Exploring the Gating Mechanism of the Human Copper Transporter, hCtr1, Using EPR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning, Expression, and Purification of hCtr1 for In Vitro Experiments
2.2. hCtr1 In Situ and Cell Membrane Fragment Experiments
2.3. Q-Band Double Electron–Electron Resonance (DEER) Experiments
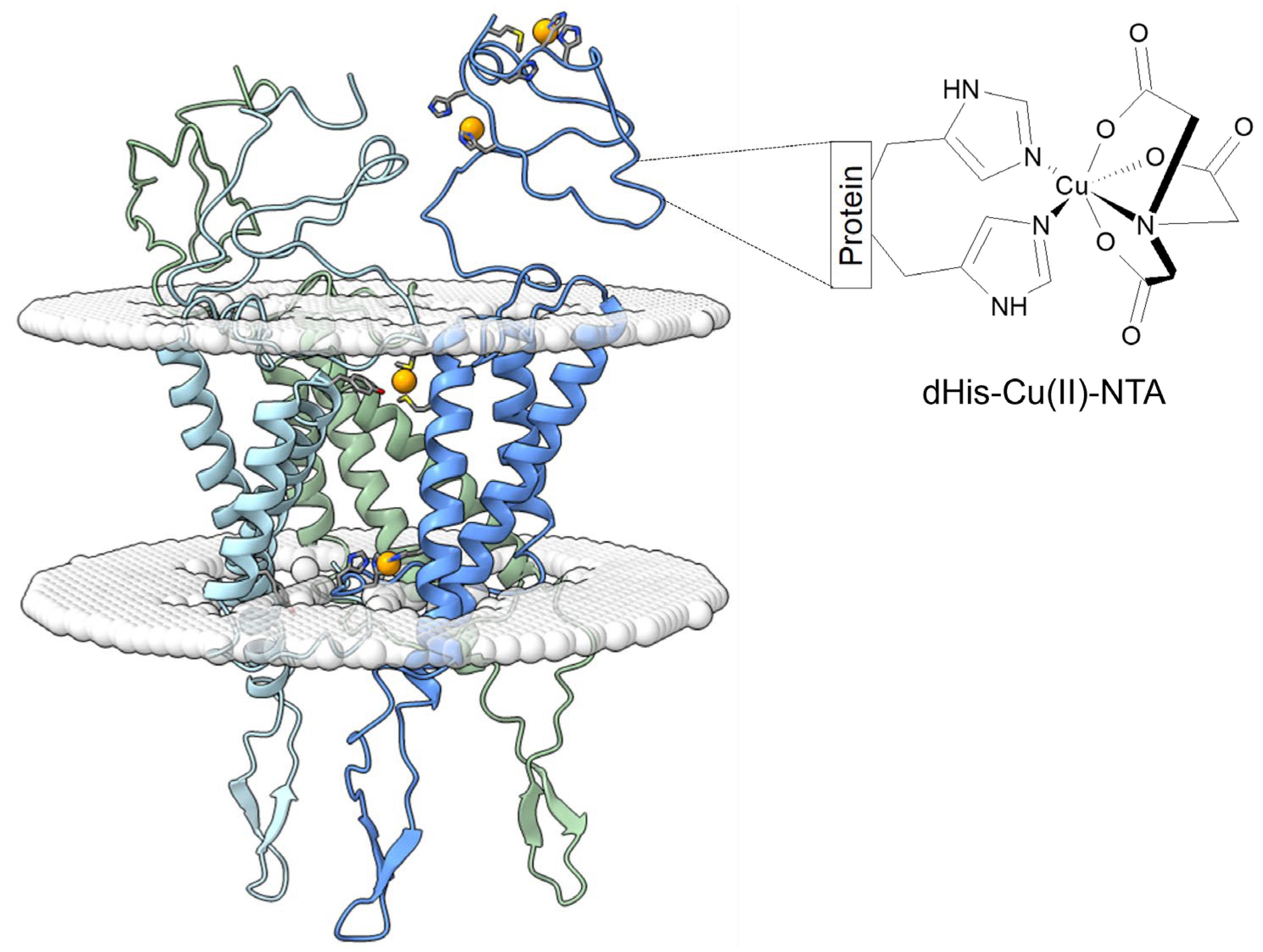
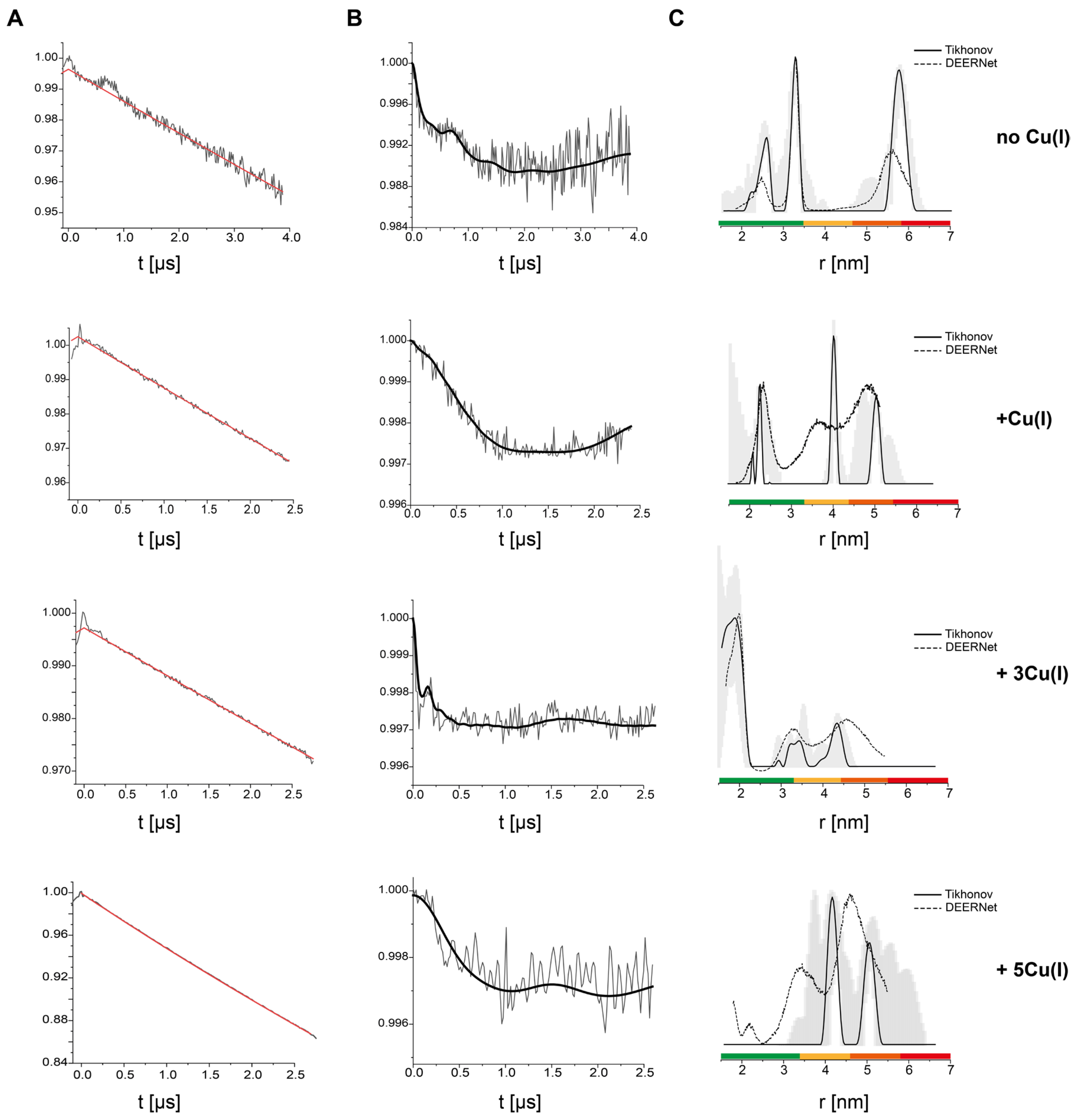
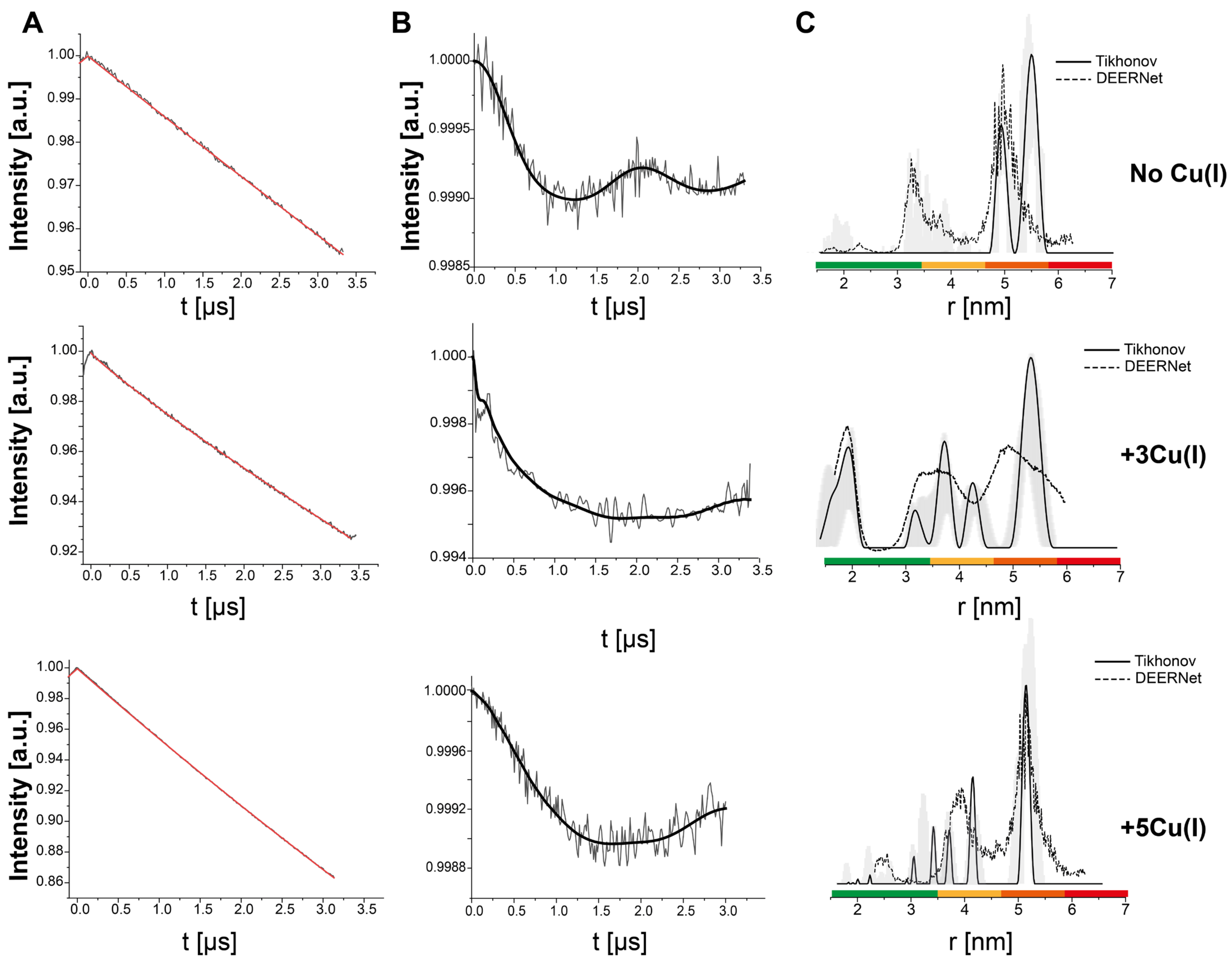
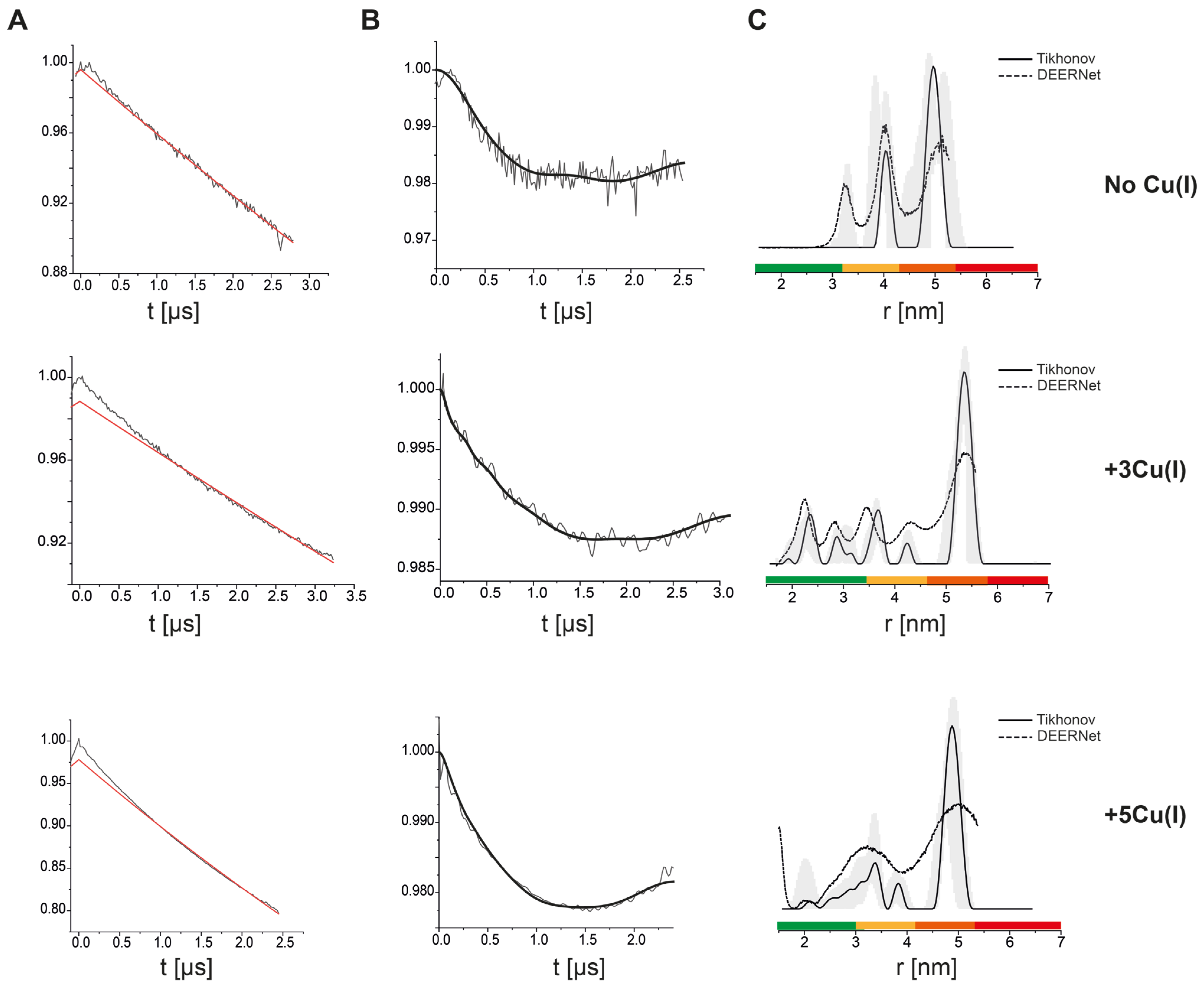
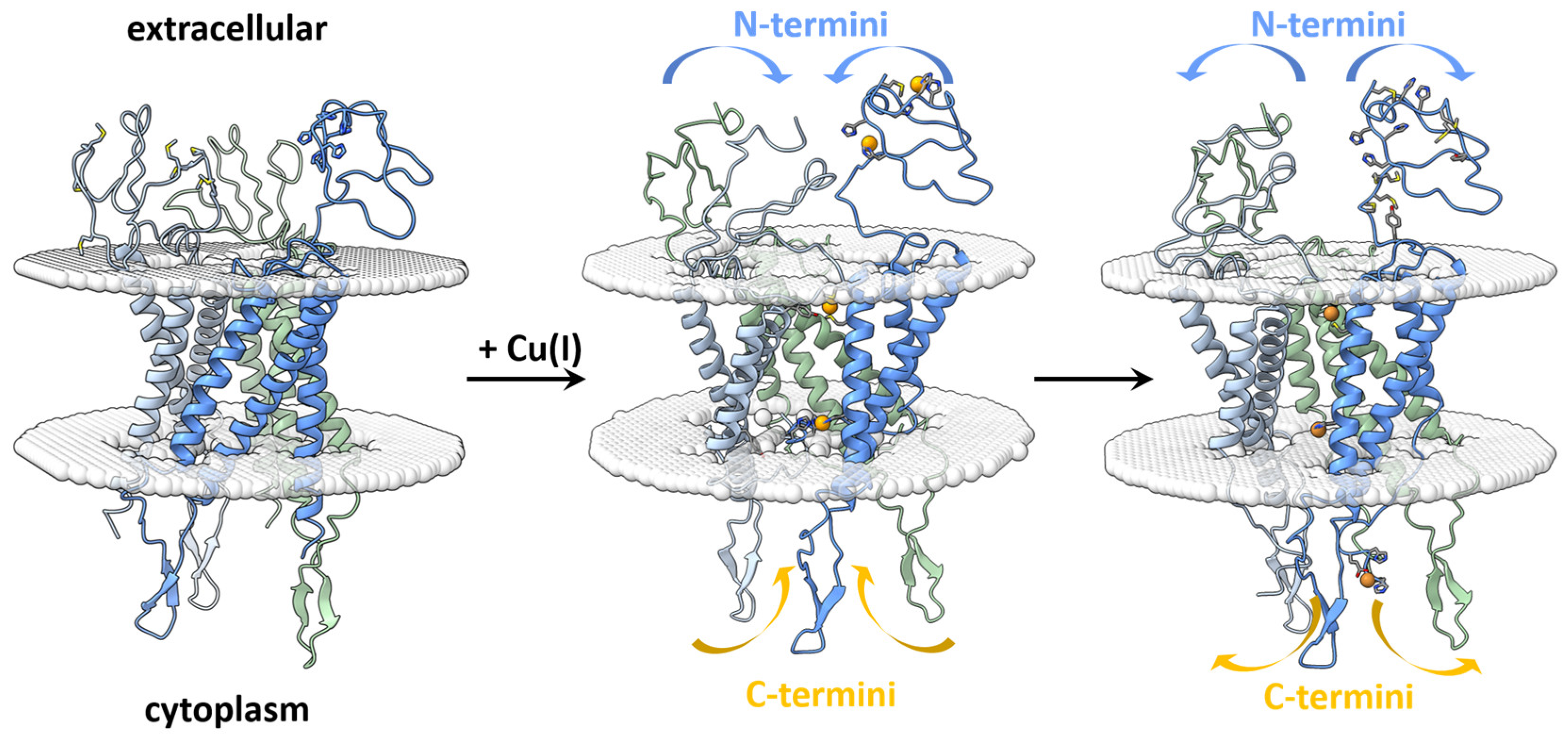
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.T.; Okunola, O.; Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 2010, 39, 3843–3862. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Zagotta, W.N.; Aldrich, R.W. Biophysical and Molecular Mechanisms of Shaker Potassium Channel Inactivation. Science 1990, 250, 533–538. [Google Scholar] [CrossRef]
- Meron, S.; Peleg, S.; Shenberger, Y.; Hofmann, L.; Gevorkyan-Airapetov, L.; Ruthstein, S. Tracking Disordered Extracellular Domains of Membrane Proteins in the Cell with Cu(II)-Based Spin Labels. J. Phys. Chem. B 2024, 128, 8908–8914. [Google Scholar] [CrossRef]
- Nyenhuis, D.A.; Nilaweera, T.D.; Cafiso, D.S. Native Cell Environment Constrains Loop Structure in the Escherichia coli Cobalamin Transporter BtuB. Biophys. J. 2020, 119, 1550–1557. [Google Scholar] [CrossRef]
- Haysom, S.F.; Machin, J.; Whitehouse, J.M.; Horne, J.E.; Fenn, K.; Ma, Y.; El Mkami, H.; Bohringer, N.; Schaberle, T.F.; Ranson, N.A.; et al. Darobactin B Stabilises a Lateral-Closed Conformation of the BAM Complex in E. coli Cells. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218783. [Google Scholar] [CrossRef]
- Nyenhuis, D.A.; Nilaweera, T.D.; Niblo, J.K.; Nguyen, N.Q.; DuBay, K.H.; Cafiso, D.S. Evidence for the Supramolecular Organization of a Bacterial Outer-Membrane Protein from In Vivo Pulse Electron Paramagnetic Resonance Spectroscopy. J. Am. Chem. Soc. 2020, 142, 10715–10722. [Google Scholar] [CrossRef] [PubMed]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Nat. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Maryon, E.B.; Kaplan, J.H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: Implications for copper homeostasis. J. Biol. Chem. 2007, 282, 26471–26480. [Google Scholar] [CrossRef]
- Zhou, B.; Gitschier, J. hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. USA 1997, 94, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, E.; Plonka, D.; Drew, S.C.; Bossak-Ahmad, K.; Haas, K.L.; Pushie, M.J.; Faller, P.; Wezynfeld, N.E.; Bal, W. The N-terminal 14-mer model peptide of human Ctr1 can collect Cu(ii) from albumin. Implications for copper uptake by Ctr1. Metallomics 2018, 10, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Bossak, K.; Drew, S.C.; Stefaniak, E.; Plonka, D.; Bonna, A.; Bal, W. The Cu(II) affinity of the N-terminus of human copper transporter CTR1: Comparison of human and mouse sequences. J. Inorg. Biochem. 2018, 182, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shenberger, Y.; Shimshi, A.; Ruthstein, S. EPR spectroscopy shows that the blood carrier protein, human serum albumin, closely interacts with the N-terminal domain of the copper transporter, Ctr1. J. Phys. Chem. B 2015, 119, 4824–4830. [Google Scholar] [CrossRef]
- Maryon, E.B.; Molloy, S.A.; Ivy, K.; Yu, H.; Kaplan, J.H. Rate and regulation of copper transport by human copper transporter (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Wang, X.; Liu, Q.; Ni, J.; Sun, H. Kinetics and thermodynamics of metal binding to the N-terminus of a human copper transporter, hCTR1. Chem. Commun. 2013, 49, 9134–9136. [Google Scholar] [CrossRef]
- Aller, S.G.; Unger, V.M. Projection structure of the human copper transporter CTR1 at 6A resolution structure reveals a compact trimer with a novel channel-like architecture. Proc. Nat. Acad. Sci. USA 2006, 103, 3627–3632. [Google Scholar] [CrossRef]
- Lee, J.; Pena, M.M.O.; Nose, Y.; Thiele, D.J. Biochemical Characterization of the Human Copper Transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef]
- Jiang, J.; Nadas, I.A.; Kim, J.M.; Franz, K.J. A mets motif peptide found in copper transport proteins selectively binds Cu(I) with methionine-only coordination. Inorg. Chem. 2005, 44, 9787–9794. [Google Scholar] [CrossRef]
- Shenberger, Y.; Marciano, O.; Gottlieb, H.; Ruthstein, S. Insights into the N-terminal Cu(II) and Cu(I) binding sites of the human copper transporter CTR1. J. Coord. Chem. 2018, 71, 1985–2002. [Google Scholar] [CrossRef]
- Magistrato, A.; Pavlin, M.; Qasem, Z.; Ruthstein, S. Copper trafficking in eukaryotic systems: Current knowledge from experimental and computational efforts. Curr. Opin. Struct. Biol. 2019, 58, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Logeman, B.L.; Zhang, X.; Liu, Y.; Thiele, D.J.; Yuan, P. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 2019, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Aupic, J.; Lapenta, F.; Janos, P.; Magistrato, A. Intrinsically disordered ectodomain modulates ion permeation through a metal transporter. Proc. Natl. Acad. Sci. USA 2022, 119, e2214602119. [Google Scholar] [CrossRef] [PubMed]
- Walke, G.; Aupic, J.; Kashoua, H.; Janos, P.; Meron, S.; Shenberger, Y.; Qasem, Z.; Gevorkyan-Airapetov, L.; Magistrato, A.; Ruthstein, S. Dynamical interplay between the human high-affinity copper transporter hCtr1 and its cognate metal ion. Biophys. J. 2022, 121, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Shenberger, Y.; Gevorkyan-Airapetov, L.; Hirsch, M.; Hofmann, L.; Ruthstein, S. An in-cell spin-labelling methodology provides structural information on cytoplasmic proteins in bacteria. Chem. Commun. 2023, 59, 10524–10527. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.F.; Putterman, M.R.; Desai, A.; Horne, W.S.; Saxena, S. The Double Histidine Cu2+-Binding Motif: A Highly Rigid, Site-Specific Spin Probe for Electron Spin Resonance Distance Measurements. Angew. Chem. (Int. Ed.) 2015, 54, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Casto, J.; Bogetti, X.; Hunter, H.R.; Hasanbasri, Z.; Saxena, S. “Store-bought is fine”: Sensitivity considerations using shaped pulses for DEER measurements on Cu(II) labels. J. Magn. Reson. 2023, 349, 107413. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, K.; Heubach, C.A.; Schiemann, O.; Bode, B.E. Pulse Dipolar Electron Paramagnetic Resonance Spectroscopy Distance Measurements at Low Nanomolar Concentrations: The Cu(II)-Trityl Case. J. Phys. Chem. Lett. 2024, 15, 1455–1461. [Google Scholar] [CrossRef]
- Ackermann, K.; Wort, J.L.; Bode, B.E. Nanomolar Pulse Dipolar EPR Spectroscopy in Proteins: Cu(II)-Cu(II) and Nitroxide-Nitroxide Cases. J. Phys. Chem. B 2021, 125, 5358–5364. [Google Scholar] [CrossRef]
- Wort, J.L.; Arya, S.; Ackermann, K.; Stewart, A.J.; Bode, B.E. Pulse Dipolar EPR Reveals Double-Histidine Motif Cu(II)-NTA Spin-Labeling Robustness against Competitor Ions. J. Phys. Chem. Lett. 2021, 12, 2815–2819. [Google Scholar] [CrossRef]
- Gamble Jarvi, A.; Bogetti, X.; Singewald, K.; Ghosh, S.; Saxena, S. Going the dHis-tance: Site-Directed Cu(2+) Labeling of Proteins and Nucleic Acids. Acc. Chem. Res. 2021, 54, 1481–1491. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Sameach, H.; Ghosh, S.; Gevorkyan-Airapetov, L.; Saxena, S.; Ruthstein, S. EPR Spectroscopy Detects Various Active State Conformations of the Transcriptional Regulator CueR. Angew. Chem. Int. Ed. Engl. 2019, 58, 3053–3056. [Google Scholar] [CrossRef]
- Jarvi, A.G.; Ranguelova, K.; Ghosh, S.; Weber, R.T.; Saxena, S. On the Use of Q-Band Double Electron-Electron Resonance to Resolve the Relative Orientations of Two Double Histidine-Bound Cu2+ Ions in a Protein. J. Phys. Chem. B 2018, 122, 10669–10677. [Google Scholar] [CrossRef] [PubMed]
- Worswick, S.G.; Spencer, J.A.; Jeschke, G.; Kuprov, I. Deep neural network processing of DEER data. Sci. Adv. 2018, 4, eaat5218. [Google Scholar] [CrossRef] [PubMed]
- Gamper, N.; Shapiro, M.S. Regulation of ion transport proteins by membrane phosphoinositides. Nat. Rev. Neurosci. 2007, 8, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Hunter, H.R.; Kankati, S.; Hasanbasri, Z.; Saxena, S.K. Endogenous Cu(II) Labeling for Distance Measurements on Proteins by EPR. Chemistry 2024, 30, e202403160. [Google Scholar] [CrossRef]
- Gu, R.X.; de Groot, B.L. Lipid-protein interactions modulate the conformational equilibrium of a potassium channel. Nat. Commun. 2020, 11, 2162. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Mandato, A.; Saxena, S.; Ruthstein, S. The use of EPR spectroscopy to study transcription mechanisms. Biophys. Rev. 2022, 14, 1141–1159. [Google Scholar] [CrossRef]
- Sukomon, N.; Fan, C.; Nimigean, C.M. Ball-and-Chain Inactivation in Potassium Channels. Annu. Rev. Biophys. 2023, 52, 91–111. [Google Scholar] [CrossRef]
- Fan, C.; Sukomon, N.; Flood, E.; Rheinberger, J.; Allen, T.W.; Nimigean, C.M. Ball-and-chain inactivation in a calcium-gated potassium channel. Nature 2020, 580, 288–293. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peleg, S.; Meron, S.; Shenberger, Y.; Hofmann, L.; Gevorkyan-Airapetov, L.; Ruthstein, S. Exploring the Gating Mechanism of the Human Copper Transporter, hCtr1, Using EPR Spectroscopy. Biomolecules 2025, 15, 127. https://doi.org/10.3390/biom15010127
Peleg S, Meron S, Shenberger Y, Hofmann L, Gevorkyan-Airapetov L, Ruthstein S. Exploring the Gating Mechanism of the Human Copper Transporter, hCtr1, Using EPR Spectroscopy. Biomolecules. 2025; 15(1):127. https://doi.org/10.3390/biom15010127
Chicago/Turabian StylePeleg, Shahaf, Shelly Meron, Yulia Shenberger, Lukas Hofmann, Lada Gevorkyan-Airapetov, and Sharon Ruthstein. 2025. "Exploring the Gating Mechanism of the Human Copper Transporter, hCtr1, Using EPR Spectroscopy" Biomolecules 15, no. 1: 127. https://doi.org/10.3390/biom15010127
APA StylePeleg, S., Meron, S., Shenberger, Y., Hofmann, L., Gevorkyan-Airapetov, L., & Ruthstein, S. (2025). Exploring the Gating Mechanism of the Human Copper Transporter, hCtr1, Using EPR Spectroscopy. Biomolecules, 15(1), 127. https://doi.org/10.3390/biom15010127







