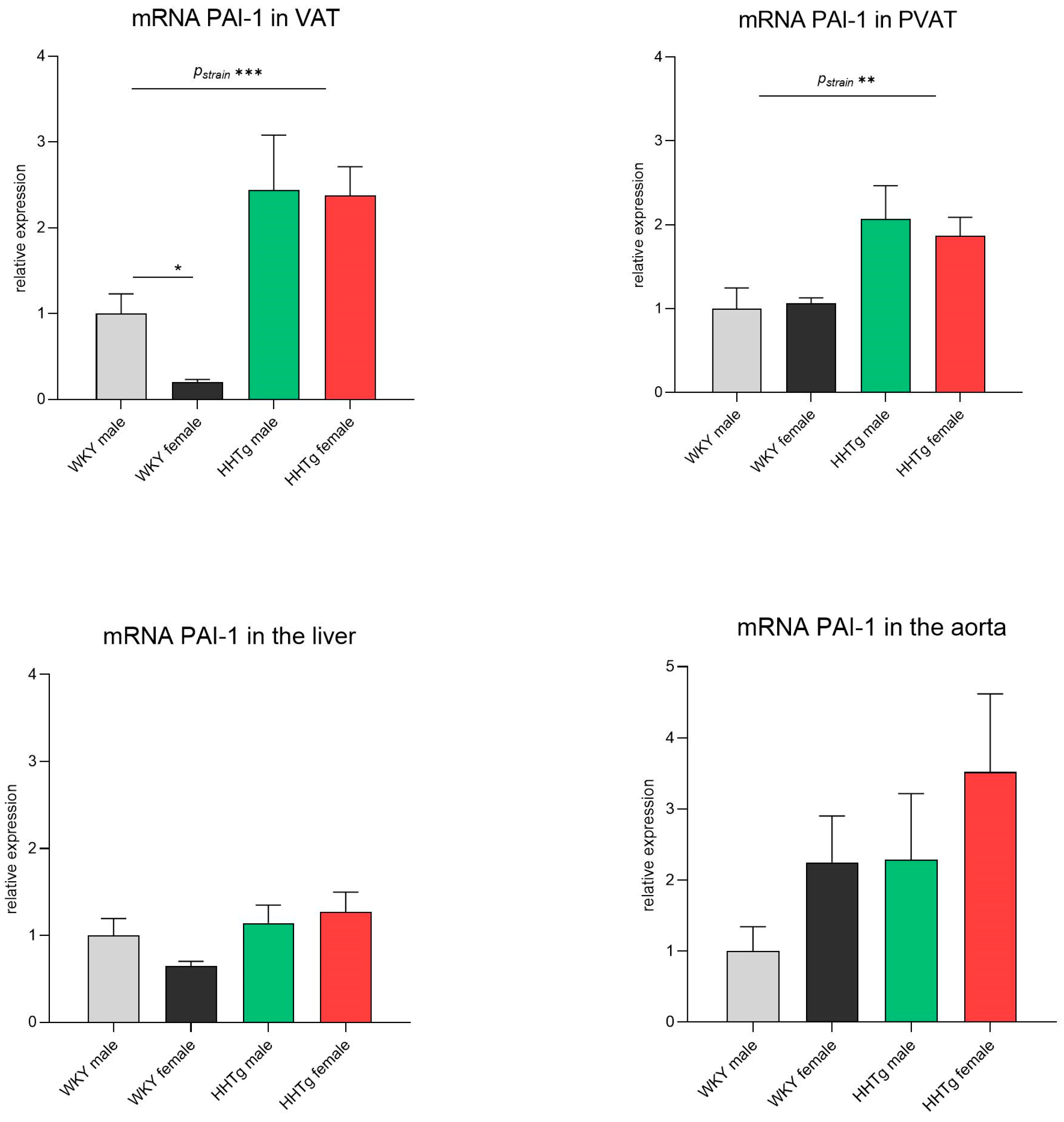
3.4. Sex-Dependent Effects of Severe Dyslipidemia on PAI-1 Gene Expression in Different Tissues
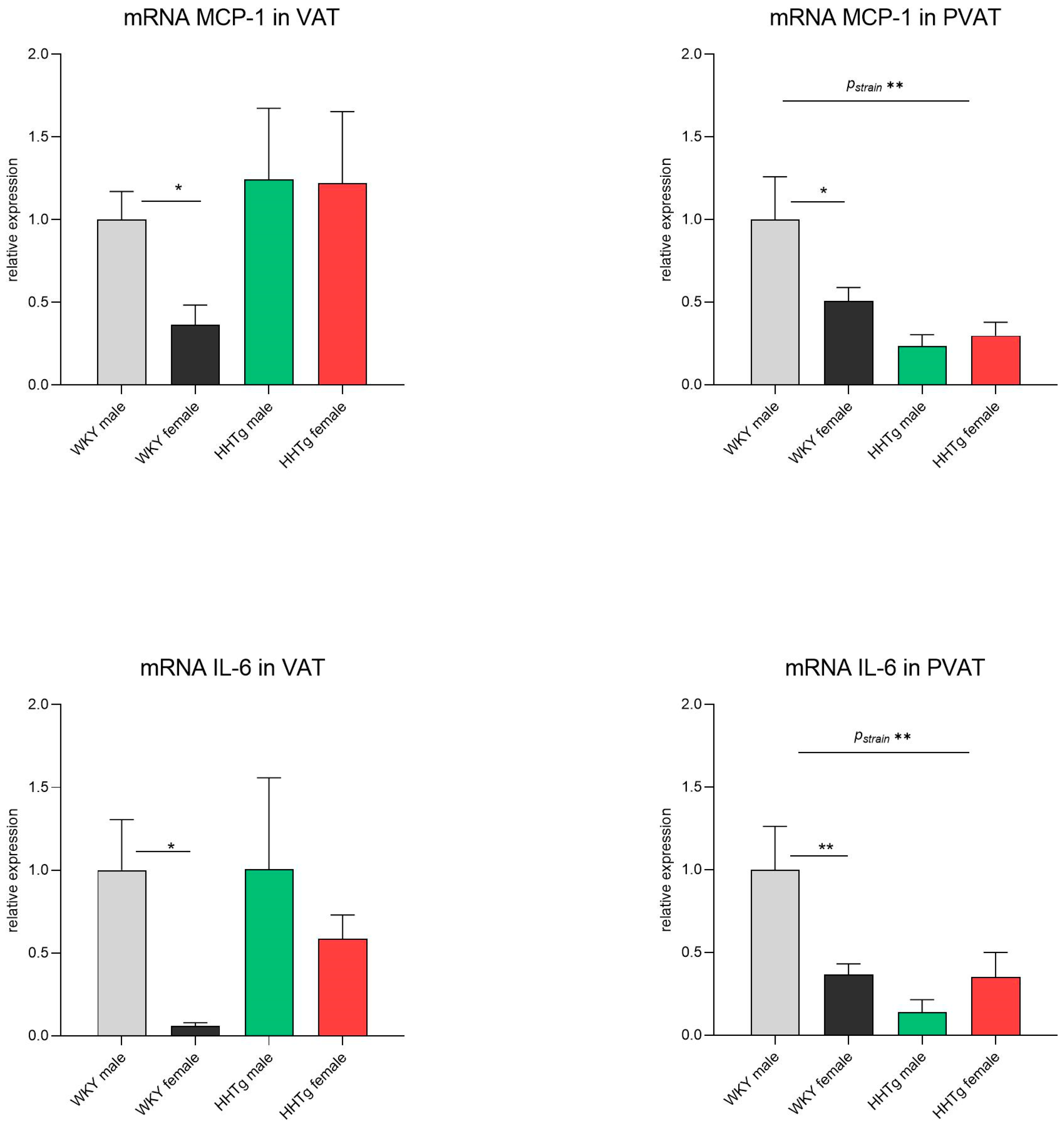
3.5. Sex-Dependent Effects of Severe Dyslipidemia on the Gene Expression of Inflammatory Markers in Visceral and Perivascular Adipose Tissues
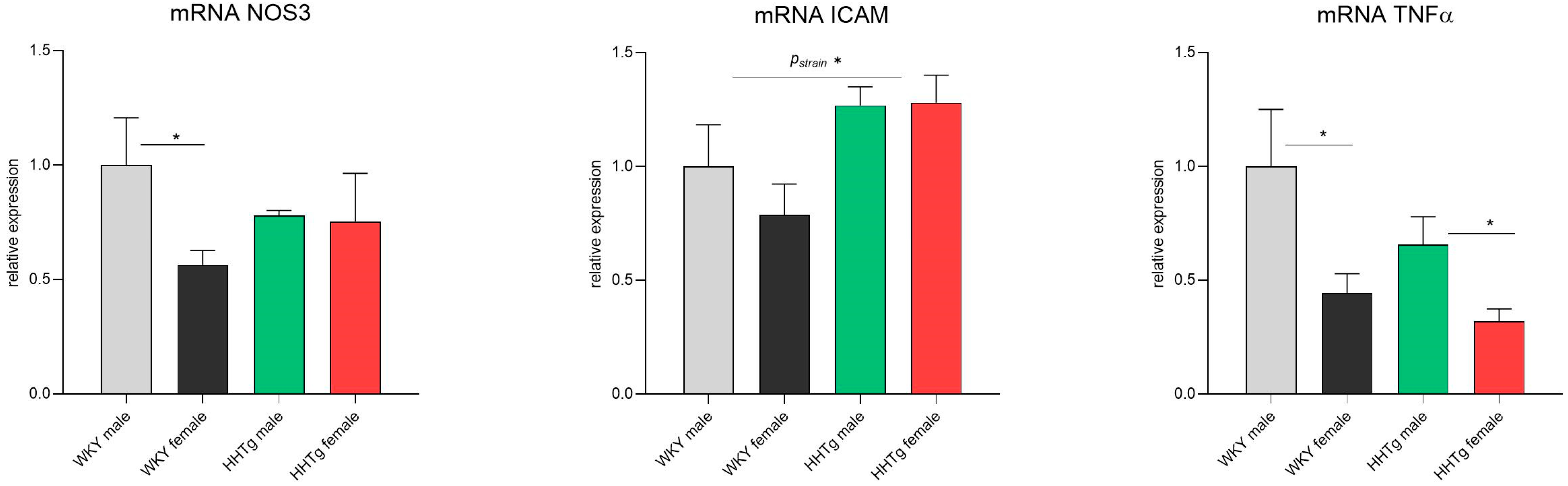
3.6. Sex-Dependent Effects of Severe Dyslipidemia on the Gene Expression of Vascular and Inflammatory Markers in the Aorta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AT | antithrombin |
| Hb | hemoglobin |
| HCT | hematocrit |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| hsCRP | high-sensitivity C-reactive protein |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL-6 | interleukin-6 |
| IRS-1 | insulin receptor substrate-1 |
| LPL | lipoprotein lipase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MCH | mean concentration of hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| MPV | mean platelet volume |
| NAFLD | nonalcoholic fatty liver disease |
| NF-κB | nuclear factor-kappa B |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| PAI-1 | plasminogen activator inhibitor-1 |
| PCT | plateletcrit |
| PLT | platelet |
| PVAT | perivascular adipose tissue |
| RBC | red blood cell |
| RDW | red blood cell distribution width |
| SREBP1 | sterol regulatory element-binding protein 1 |
| TAG | triglyceride |
| TF | tissue factor |
| tPA | tissue plasminogen activator |
| TNFα | tumor necrosis factor alpha |
| VAT | visceral adipose tissue |
| vWf | von Willebrand factor |
| WBC | white blood cell |
References
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones 2018, 17, 299–313. [Google Scholar] [CrossRef]
- Broni, E.K.; Ndumele, C.E.; Echouffo-Tcheugui, J.B.; Kalyani, R.R.; Bennett, W.L.; Michos, E.D. The Diabetes-Cardiovascular Connection in Women: Understanding the Known Risks, Outcomes, and Implications for Care. Curr. Diabetes Rep. 2022, 22, 11–25. [Google Scholar] [CrossRef]
- Russo, I. The prothrombotic tendency in metabolic syndrome: Focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica 2012, 2012, 525374. [Google Scholar] [CrossRef]
- Zhang, Z.; Rodriguez, M.; Zheng, Z. Clot or Not? Reviewing the Reciprocal Regulation between Lipids and Blood Clotting. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 533–544. [Google Scholar] [CrossRef]
- Gugliucci, A. Biomarkers of dysfunctional visceral fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar]
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The Role of Secretory Activity Molecules of Visceral Adipocytes in Abdominal Obesity in the Development of Cardiovascular Disease: A Review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar]
- Morrow, G.B.; Mutch, N.J. Past, Present, and Future Perspectives of Plasminogen Activator Inhibitor 1 (PAI-1). Semin. Thromb. Hemost. 2023, 49, 305–313. [Google Scholar] [CrossRef]
- Zicha, J.; Pechanova, O.; Cacanyiova, S.; Cebova, M.; Kristek, F.; Torok, J.; Simko, F.; Dobesova, Z.; Kunes, J. Hereditary hypertriglyceridemic rat: A suitable model of cardiovascular disease and metabolic syndrome? Physiol. Res. 2006, 55 (Suppl. S1), S49–S63. [Google Scholar] [CrossRef]
- Vrana, A.; Kazdova, L. The hereditary hypertriglyceridemic nonobese rat: An experimental model of human hypertriglyceridemia. Transplant. Proc. 1990, 22, 2579. [Google Scholar]
- Cacanyiova, S.; Berenyiova, A.; Malinska, H.; Huttl, M.; Markova, I.; Aydemir, B.G.; Garaiova, V.; Cebova, M. Female prediabetic rats are protected from vascular dysfunction: The role of nitroso and sulfide signaling. Biol. Res. 2024, 57, 91. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Huttl, M.; Markova, I.; Berenyiova, A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules 2021, 11, 108. [Google Scholar] [CrossRef]
- Malinska, H.; Huttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015, 31, 1045–1051. [Google Scholar] [CrossRef]
- Cucuianu, M.; Knauer, O.; Roman, S. Alpha 2-antiplasmin, plasminogen activator inhibitor (PAI) and dilute blood clot lysis time in selected disease states. Thromb. Haemost. 1991, 66, 586–591. [Google Scholar]
- Deng, Z.Y.; Shan, W.G.; Wang, S.F.; Hu, M.M.; Chen, Y. Effects of astaxanthin on blood coagulation, fibrinolysis and platelet aggregation in hyperlipidemic rats. Pharm. Biol. 2017, 55, 663–672. [Google Scholar] [CrossRef]
- Barale, C.; Russo, I. Influence of Cardiometabolic Risk Factors on Platelet Function. Int. J. Mol. Sci. 2020, 21, 623. [Google Scholar] [CrossRef]
- Jones, W.L.; Ramos, C.R.; Banerjee, A.; Moore, E.E.; Hansen, K.C.; Coleman, J.R.; Kelher, M.; Neeves, K.B.; Silliman, C.C.; Di Paola, J.; et al. Apolipoprotein A-I, elevated in trauma patients, inhibits platelet activation and decreases clot strength. Platelets 2022, 33, 1119–1131. [Google Scholar] [CrossRef]
- Seixas, M.O.; Rocha, L.C.; Carvalho, M.B.; Menezes, J.F.; Lyra, I.M.; Nascimento, V.M.; Couto, R.D.; Atta, A.M.; Reis, M.G.; Goncalves, M.S. Levels of high-density lipoprotein cholesterol (HDL-C) among children with steady-state sickle cell disease. Lipids Health Dis. 2010, 9, 91. [Google Scholar] [CrossRef]
- Murphy, A.J.; Bijl, N.; Yvan-Charvet, L.; Welch, C.B.; Bhagwat, N.; Reheman, A.; Wang, Y.; Shaw, J.A.; Levine, R.L.; Ni, H.; et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat. Med. 2013, 19, 586–594. [Google Scholar] [CrossRef]
- Cui, M.Z.; Zhao, G.; Winokur, A.L.; Laag, E.; Bydash, J.R.; Penn, M.S.; Chisolm, G.M.; Xu, X. Lysophosphatidic acid induction of tissue factor expression in aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 224–230. [Google Scholar] [CrossRef]
- Banfi, C.; Mussoni, L.; Rise, P.; Cattaneo, M.G.; Vicentini, L.; Battaini, F.; Galli, C.; Tremoli, E. Very low density lipoprotein-mediated signal transduction and plasminogen activator inhibitor type 1 in cultured HepG2 cells. Circ. Res. 1999, 85, 208–217. [Google Scholar] [CrossRef]
- Zheng, Z.; Nakamura, K.; Gershbaum, S.; Wang, X.; Thomas, S.; Bessler, M.; Schrope, B.; Krikhely, A.; Liu, R.M.; Ozcan, L.; et al. Interacting hepatic PAI-1/tPA gene regulatory pathways influence impaired fibrinolysis severity in obesity. J. Clin. Investig. 2020, 130, 4348–4359. [Google Scholar] [CrossRef]
- Slatter, D.A.; Percy, C.L.; Allen-Redpath, K.; Gajsiewicz, J.M.; Brooks, N.J.; Clayton, A.; Tyrrell, V.J.; Rosas, M.; Lauder, S.N.; Watson, A.; et al. Enzymatically oxidized phospholipids restore thrombin generation in coagulation factor deficiencies. JCI Insight 2018, 3, e98459. [Google Scholar] [CrossRef]
- Hur, W.S.; King, K.C.; Patel, Y.N.; Nguyen, Y.V.; Wei, Z.; Yang, Y.; Juang, L.J.; Leung, J.; Kastrup, C.J.; Wolberg, A.S.; et al. Elimination of fibrin polymer formation or crosslinking, but not fibrinogen deficiency, is protective against diet-induced obesity and associated pathologies. J. Thromb. Haemost. 2022, 20, 2873–2886. [Google Scholar] [CrossRef]
- Iwaki, T.; Arakawa, T.; Sandoval-Cooper, M.J.; Smith, D.L.; Donahue, D.; Ploplis, V.A.; Umemura, K.; Castellino, F.J. Plasminogen Deficiency Significantly Reduces Vascular Wall Disease in a Murine Model of Type IIa Hypercholesterolemia. Biomedicines 2021, 9, 1832. [Google Scholar] [CrossRef]
- Rodriguez, M.; Zheng, Z. Connecting impaired fibrinolysis and dyslipidemia. Res. Pract. Thromb. Haemost. 2024, 8, 102394. [Google Scholar] [CrossRef]
- Kelem, A.; Adane, T.; Shiferaw, E. Insulin Resistance-Induced Platelet Hyperactivity and a Potential Biomarker Role of Platelet Parameters: A Narrative Review. Diabetes Metab. Syndr. Obes. 2023, 16, 2843–2853. [Google Scholar] [CrossRef]
- Huttl, M.; Markova, I.; Miklankova, D.; Zapletalova, I.; Kujal, P.; Silhavy, J.; Pravenec, M.; Malinska, H. Hypolipidemic and insulin sensitizing effects of salsalate beyond suppressing inflammation in a prediabetic rat model. Front. Pharmacol. 2023, 14, 1117683. [Google Scholar] [CrossRef]
- Gerrits, A.J.; Gitz, E.; Koekman, C.A.; Visseren, F.L.; van Haeften, T.W.; Akkerman, J.W. Induction of insulin resistance by the adipokines resistin, leptin, plasminogen activator inhibitor-1 and retinol binding protein 4 in human megakaryocytes. Haematologica 2012, 97, 1149–1157. [Google Scholar] [CrossRef]
- Alamri, B.N.; Bahabri, A.; Aldereihim, A.A.; Alabduljabbar, M.; Alsubaie, M.M.; Alnaqeb, D.; Almogbel, E.; Metias, N.S.; Alotaibi, O.A.; Al-Rubeaan, K. Hyperglycemia effect on red blood cells indices. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2139–2150. [Google Scholar]
- Krisnamurti, D.G.B.; Purwaningsih, E.H.; Tarigan, T.J.E.; Soetikno, V.; Louisa, M. Hematological indices and their correlation with glucose control parameters in a prediabetic rat model. Vet. World 2022, 15, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K.; Konstantinides, S. Adipokines and thrombosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, P.; Eisenreich, A.; Weithauser, A.; Schultheiss, H.P.; Rauch, U. Leptin and resistin induce increased procoagulability in diabetes mellitus. Cytokine 2011, 56, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Cardillo, C.; Della Morte, D.; Tesauro, M. The Role of Perivascular Adipose Tissue in the Pathogenesis of Endothelial Dysfunction in Cardiovascular Diseases and Type 2 Diabetes Mellitus. Biomedicines 2023, 11, 3006. [Google Scholar] [CrossRef]
- Ahmed, A.; Bibi, A.; Valoti, M.; Fusi, F. Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes? Cells 2023, 12, 1196. [Google Scholar] [CrossRef]
- Jansen, H.J.; Vervoort, G.M.; van der Graaf, M.; Stienstra, R.; Tack, C.J. Liver fat content is linked to inflammatory changes in subcutaneous adipose tissue in type 2 diabetes patients. Clin. Endocrinol. 2013, 79, 661–666. [Google Scholar] [CrossRef]
- Alsharoh, H.; Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Dumitrascu, D.L. Plasminogen Activator Inhibitor-1 Levels in Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Gastrointest. Liver Dis. 2022, 31, 206–214. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Sabetta, A.; Lombardi, L.; Stefanini, L. Sex differences at the platelet-vascular interface. Intern. Emerg. Med. 2022, 17, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Wong, S.K.; Hasan, W.N.W.; Jolly, J.J.; Nur-Farhana, M.F.; Irma-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kawao, N.; Okada, K.; Yano, M.; Okumoto, K.; Matsuo, O.; Kaji, H. Plasminogen activator inhibitor-1 is involved in streptozotocin-induced bone loss in female mice. Diabetes 2013, 62, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Nozell, S.; Chen, Y.F.; Hage, F.; Oparil, S. Estrogen and mechanisms of vascular protection. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 289–295. [Google Scholar] [CrossRef] [PubMed]


| WKY Male | WKY Female | HHTg Male | HHTg Female | p-STRAIN | p-SEX | p-INT | |
|---|---|---|---|---|---|---|---|
| Body weight | 375 ± 4 | 251 ± 3 *** | 432 ± 7 | 257 ± 2 *** | <0.001 | <0.001 | <0.001 |
| Adiposity index | 2.00 ± 0.10 | 1.96 ± 0.12 ** | 4.16 ± 0.10 | 4.74 ± 0.17 ** | <0.001 | <0.05 | <0.05 |
| Left ventricle weight | 0.165 ± 0.004 | 0.169 ± 0.009 | 0.116 ± 0.004 | 0.148 ± 0.004 *** | <0.001 | <0.001 | <0.001 |
| Fasting glucose | 5.6 ± 0.1 | 5.5 ± 0.2 | 6.8 ± 0.1 | 6.5 ± 0.3 | <0.001 | n.s. | n.s. |
| Non-fasting glucose | 9.09 ± 0.37 | 8.08 ± 0.25 | 10.46 ± 0.64 | 8.51 ± 0.70 * | <0.05 | <0.01 | n.s. |
| Insulin | 0.236 ± 0.032 | 0.167 ± 0.029 | 0.372 ± 0.059 | 0.233 ± 0.068 * | <0.05 | <0.05 | n.s. |
| Serum TAG | 0.64 ± 0.06 | 0.66 ± 0.07 | 4.19 ± 0.30 | 5.41 ± 0.38 ** | <0.001 | <0.05 | <0.05 |
| Serum cholesterol | 2.35 ± 0.03 | 2.81 ± 0.08 | 1.71 ± 0.04 | 1.48 ± 0.08 * | <0.001 | n.s. | n.s. |
| HDL-cholesterol | 0.73 ± 0.01 | 0.83 ± 0.03 | 0.57 ± 0.01 | 0.46 ± 0.02 *** | <0.01 | n.s. | n.s. |
| HOMA-IR | 2.01 ± 0.08 | 1.84 ± 0.08 | 2.44 ± 0.09 | 2.29 ± 0.03 | <0.001 | <0.05 | n.s. |
| WKY Male | WKY Female | HHTg Male | HHTg Female | p-STRAIN | p-SEX | p-INT | |
|---|---|---|---|---|---|---|---|
| Hb (g/L) | 141 ± 1 | 129 ± 1 *** | 142 ± 2 | 137 ± 2 * | <0.01 | <0.001 | <0.05 |
| HCT (L/L) | 0.44 ± 0.01 | 0.40 ± 0.01 ** | 0.45 ± 0.01 | 0.42 ± 0.01 * | n.s. | <0.01 | n.s. |
| RBC (×1012/L) | 8.77 ± 0.08 | 7.61 ± 0.10 *** | 8.50 ± 0.07 | 7.50 ± 0.10 *** | <0.05 | <0.001 | n.s. |
| MCV (fL) | 50.6 ± 0.1 | 52.8 ± 0.2 *** | 52.4 ± 0.2 | 55.6 ± 0.1 *** | <0.001 | <0.001 | <0.01 |
| MCH (pg) | 16.0 ± 0.1 | 17.0 ± 0.1 *** | 16.7 ± 0.1 | 18.2 ± 0.1 *** | <0.001 | <0.001 | <0.05 |
| MCHC (g/L) | 316 ± 1 | 322 ± 1 *** | 320 ± 1 | 328 ± 1 *** | <0.001 | <0.001 | n.s. |
| RDW (%) | 17.9 ± 0.1 | 13.8 ± 0.1 *** | 17.3 ± 0.1 | 13.8 ± 0.1 *** | <0.01 | <0.001 | <0.01 |
| Fibrinogen (g/L) | 1.97 ± 0.05 | 1.71 ± 0.03 *** | 2.10 ± 0.04 | 1.64 ± 0.05 *** | n.s. | <0.001 | <0.05 |
| Factor VIII (%) | 602 ± 56 | 632 ± 26 | 556 ± 39 | 560 ± 55 | n.s. | n.s. | n.s. |
| vWf (%) | 84.8 ± 2.2 | 67.8 ± 1.7 *** | 72.9 ± 1.8 | 58.5 ± 4.7 ** | <0.01 | <0.001 | n.s. |
| AT (%) | 119 ± 3 | 143 ± 4 *** | 107 ± 2 | 111 ± 1 | <0.001 | <0.001 | <0.01 |
| PLT (×109/L) | 653 ± 10 | 701 ± 16 | 867 ± 11 | 924 ± 28 * | <0.001 | <0.01 | n.s. |
| MPV (fL) | 7.31 ± 0.03 | 7.37 ± 0.03 | 7.79 ± 0.04 | 7.75 ± 0.03 | <0.001 | n.s. | n.s. |
| PCT (mL/L) | 4.35 ± 0.06 | 4.50 ± 0.10 | 6.56 ± 0.14 | 6.53 ± 0.18 | <0.001 | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markova, I.; Hüttl, M.; Gayova, N.; Miklankova, D.; Cerna, K.; Kavanova, M.; Skaroupkova, P.; Cacanyiova, S.; Malinska, H. Visceral Adipose Tissue Inflammation and Vascular Complications in a Rat Model with Severe Dyslipidemia: Sex Differences and PAI-1 Tissue Involvement. Biomolecules 2025, 15, 19. https://doi.org/10.3390/biom15010019
Markova I, Hüttl M, Gayova N, Miklankova D, Cerna K, Kavanova M, Skaroupkova P, Cacanyiova S, Malinska H. Visceral Adipose Tissue Inflammation and Vascular Complications in a Rat Model with Severe Dyslipidemia: Sex Differences and PAI-1 Tissue Involvement. Biomolecules. 2025; 15(1):19. https://doi.org/10.3390/biom15010019
Chicago/Turabian StyleMarkova, Irena, Martina Hüttl, Natalie Gayova, Denisa Miklankova, Kristyna Cerna, Martina Kavanova, Petra Skaroupkova, Sona Cacanyiova, and Hana Malinska. 2025. "Visceral Adipose Tissue Inflammation and Vascular Complications in a Rat Model with Severe Dyslipidemia: Sex Differences and PAI-1 Tissue Involvement" Biomolecules 15, no. 1: 19. https://doi.org/10.3390/biom15010019
APA StyleMarkova, I., Hüttl, M., Gayova, N., Miklankova, D., Cerna, K., Kavanova, M., Skaroupkova, P., Cacanyiova, S., & Malinska, H. (2025). Visceral Adipose Tissue Inflammation and Vascular Complications in a Rat Model with Severe Dyslipidemia: Sex Differences and PAI-1 Tissue Involvement. Biomolecules, 15(1), 19. https://doi.org/10.3390/biom15010019







