IL-4, IL-7, IL-9, NT, NRP1 May Be Useful Markers in the Diagnosis of Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participation in the Study
2.3. Laboratory Analysis
2.4. Statistical Calculations
3. Results
3.1. Serum Concentrations of the Studied Proteins in the Study Group and the Control Group
3.2. Correlations between Studied Proteins
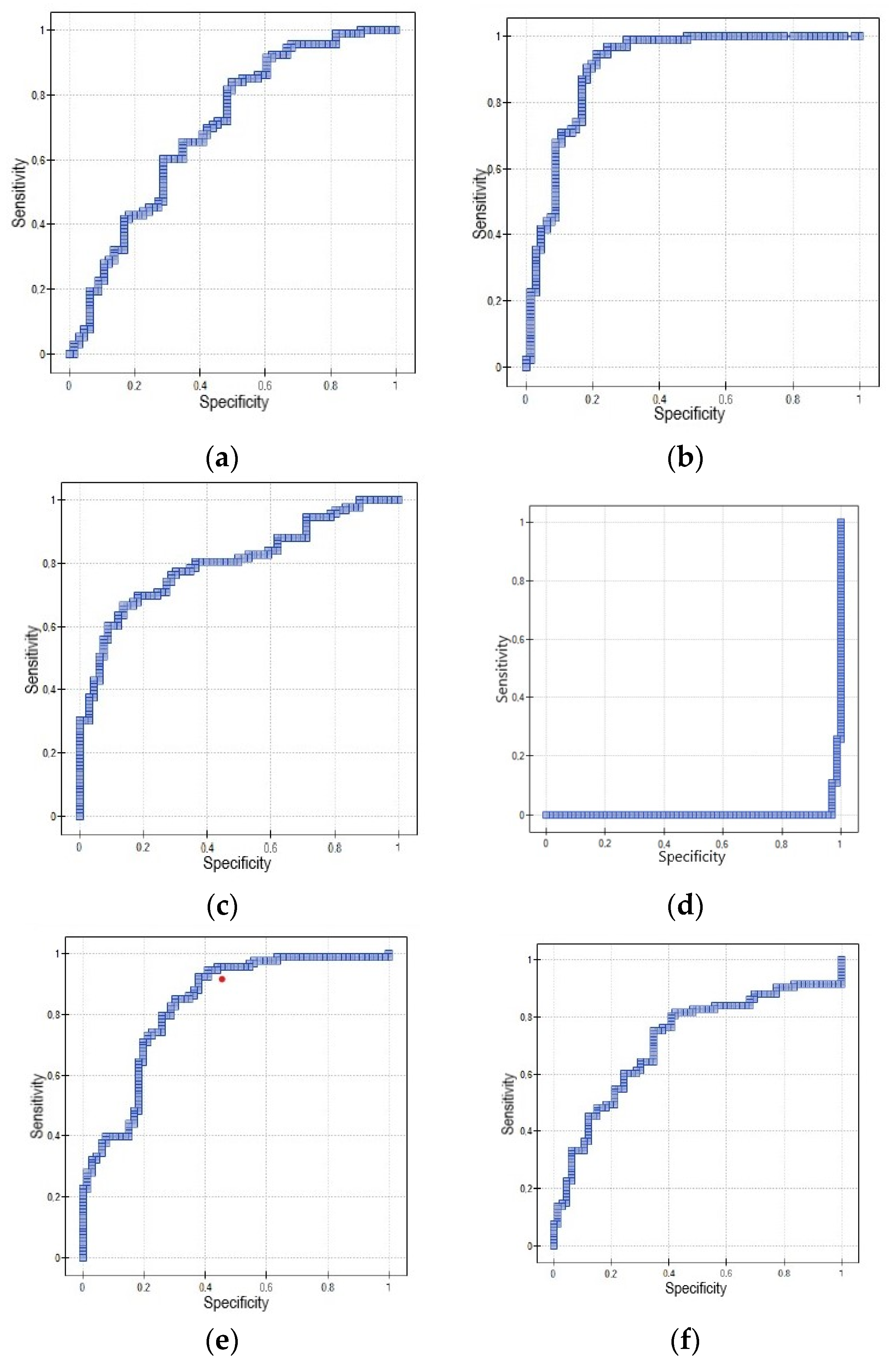
3.3. Receiver Operating Characteristic (ROC) Curve for Using IL-4, IL-7, IL-9, IL-10, NT, NRP1 Distinguishing between Endometrial Cancer and Noncancerous Endometrial Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.; Wang, B.; Xu, W.; Liu, K.; Gao, Y.; Guo, C.; Chen, J.; Kamal, M.A.; Yuan, C. Endometrial Cancer: Genetic, Metabolic Characteristics, Therapeutic Strategies and Nanomedicine. Curr. Med. Chem. 2021, 28, 8755–8781. [Google Scholar] [CrossRef]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and management of endometrial cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Endometrial Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/ (accessed on 8 August 2024).
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- PDQ Screening and Prevention Editorial Board. Endometrial Cancer Screening (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Cai, J.; Cui, K.; Niu, F.; Jin, T.; Huang, S.; Zhang, Y.; Bao, S. Genetics of IL6 polymorphisms: Case–control study of the risk of endometrial cancer. Mol. Genet. Genom. Med. 2019, 7, e00600. [Google Scholar] [CrossRef]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Ma, J.; Liu, D.; Mao, X.; Huang, L.; Ren, Y.; Xu, X.; Huang, X.; Deng, C.; Shi, F.; Sun, P. Enhanced Diagnostic Efficiency of Endometrial Carcinogenesis and Progression in Women with Abnormal Uterine Bleeding through Peripheral Blood Cytokine Testing: A Multicenter Retrospective Cohort Study. Int. J. Med. Sci. 2024, 21, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Sompolska-Rzechuła, A.; Machaliński, B.; Surowiec, A.; Menkiszak, J. Clinical importance of serum HE4 and MMP2 levels in endometrial cancer patients. Onco Targets Ther. 2017, 10, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Brown, A.K.; Craig Miller, A.; Badgwell, D.; Lu, Z.; Jeffrey Allard, W.; Granai, C.O.; Bast Jr, R.C.; Lu, K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol. Oncol. 2008, 110, 196–201. [Google Scholar] [CrossRef]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible roles of interleukin-4 and-13 and their receptors in gastric and colon cancer. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, P.; Liu, S.; Jin, Z.; Li, D.; Zhao, H.; Zhu, X.; Shu, C.; Yan, D.; Dong, Z. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 2019, 28, 421–430. [Google Scholar] [CrossRef]
- Seki, N.; Kodama, J.; Hashimoto, I.; Hongo, A.; Yoshinouchi, M.; Kudo, T. Thrombospondin-1 and -2 messenger RNA expression in normal and neoplastic endometrial tissues: Correlation with angiogenesis and prognosis. Int. J. Oncol. 2001, 19, 305–310. [Google Scholar] [CrossRef]
- Lee, J.E.; Zhu, Z.; Bai, Q.; Brady, T.J.; Xiao, H.; Wakefield, M.R.; Fang, Y. The Role of Interleukin-9 in Cancer. Pathol. Oncol. Res. 2020, 26, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Lam, H.Y.P.; Hsu, H.J.; Jiang, S.J. Interleukin-10: A double-edged sword in breast cancer. Tzu Chi Med. J. 2021, 33, 203–211. [Google Scholar] [CrossRef]
- Wang, C.; Kong, L.; Kim, S.; Lee, S.; Oh, S.; Jo, S.; Jang, I.; Kim, T.-D. The Role of IL-7 and IL-7R in Cancer Pathophysiology and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 10412. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, Z.; Xiao, H.; Wakefield, M.R.; Ding, V.A.; Bai, Q.; Fang, Y. The role of il-7 in immunity and cancer. Anticancer. Res. 2017, 37, 963–968. [Google Scholar] [CrossRef]
- Formentini, A.; Braun, P.; Fricke, H.; Link, K.H.; Henne-Bruns, D.; Kornmann, M. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int. J. Color. Dis. 2012, 27, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Wieder-Huszla, S.; Chudecka-Głaz, A.; Gutowska, I.; Karakiewicz, B.; Jurczak, A. Effect of the treatment stage on the serum levels of selected cytokines and antioxidant enzymes in patients with tumors of the reproductive organs. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3117–3133. [Google Scholar] [CrossRef] [PubMed]
- Perambakam, S.M.; Srivastava, R.; Peace, D.J. Distinct cytokine patterns exist in peripheral blood mononuclear cell cultures of patients with prostate cancer. Clin. Immunol. 2005, 117, 94–99. [Google Scholar] [CrossRef]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Toi, M. Interleukin-4 and breast cancer. Breast Cancer 2000, 7, 181–186. [Google Scholar] [CrossRef]
- Fry, T.J.; Mackall, C.L. Interleukin-7: From bench to clinic. Blood 2002, 99, 3892–3904. [Google Scholar] [CrossRef]
- Tong, H.; Feng, H.; Hu, X.; Wang, M.-F.; Song, Y.-F.; Wen, X.-L.; Li, Y.-R.; Wan, X.-P. Identification of Interleukin-9 Producing Immune Cells in Endometrial Carcinoma and Establishment of a Prognostic Nomogram. Front. Immunol. 2020, 11, 544248. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chien, W.C.; Liao, J.M.; Tsai, C.W.; Chang, W.S.; Su, C.H.; Hsu, S.W.; Wang, H.C.; Bau, D.T. Contribution of interleukin-10 genotype to triple negative breast cancer risk. Anticancer. Res. 2021, 41, 2451–2457. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Q.; Wei, S.; Zhang, Y.; Zhou, T.; Zhang, Q.; Shi, R.; Zinovkin, D.; Pranjol, Z.I.; Zhang, J.; et al. CD146+CAFs promote progression of endometrial cancer by inducing angiogenesis and vasculogenic mimicry via IL-10/JAK1/STAT3 pathway. Cell Commun. Signal. 2024, 22, 170. [Google Scholar] [CrossRef]
- Nikolaou, S.; Qui, S.; Fiorentino, F.; Simillis, C.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The role of Neurotensin and its receptors in non-gastrointestinal cancers: A review. Cell Commun. Signal. 2020, 18, 68. [Google Scholar] [CrossRef]
- Grill, S.; Yahiaoui-Doktor, M.; Basrai, M.; Struck, J.; Schulte, J.; Berling-Ernst, A.; Engel, C.; Ullrich, M.; Lammert, J.; Bischoff, S.C.; et al. Precursor fractions of neurotensin and enkephalin might point to molecular mechanisms of cancer risk modulation during a lifestyle-intervention in germline BRCA1/2 gene mutation carriers. Breast Cancer Res. Treat. 2021, 186, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Wu, Z.; Dupouy, S.; Lupo, A.M.; Mourra, N.; Takahashi, T.; Fléjou, J.F.; Trédaniel, J.; Régnard, J.F.; Damotte, D.; et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 2014, 5, 8252–8269. [Google Scholar] [CrossRef]
- Agopiantz, M.; Forgez, P.; Casse, J.M.; Lacomme, S.; Charra-Brunaud, C.; Clerc-Urmès, I.; Morel, O.; Bonnet, C.; Guéant, J.L.; Vignaud, J.M.; et al. Expression of neurotensin receptor 1 in endometrial adenocarcinoma is correlated with histological grade and clinical outcome. Virchows Arch. 2017, 471, 521–530. [Google Scholar] [CrossRef]
- Liu, J.; Agopiantz, M.; Poupon, J.; Wu, Z.; Just, P.A.; Borghese, B.; Ségal-Bendirdjian, E.; Gauchotte, G.; Gompel, A.; Forgez, P. neurotensin receptor 1 antagonist SR48692 improves response to carboplatin by enhancing apoptosis and inhibiting drug efflux in ovarian cancer. Clin. Cancer Res. 2017, 23, 6516–6528. [Google Scholar] [CrossRef]
- Liu, J.F.; Lee, C.W.; Tsai, M.H.; Tang, C.H.; Chen, P.C.; Lin, L.W.; Lin, C.Y.; Lu, C.H.; Lin, Y.F.; Yang, S.H.; et al. Thrombospondin 2 promotes tumor metastasis by inducing matrix metalloproteinase-13 production in lung cancer cells. Biochem. Pharmacol. 2018, 155, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Okon, I.S.; Ding, Y.; Coughlan, K.A.; Wang, Q.; Song, P.; Benbrook, D.M.; Zou, M.H. Aberrant NRP-1 expression serves as predicator of metastatic endometrial and lung cancers. Oncotarget 2016, 7, 7970–7978. [Google Scholar] [CrossRef]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2014, 63, 81–99. [Google Scholar] [CrossRef]
- Oplawski, M.; Dziobek, K.; Brabarek, B.; Zmarzły, N.; Dąbruś, D.; Januszyk, P.; Brus, R.; Tomala, B.; Boroń, D. Expression of NRP-1 and NRP-2 in Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Rzepakowska, A.; Żurek, M.; Grzybowski, J.; Kotula, I.; Pihowicz, P.; Górnicka, B.; Demkow, U.; Niemczyk, K. Serum and tissue expression of neuropilin 1 in precancerous and malignant vocal fold lesions. PLoS ONE 2020, 15, e0239550. [Google Scholar] [CrossRef]
- Kenawy, M.Z.; Mikhael, N.W.; El-fallah, A.A.; Shehata, R.A. Serum Levels of Insulin Like Growth Factor-1(IGF-1) in Patients with Seborrheic Dermatitis. Benha J. Appl. Sci. 2020, 5, 229–231. [Google Scholar] [CrossRef]
- Wang, H.W.; Joyce, J.A. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle 2010, 9, 4824–4835. [Google Scholar] [CrossRef] [PubMed]
- Baniyash, M. Chronic inflammation, immunosuppression and cancer: New insights and outlook. Semin. Cancer Biol. 2006, 16, 80–88. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, L.; Hao, S.; Liu, Z.; Ding, S.; Zhang, W.; Yang, X.; Li, S. Activation of the IL-4/STAT6 Signaling Pathway Promotes Lung Cancer Progression by Increasing M2 Myeloid Cells. Front. Immunol. 2019, 10, 2638. [Google Scholar] [CrossRef]
- Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Alcocer-González, J.M.; Moreno, J.; Madrid-Marina, V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep. 2011, 4, 369–375. [Google Scholar] [CrossRef][Green Version]
- Ramspott, J.P.; Bekkat, F.; Bod, L.; Favier, M.; Terris, B.; Salomon, A.; Djerroudi, L.; Zaenker, K.S.; Richard, Y.; Molinier-Frenkel, V.; et al. Emerging Role of IL-4–Induced Gene 1 as a Prognostic Biomarker Affecting the Local T-Cell Response in Human Cutaneous Melanoma. J. Investig. Dermatol. 2018, 138, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Circulating serum levels of cytokines and angiogenic factors in patients with cervical cancer. Cancer Investig. 1998, 16, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lambeck, A.J.A.; Crijns, A.P.G.; Leffers, N.; Sluiter, W.J.; ten Hoor, K.A.; Braid, M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W.; Kast, W.M. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: A potential role for interleukin 7. Clin. Cancer Res. 2007, 13, 2385–2391. [Google Scholar] [CrossRef]
- Xie, X.; Ye, D.; Chen, H.; Lu, W.; Cheng, B.; Zhong, H. Interleukin-7 and suppression of local peritoneal immunity in ovarian carcinoma. Int. J. Gynecol. Obstet. 2004, 85, 151–158. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Lampaki, S.; Yarmus, L.; Kioumis, I.; Pitsiou, G.; Katsikogiannis, N.; Hohenforst-Schmidt, W.; Li, Q.; Huang, H.; Sakkas, A.; et al. Interleukin-7 and interleukin-15 for cancer. J. Cancer 2014, 5, 765–773. [Google Scholar] [CrossRef]
- Chopra, V.; Ding, T.V.; Hanningan, E.V. Serum levels of interleukins, growth factors and anglogenin in patients with endometrial cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 167–172. [Google Scholar] [CrossRef]
- Zang, Y.; Li, H.; Liu, S.; Zhao, R.; Zhang, K.; Zang, Y.; Wang, Y.; Xue, F. The roles and clinical applications of interleukins in endometrial carcinoma. Front. Oncol. 2022, 12, 1001693. [Google Scholar] [CrossRef]
- Habel, A.; Xu, W.; Ahmed, M.H.; Stayoussef, M.; Bouaziz, H.; Ayadi, M.; Mezlini, A.; Larbi, A.; Yaacoubi-Loueslati, B. Identification of two theranostic biomarker panels for epithelial ovarian cancer. Cytokine 2023, 161, 156051. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Song, M.; Zhang, R.; Chen, F. Expression and clinical significance of peripheral blood IL-9 and IL-22 in patients with endometrial cancer. Minerva Gastroenterol. 2024, 70, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Souazé, F.; Dupouy, S.; Viardot-Foucault, V.; Bruyneel, E.; Attoub, S.; Gespach, C.; Gompel, A.; Forgez, P. Expression of neurotensin and NT1 receptor in human breast cancer: A potential role in tumor progression. Cancer Res. 2006, 66, 6243–6249. [Google Scholar] [CrossRef] [PubMed]
- Soma, S.; Gompel, A.; Rostène, W.; Forgez, P. Neurotensin counteracts apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 295, 482–488. [Google Scholar] [CrossRef]
- Morgat, C.; Brouste, V.; Chastel, A.; Vélasco, V.; Macgrogan, G.; Hindié, E. Expression of neurotensin receptor-1 (NTS1) in primary breast tumors, cellular distribution, and association with clinical and biological factors. Breast Cancer Res. Treat. 2021, 190, 403–413. [Google Scholar] [CrossRef]
- Takahashi, K.; Ehata, S.; Miyauchi, K.; Morishita, Y.; Miyazawa, K.; Miyazono, K. Neurotensin receptor 1 signaling promotes pancreatic cancer progression. Mol. Oncol. 2021, 15, 151–166. [Google Scholar] [CrossRef]
- Kofler, N.M.; Simons, M. Angiogenesis versus arteriogenesis: Neuropilin 1 modulation of VEGF signaling. F1000Prime Rep. 2015, 7, 26. [Google Scholar] [CrossRef]
- Glinka, Y.; Stoilova, S.; Mohammed, N.; Prud’homme, G.J. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis 2011, 32, 613–621. [Google Scholar] [CrossRef]
- Ding, Z.; Du, W.; Lei, Z.; Zhang, Y.; Zhu, J.; Zeng, Y.; Wang, S.; Zheng, Y.; Liu, Z.; Huang, J.A. Neuropilin 1 modulates TGF-β1-induced epithelial-mesenchymal transition in non-small cell lung cancer. Int. J. Oncol. 2020, 56, 531–543. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.; Huang, L.; Bai, B.; Xu, Z. Clinicopathological Significance of Neuropilin 1 Expression in Gastric Cancer: A Meta-Analysis. Dis. Markers 2020, 2020, 4763492. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Patients (%) |
|---|---|
| Endometrial cancer | |
| Yes | 93 (58) |
| No | 66 (42) |
| Endometrial Cancer | |
| Endometrioid endometrial carcinoma | 82 (88) |
| Nonendometrioid endometrial carcinoma | 11 (12) |
| Clinical staging | |
| FIGO I and II | 88 (95) |
| FIGO III and IV | 5 (5) |
| Histopathological grading | |
| Grade 1 | 37 (40) |
| Grade 2 | 34 (37) |
| Grade 3 | 22 (24) |
| Clinic Demographic Characteristics | Total Cohort (n = 159) | Endometrial Cancer (n = 93) | NCEL (n = 66) | p-Value | Statistical Power |
|---|---|---|---|---|---|
| Median (IQR) | |||||
| Age (years old) | 55 (43–67) | 51 (43–63) | 59.5 (43–71) | 0.1106 | 0.995 |
| BMI (kg/m2) | 27.9 (24.2–31.7) | 28.6 (25.4–31.7) | 27 (23–31) | 0.0397 | 0.1671 |
| Number (%) | |||||
| Age (years old) | |||||
| <65 | 114 (72) | 74 (80) | 40 (61) | 0.0967 | 0.999 |
| ≥65 | 45 (28) | 19 (20) | 26 (39) | 0.2889 | 0.991 |
| BMI | |||||
| <25 | 43 (27) | 22 (24) | 21 (32) | 0.5922 | 0.0947 |
| ≥25 | 116 (73) | 71 (76) | 45 (68) | 0.1176 | 0.999 |
| Menopausal status | |||||
| Premenopausal | 52 (33) | 34 (37) | 18 (27) | 0.0387 | 0.999 |
| Postmenopausal | 107 (67) | 59 (63) | 48 (73) | 0.3035 | 0.999 |
| Hypertension | |||||
| Yes | 82 (52) | 49 (53) | 33 (50) | 0.3600 | 0.999 |
| No | 77 (48) | 44 (47) | 33 (50) | 0.5807 | 0.999 |
| Diabetes mellitus | |||||
| Yes | 73 (46) | 42 (45) | 31 (47) | 0.5577 | 0.999 |
| No | 86 (54) | 51 (55) | 35 (53) | 0.3123 | 0.999 |
| Characteristics | EC | NCEL | p-Value | Statistical Power | |
|---|---|---|---|---|---|
| IL-4 [pg/mL] | Median | 982.36 | 806.42 | 0.000023 | 0.999 |
| Q1–Q3 | 855.79–1171.3 | 679.57–991.25 | |||
| IL-7 [ng/L] | Median | 190.94 | 106.79 | 0.000000 | 0.999 |
| Q1–Q3 | 164.55–232.07 | 89.9–131.77 | |||
| IL-9 [pg/mL] | Median | 257.15 | 198.32 | 0.000000 | 0.999 |
| Q1–Q3 | 220.92–307.78 | 163.79–223.46 | |||
| IL-10 [pg/mL] | Median | 1594.97 | 3245.68 | 0.000000 | 0.999 |
| Q1–Q3 | 1257.75–1989.90 | 2998.79–3889.77 | |||
| NT [pmol/L] | Median | 367.46 | 242.33 | 0.000000 | 0.999 |
| Q1–Q3 | 321.16–461.62 | 177.79–322.46 | |||
| TSP-2 [µg/mL] | Median | 88.48 | 87.6 | 0.6787 | 0.0845 |
| Q1–Q3 | 71.27–113.41 | 77.57–109.57 | |||
| NRP1 [ng/mL] | Median | 40.86 | 28.74 | 0.000004 | 0.999 |
| Q1–Q3 | 33.59–48.59 | 15.68–38.46 | |||
| Variable | IL-4 | IL-7 | IL-9 | IL-10 | NT | NRP1 | |
|---|---|---|---|---|---|---|---|
| IL-4 | rs | 1 | 0.395 | 0.396 | −0.149 | 0.394 | 0.304 |
| p-Value | - | 0.0000001 | 0.0000001 | 0.061206 | 0.0000001 | 0.0001 | |
| Statistical power | - | 0.979 | 0.979 | 0.299 | 0.978 | 0.850 | |
| IL-7 | rs | 0.395 | 1 | 0.628 | −0.420 | 0.529 | 0.259 |
| p-Value | 0.0000001 | - | 0.0000001 | 0.0000001 | 0.0000001 | 0.000982 | |
| Statistical power | 0.979 | - | 0.999 | 0.990 | 0.999 | 0.715 | |
| IL-9 | rs | 0.396 | 0.628 | 1 | −0.229 | 0.629 | 0.264 |
| p-Value | 0.0000001 | 0.0000001 | - | 0.003764 | 0.0000001 | 0.000788 | |
| Statistical power | 0.979 | 0.999 | - | 0.604 | 0.999 | 0.732 | |
| IL-10 | rs | −0.149 | −0.420 | −0.229 | 1 | −0.348 | −0.232 |
| p-Value | 0.061206 | 0.0000001 | 0.003764 | - | 0.000007 | 0.003313 | |
| Statistical power | 0.299 | 0.990 | 0.604 | - | 0.934 | 0.616 | |
| NT | rs | 0.394 | 0.529 | 0.629 | −0.348 | 1 | 0.251 |
| p-Value | 0.0000001 | 0.0000001 | 0.0000001 | 0.000007 | - | 0.001391 | |
| Statistical power | 0.978 | 0.999 | 0.999 | 0.934 | - | 0.687 | |
| NRP1 | rs | 0.304 | 0.259 | 0.264 | −0.232 | 0.251 | 1 |
| p-Value | 0.0001 | 0.000982 | 0.000788 | 0.003313 | 0.001391 | - | |
| Statistical power | 0.850 | 0.715 | 0.732 | 0.616 | 0.687 | - | |
| Marker | AUC (95% CI) | Sensitivity [%] | Specificity [%] | PPV [%] | NPV [%] | p-Value | Cut-Off Value |
|---|---|---|---|---|---|---|---|
| IL-4 | 0.7 (0.61–0.78) | 83.87 | 50 | 70.27 | 68.75 | 0.000023 | 802.26 pg/mL |
| IL-7 | 0.91 (0.85–0.96) | 96.77 | 75.76 | 84.91 | 94.34 | <0.000001 | 133.63 ng/L |
| IL-9 | 0.8 (0.73–0.87) | 69.89 | 81.82 | 84.42 | 65.85 | <0.000001 | 228.79 pg/mL |
| IL-10 | 0.006 (0–0.014) | 100 | 0 | 58.49 | NA | <0.000001 | 187.4 pg/mL |
| NT | 0.83 (0.76–0.9) | 94.62 | 59.09 | 76.52 | 88.64 | <0.000001 | 275.43 pmol/L |
| NRP1 | 0.71 (0.63–0.8) | 81.72 | 57.58 | 73.08 | 69.09 | 0.000004 | 30.37 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, M.; Borzyszkowska, D.; Lerch, N.; Turoń-Skrzypińska, A.; Tkacz, M.; Lubikowski, J.; Tarnowski, M.; Rotter, I.; Cymbaluk-Płoska, A. IL-4, IL-7, IL-9, NT, NRP1 May Be Useful Markers in the Diagnosis of Endometrial Cancer. Biomolecules 2024, 14, 1095. https://doi.org/10.3390/biom14091095
Kozłowski M, Borzyszkowska D, Lerch N, Turoń-Skrzypińska A, Tkacz M, Lubikowski J, Tarnowski M, Rotter I, Cymbaluk-Płoska A. IL-4, IL-7, IL-9, NT, NRP1 May Be Useful Markers in the Diagnosis of Endometrial Cancer. Biomolecules. 2024; 14(9):1095. https://doi.org/10.3390/biom14091095
Chicago/Turabian StyleKozłowski, Mateusz, Dominika Borzyszkowska, Natalia Lerch, Agnieszka Turoń-Skrzypińska, Marta Tkacz, Jerzy Lubikowski, Maciej Tarnowski, Iwona Rotter, and Aneta Cymbaluk-Płoska. 2024. "IL-4, IL-7, IL-9, NT, NRP1 May Be Useful Markers in the Diagnosis of Endometrial Cancer" Biomolecules 14, no. 9: 1095. https://doi.org/10.3390/biom14091095
APA StyleKozłowski, M., Borzyszkowska, D., Lerch, N., Turoń-Skrzypińska, A., Tkacz, M., Lubikowski, J., Tarnowski, M., Rotter, I., & Cymbaluk-Płoska, A. (2024). IL-4, IL-7, IL-9, NT, NRP1 May Be Useful Markers in the Diagnosis of Endometrial Cancer. Biomolecules, 14(9), 1095. https://doi.org/10.3390/biom14091095







