Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions
Abstract
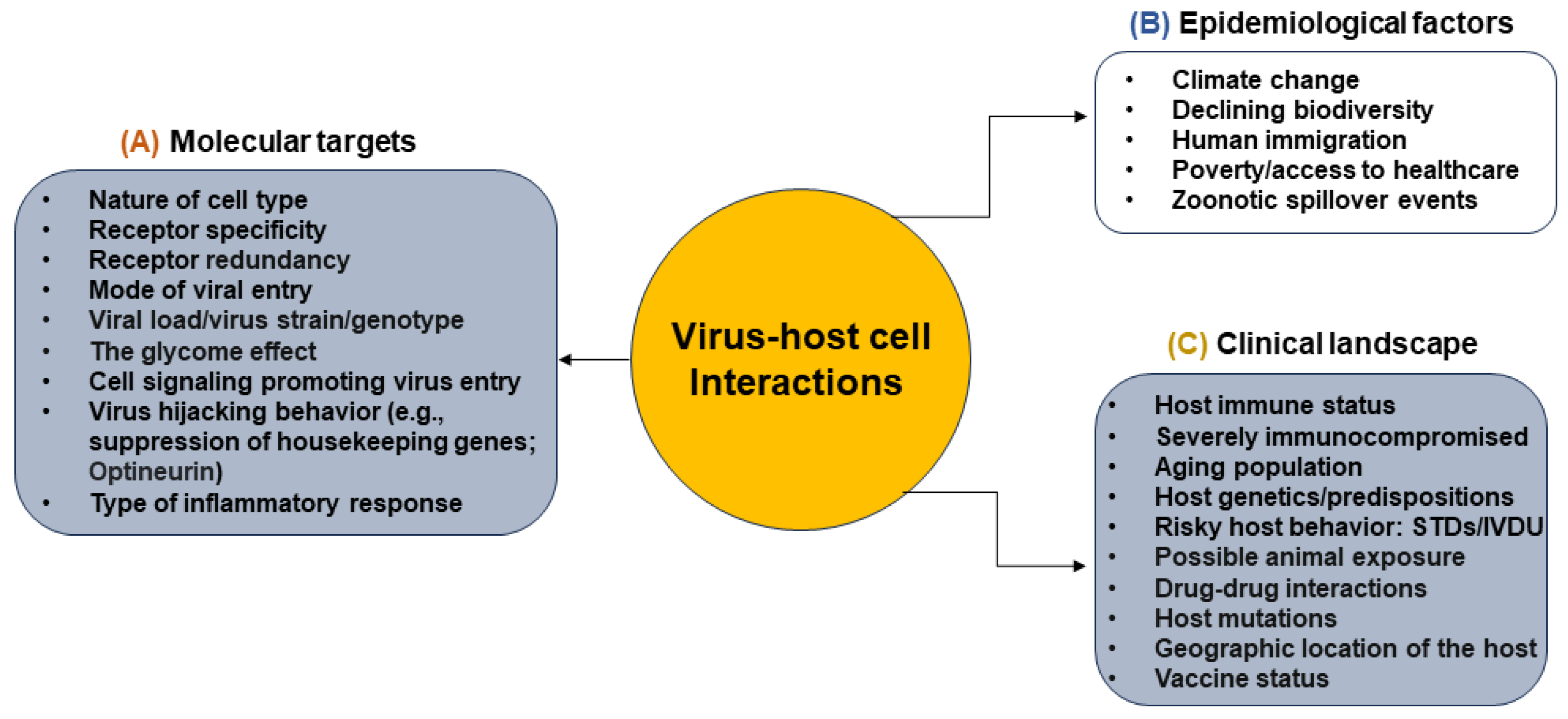
1. Diverse Landscape of Virus–Host Cell Interactions
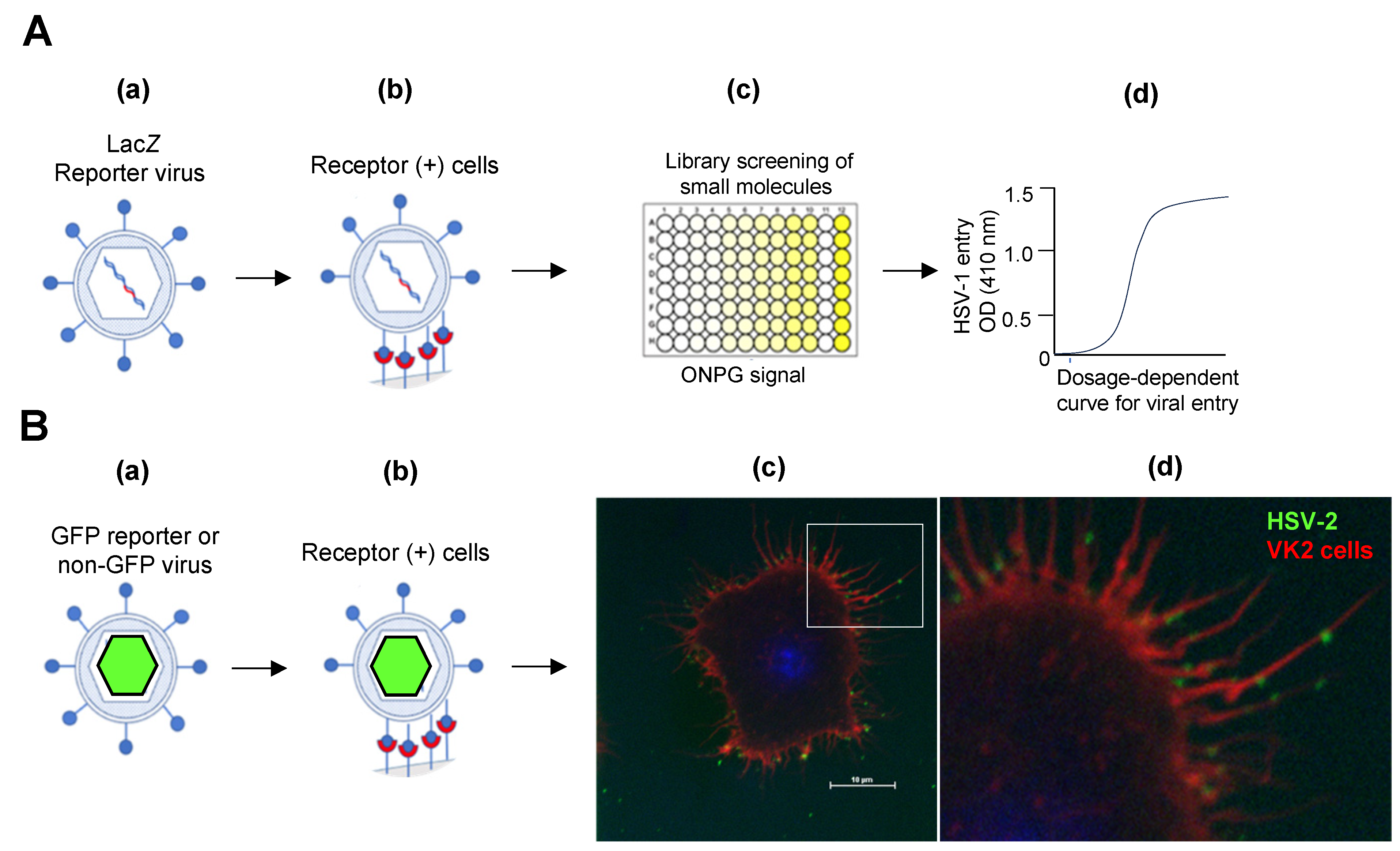
2. Signaling Pathways That Promote Viral Entry into the Host Cell
3. Role of Artificial Intelligence in the Study of Virus Infections
4. General Application of Artificial Intelligence in Infectious Disease
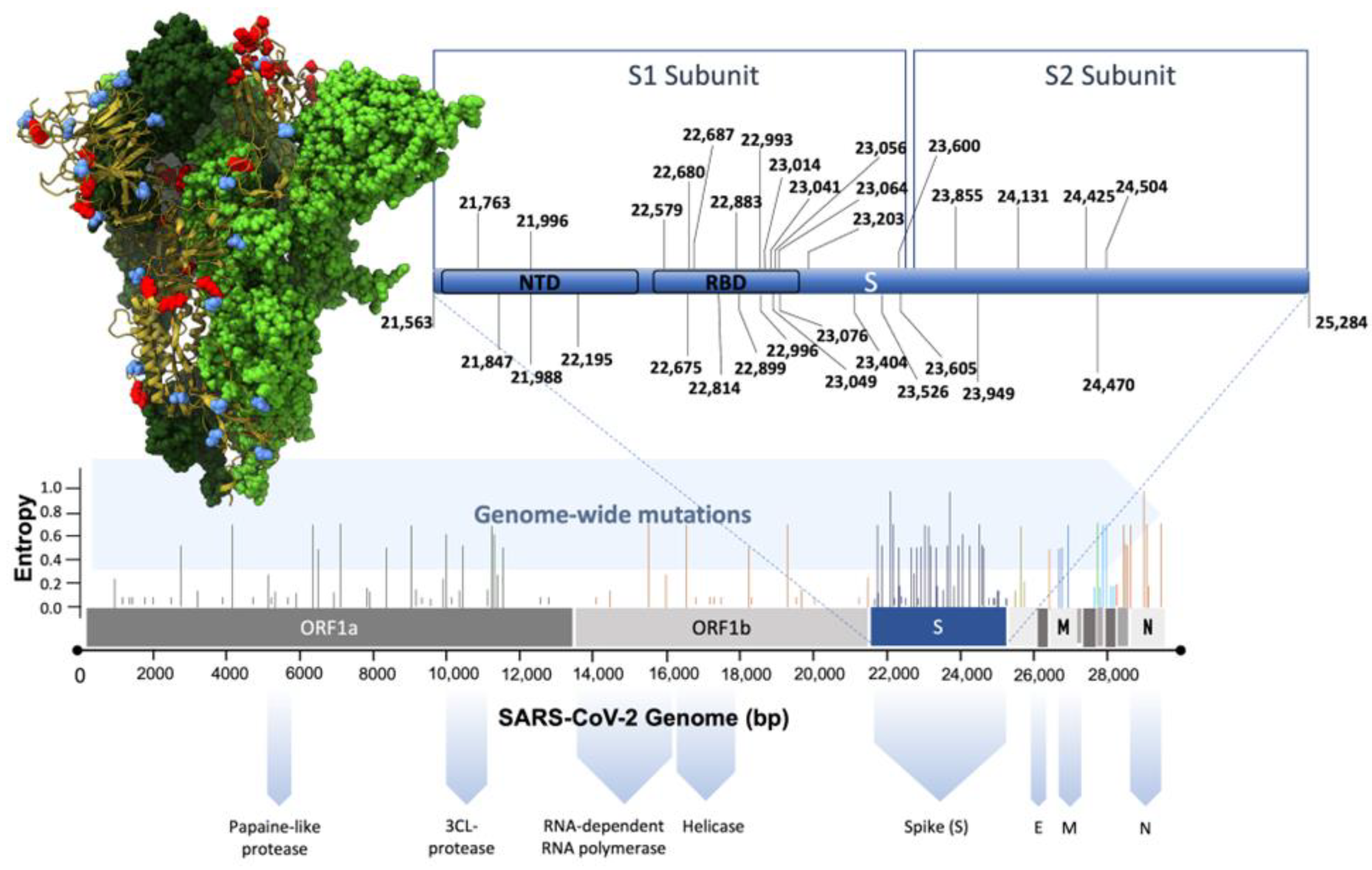
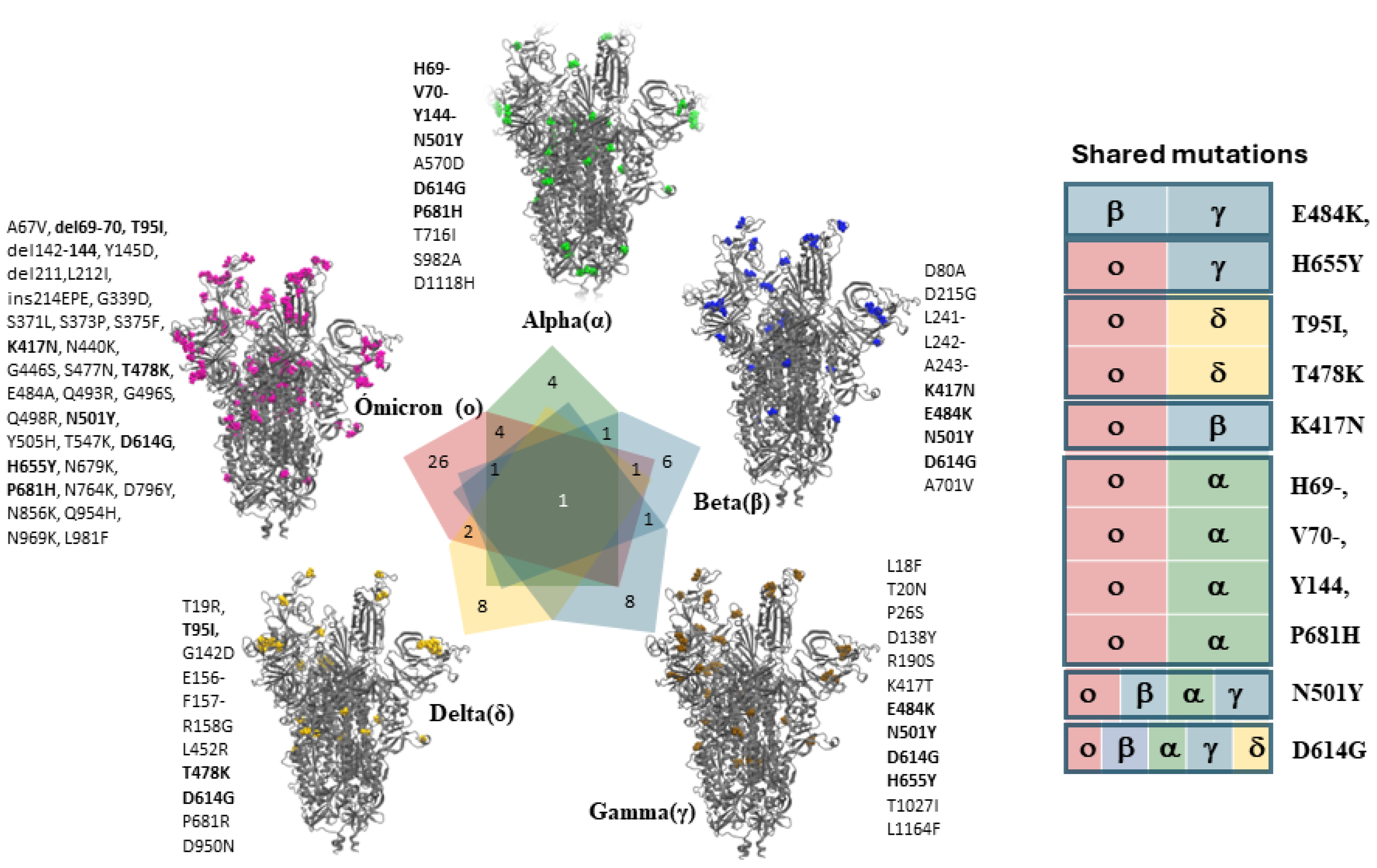
5. Current Research on Artificial Intelligence and SARS-CoV-2
6. Current Research on Artificial Intelligence in Infectious Disease Population Surveillance to Predict Future Pandemic Scenarios
7. Limitations and Challenges of Using Artificial Intelligence in Viral Pathogenesis Research
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Payne, S. Virus Interactions with the Cell. Viruses 2017, 23–35. [Google Scholar] [CrossRef]
- Sieczkarski, S.B.; Whittaker, G.R. Viral entry. Curr. Top. Microbiol. Immunol. 2005, 285, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Carette, J.E. Hunting Viral Receptors Using Haploid Cells. Annu. Rev. Virol. 2015, 2, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.G. Herpes simplex virus: Receptors and ligands for cell entry. Cell. Microbiol. 2004, 6, 401–410. [Google Scholar] [CrossRef]
- Zimmerman, O.; Holmes, A.C.; Kafai, N.M.; Adams, L.J.; Diamond, M.S. Entry receptors—The gateway to alphavirus infection. J. Clin. Investig. 2023, 133, e165307. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J.; Spear, P.G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 1998, 280, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.I.; Warner, M.S.; Lum, B.J.; Spear, P.G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 1996, 87, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Liu, J.; Blaiklock, P.; Shworak, N.W.; Bai, X.; Esko, J.D.; Cohen, G.H.; Eisenberg, R.J.; Rosenberg, R.D.; Spear, P.G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 1999, 99, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Haines, K.M.; Vande Burgt, N.H.; Francica, J.R.; Kaletsky, R.L.; Bates, P. Chinese hamster ovary cell lines selected for resistance to ebolavirus glycoprotein mediated infection are defective for NPC1 expression. Virology 2012, 432, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nagarajan, H.; Lewis, N.E.; Pan, S.; Cai, Z.; Liu, X.; Chen, W.; Xie, M.; Wang, W.; Hammond, S.; et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011, 29, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Yamano-Adachi, N.; Arishima, R.; Puriwat, S.; Omasa, T. Establishment of fast-growing serum-free immortalised cells from Chinese hamster lung tissues for biopharmaceutical production. Sci. Rep. 2020, 10, 17612. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Shukla, D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009, 276, 7228–7236. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Helenius, A. Virus entry at a glance. J. Cell Sci. 2013, 126, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Tarbutton, M.S.; Shukla, D. Diversity of heparan sulfate and HSV entry: Basic understanding and treatment strategies. Molecules 2015, 20, 2707–2727. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Partridge, L.J.; Urwin, L.; Nicklin, M.J.H.; James, D.C.; Green, L.R.; Monk, P.N. ACE2-Independent Interaction of SARS-CoV-2 Spike Protein with Human Epithelial Cells Is Inhibited by Unfractionated Heparin. Cells 2021, 10, 1419. [Google Scholar] [CrossRef]
- Yue, J.; Jin, W.; Yang, H.; Faulkner, J.; Song, X.; Qiu, H.; Teng, M.; Azadi, P.; Zhang, F.; Linhardt, R.J.; et al. Heparan Sulfate Facilitates Spike Protein-Mediated SARS-CoV-2 Host Cell Invasion and Contributes to Increased Infection of SARS-CoV-2 G614 Mutant and in Lung Cancer. Front. Mol. Biosci. 2021, 8, 649575. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Sztain, T.; Ahn, S.H.; Bogetti, A.T.; Casalino, L.; Goldsmith, J.A.; Seitz, E.; McCool, R.S.; Kearns, F.L.; Acosta-Reyes, F.; Maji, S.; et al. A glycan gate controls opening of the SARS-CoV-2 spike protein. Nat. Chem. 2021, 13, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xu, M.; Zhang, Q.; Chen, C.Z.; Guo, H.; Ye, Y.; Zheng, W.; Shen, M. Graph Convolutional Network-Based Screening Strategy for Rapid Identification of SARS-CoV-2 Cell-Entry Inhibitors. J. Chem. Inf. Model. 2022, 62, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pavlinov, I.; Ye, Y.; Zheng, W. Therapeutic development targeting host heparan sulfate proteoglycan in SARS-CoV-2 infection. Front. Med. 2024, 11, 1364657. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Kusche-Gullberg, M. Heparan Sulfate: Biosynthesis, Structure, and Function. Int. Rev. Cell Mol. Biol. 2016, 325, 215–273. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Powers, R.K.; Camacho, D.M.; Collins, J.J. Deep-Learning Resources for Studying Glycan-Mediated Host-Microbe Interactions. Cell Host Microbe 2021, 29, 132–144.e3. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chopra, P.; Tomris, I.; van der Woude, R.; Liu, L.; de Vries, R.P.; Boons, G.J. Well-Defined Heparin Mimetics Can Inhibit Binding of the Trimeric Spike of SARS-CoV-2 in a Length-Dependent Manner. JACS Au 2023, 3, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Jin, C.; Thomsson, K.A.; Karlsson, N.G.; Ives, C.M.; Fadda, E.; Bojar, D. Predicting glycan structure from tandem mass spectrometry via deep learning. Nat. Methods 2024, 21, 1206–1215. [Google Scholar] [CrossRef]
- Chang, K.; Baginski, J.; Hassan, S.F.; Volin, M.; Shukla, D.; Tiwari, V. Filopodia and Viruses: An Analysis of Membrane Processes in Entry Mechanisms. Front. Microbiol. 2016, 7, 300. [Google Scholar] [CrossRef]
- Chang, K.; Majmudar, H.; Tandon, R.; Volin, M.V.; Tiwari, V. Induction of Filopodia During Cytomegalovirus Entry Into Human Iris Stromal Cells. Front. Microbiol. 2022, 13, 834927. [Google Scholar] [CrossRef]
- Tiwari, V.; Shukla, D. Phosphoinositide 3 kinase signalling may affect multiple steps during herpes simplex virus type-1 entry. J. Gen. Virol. 2010, 91, 3002–3009. [Google Scholar] [CrossRef]
- Lehmann, M.J.; Sherer, N.M.; Marks, C.B.; Pypaert, M.; Mothes, W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 2005, 170, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Akhtar, J.; Desai, P.; Shukla, D. A role for heparan sulfate in viral surfing. Biochem. Biophys. Res. Commun. 2010, 391, 176–181. [Google Scholar] [CrossRef]
- Zhang, H.; Zang, C.; Xu, Z.; Zhang, Y.; Xu, J.; Bian, J.; Morozyuk, D.; Khullar, D.; Zhang, Y.; Nordvig, A.S.; et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat. Med. 2023, 29, 226–235. [Google Scholar] [CrossRef]
- Choudhary, S.; Burnham, L.; Thompson, J.M.; Shukla, D.; Tiwari, V. Role of Filopodia in HSV-1 Entry into Zebrafish 3-O-Sulfotransferase-3-Expressing Cells. Open Virol. J. 2013, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Chan, A.; Patil, C.; Tripathi, V.; Shadrack, D.M.; Jaishankar, D.; Hawkey, A.; Mungerson, M.S.; Shukla, D.; Tiwari, V. Archaic connectivity between the sulfated heparan sulfate and the herpesviruses—An evolutionary potential for cross-species interactions. Comput. Struct. Biotechnol. J. 2023, 21, 1030–1040. [Google Scholar] [CrossRef]
- LeBlanc, E.V.; Colpitts, C.C. The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus attachment inhibitor. Sci. Rep. 2022, 12, 12899. [Google Scholar] [CrossRef]
- Tan, C.W.; Gamage, A.M.; Yap, W.C.; Wei Tang, L.J.; Sun, Y.; Yang, X.L.; Pyke, A.; Chua, K.B.; Wang, L.F. Pteropine orthoreoviruses use cell surface heparan sulphate as an attachment receptor. Emerg. Microbes Infect. 2023, 12, 2208683. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Song, Y.; Xia, K.; He, P.; Zhang, F.; Chen, S.; Pouliot, R.; Weiss, D.J.; Tandon, R.; Bates, J.T.; et al. Heparan sulfates from bat and human lung and their binding to the spike protein of SARS-CoV-2 virus. Carbohydr. Polym. 2021, 260, 117797. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Liu, X.; Zhang, Y.; Wang, C.; Guo, Y. Attachment, Entry, and Intracellular Trafficking of Classical Swine Fever Virus. Viruses 2023, 15, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Dhondt, K.P.; Chalons, M.; Mely, S.; Raoul, H.; Negre, D.; Cosset, F.L.; Gerlier, D.; Vives, R.R.; Horvat, B. Heparan sulfate-dependent enhancement of henipavirus infection. mBio 2015, 6, e02427. [Google Scholar] [CrossRef]
- Sasaki, M.; Anindita, P.D.; Ito, N.; Sugiyama, M.; Carr, M.; Fukuhara, H.; Ose, T.; Maenaka, K.; Takada, A.; Hall, W.W.; et al. The Role of Heparan Sulfate Proteoglycans as an Attachment Factor for Rabies Virus Entry and Infection. J. Infect. Dis. 2018, 217, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Valero-Rello, A.; Baeza-Delgado, C.; Andreu-Moreno, I.; Sanjuan, R. Cellular receptors for mammalian viruses. PLoS Pathog. 2024, 20, e1012021. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Maginnis, M.S. Virus-Receptor Interactions: The Key to Cellular Invasion. J. Mol. Biol. 2018, 430, 2590–2611. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Sathiyamoorthy, K.; Chen, J.; Longnecker, R.; Jardetzky, T.S. The COMPLEXity in herpesvirus entry. Curr. Opin. Virol. 2017, 24, 97–104. [Google Scholar] [CrossRef]
- Gobeil, S.M.; Janowska, K.; McDowell, S.; Mansouri, K.; Parks, R.; Manne, K.; Stalls, V.; Kopp, M.F.; Henderson, R.; Edwards, R.J.; et al. D614G Mutation Alters SARS-CoV-2 Spike Conformation and Enhances Protease Cleavage at the S1/S2 Junction. Cell Rep. 2021, 34, 108630. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Xiao, T.; Lu, J.; Peng, H.; Sterling, S.M.; Walsh, R.M., Jr.; Rits-Volloch, S.; Zhu, H.; Woosley, A.N.; et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science 2021, 372, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, V.; Wrobel, A.G.; Toporowska, J.; Hammerschmid, D.; Doores, K.J.; Bradshaw, R.T.; Parsons, R.B.; Benton, D.J.; Roustan, C.; Reading, E.; et al. Structural dynamics in the evolution of SARS-CoV-2 spike glycoprotein. Nat. Commun. 2023, 14, 1421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Cohen, S.; Zhao, M.; Madeleine, M.; Payne, T.H.; Lybrand, T.P.; Geraghty, D.E.; Jerome, K.R.; Corey, L. Using Haplotype-Based Artificial Intelligence to Evaluate SARS-CoV-2 Novel Variants and Mutations. JAMA Netw. Open 2023, 6, e230191. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Y.; Pan, W.; Xue, Q.; Fu, J.; Qu, G.; Zhang, A. Exogenous Chemicals Impact Virus Receptor Gene Transcription: Insights from Deep Learning. Environ. Sci. Technol. 2023, 57, 18038–18047. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef]
- Bambace, C.; Dahlman, I.; Arner, P.; Kulyte, A. NPC1 in human white adipose tissue and obesity. BMC Endocr. Disord. 2013, 13, 5. [Google Scholar] [CrossRef]
- Al-Benna, S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Cast, N.; Udawatte, S.; Adhikari, K.; Payen, S.H.; Verma, S.C.; Shukla, D.; Swanson-Mungerson, M.; Tiwari, V. Co-Expression of Niemann-Pick Type C1-Like1 (NPC1L1) with ACE2 Receptor Synergistically Enhances SARS-CoV-2 Entry and Fusion. Biomedicines 2024, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.C.; Damen, M.; Madan, R.; Deepe, G.S., Jr.; Spearman, P.; Way, S.S.; Divanovic, S. Adipocyte inflammation and pathogenesis of viral pneumonias: An overlooked contribution. Mucosal Immunol. 2021, 14, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Sialobiology of influenza: Molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 2005, 28, 399–408. [Google Scholar] [CrossRef]
- Rogers, G.N.; Paulson, J.C.; Daniels, R.S.; Skehel, J.J.; Wilson, I.A.; Wiley, D.C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983, 304, 76–78. [Google Scholar] [CrossRef]
- Lucas, T.M.; Gupta, C.; Altman, M.O.; Sanchez, E.; Naticchia, M.R.; Gagneux, P.; Singharoy, A.; Godula, K. Mucin-mimetic glycan arrays integrating machine learning for analyzing receptor pattern recognition by influenza A viruses. Chem 2021, 7, 3393–3411. [Google Scholar] [CrossRef]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ren, L.; Cai, P.; Zhang, Y.; Ding, H.; Deng, K.; Yu, X.; Lin, H.; Huang, C. Accurately identifying hemagglutinin using sequence information and machine learning methods. Front. Med. 2023, 10, 1281880. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus-sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.L.; Chlanda, P. The Art of Viral Membrane Fusion and Penetration. Subcell. Biochem. 2023, 106, 113–152. [Google Scholar] [CrossRef]
- White, J.M.; Ward, A.E.; Odongo, L.; Tamm, L.K. Viral Membrane Fusion: A Dance Between Proteins and Lipids. Annu. Rev. Virol. 2023, 10, 139–161. [Google Scholar] [CrossRef]
- Dalgleish, A.G.; Beverley, P.C.; Clapham, P.R.; Crawford, D.H.; Greaves, M.F.; Weiss, R.A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 1984, 312, 763–767. [Google Scholar] [CrossRef]
- Wyatt, R.; Sodroski, J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science 1998, 280, 1884–1888. [Google Scholar] [CrossRef]
- Dragic, T.; Litwin, V.; Allaway, G.P.; Martin, S.R.; Huang, Y.; Nagashima, K.A.; Cayanan, C.; Maddon, P.J.; Koup, R.A.; Moore, J.P.; et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996, 381, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef] [PubMed]
- von Itzstein, M.; Thomson, R. Anti-influenza drugs: The development of sialidase inhibitors. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 111–154. [Google Scholar] [CrossRef]
- Yi, Y.; Ni, X.C.; Liu, G.; Yin, Y.R.; Huang, J.L.; Gan, W.; Zhou, P.Y.; Guan, R.Y.; Zhou, C.; Sun, B.Y.; et al. Clinical significance of herpes virus entry mediator expression in hepatitis B virus-related hepatocellular carcinoma. Oncol. Lett. 2020, 20, 19. [Google Scholar] [CrossRef]
- Mikulicic, S.; Finke, J.; Boukhallouk, F.; Wustenhagen, E.; Sons, D.; Homsi, Y.; Reiss, K.; Lang, T.; Florin, L. ADAM17-dependent signaling is required for oncogenic human papillomavirus entry platform assembly. Elife 2019, 8, e44345. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.J.; Bilawchuk, L.; Nish, G.; Hegele, R.G. Virus-induced signaling influences endosome trafficking, virus entry, and replication. Methods Enzymol. 2014, 534, 65–76. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhou, X.; Li, L.; Liu, Q.; Wang, Z.; Bai, X.; Zhao, Y.; Shi, H.; Zhang, X.; et al. The oncoprotein HBXIP enhances migration of breast cancer cells through increasing filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer Lett. 2014, 355, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Lidke, D.S.; Ozbun, M.A. Virus activated filopodia promote human papillomavirus type 31 uptake from the extracellular matrix. Virology 2008, 381, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Shum, D.; Konig, A.; Kim, H.; Heo, J.; Min, S.; Lee, J.; Ko, Y.; Choi, I.; Lee, H.; et al. High-throughput drug screening using the Ebola virus transcription- and replication-competent virus-like particle system. Antivir. Res. 2018, 158, 226–237. [Google Scholar] [CrossRef]
- Dicker, I.B.; Blasecki, J.W.; Seetharam, S. Herpes simplex type1:lacZ recombinant viruses. II. Microtiter plate-based colorimetric assays for the discovery of new antiherpes agents and the points at which such agents disrupt the viral replication cycle. Antivir. Res. 1995, 28, 213–224. [Google Scholar] [CrossRef]
- Clement, C.; Tiwari, V.; Scanlan, P.M.; Valyi-Nagy, T.; Yue, B.Y.; Shukla, D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 2006, 174, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.B.; Mazelier, M.; Leger, P.; Lozach, P.Y. Deciphering Virus Entry with Fluorescently Labeled Viral Particles. Methods Mol. Biol. 2018, 1836, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Shukla, D. Nonprofessional phagocytosis can facilitate herpesvirus entry into ocular cells. Clin. Dev. Immunol. 2012, 2012, 651691. [Google Scholar] [CrossRef]
- Kincaid, R.P.; Sullivan, C.S. Virus-encoded microRNAs: An overview and a look to the future. PLoS Pathog. 2012, 8, e1003018. [Google Scholar] [CrossRef] [PubMed]
- Boulant, S.; Stanifer, M.; Lozach, P.Y. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses 2015, 7, 2794–2815. [Google Scholar] [CrossRef]
- Sodhi, A.; Montaner, S.; Gutkind, J.S. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat. Rev. Mol. Cell Biol. 2004, 5, 998–1012. [Google Scholar] [CrossRef]
- Maginnis, M.S. beta-arrestins and G protein-coupled receptor kinases in viral entry: A graphical review. Cell. Signal. 2023, 102, 110558. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021, 31, 818–820. [Google Scholar] [CrossRef]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. NPJ Vaccines 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. 2019, 28, 73–81. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, K.; Zhan, W.; Deng, L. Targeting Virus-host Protein Interactions: Feature Extraction and Machine Learning Approaches. Curr. Drug Metab. 2019, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Agyenkwa-Mawuli, K.; Agyapong, O.; Wilson, M.D.; Kwofie, S.K. EBOLApred: A machine learning-based web application for predicting cell entry inhibitors of the Ebola virus. Comput. Biol. Chem. 2022, 101, 107766. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Gawriljuk, V.O.; Zin, P.P.K.; Puhl, A.C.; Zorn, K.M.; Foil, D.H.; Lane, T.R.; Hurst, B.; Tavella, T.A.; Costa, F.T.M.; Lakshmanane, P.; et al. Machine Learning Models Identify Inhibitors of SARS-CoV-2. J. Chem. Inf. Model. 2021, 61, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yang, X.; Yang, S.; Zhang, Z. Current status and future perspectives of computational studies on human-virus protein-protein interactions. Brief. Bioinform. 2021, 22, bbab029. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Zhang, Q.R.; Kong, X.; Zhang, L.; Zhang, Y.; Tang, Y.; Xu, H. Machine learning prediction of antiviral-HPV protein interactions for anti-HPV pharmacotherapy. Sci. Rep. 2021, 11, 24367. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wojtczak, D. Dive into machine learning algorithms for influenza virus host prediction with hemagglutinin sequences. Biosystems 2022, 220, 104740. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, S.; Ren, P.; Wuchty, S.; Zhang, Z. Deep Learning-Powered Prediction of Human-Virus Protein-Protein Interactions. Front. Microbiol. 2022, 13, 842976. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.C.; Vaisman, I.I. Respiratory Syncytial Virus Vaccine Design Using Structure-Based Machine-Learning Models. Viruses 2024, 16, 821. [Google Scholar] [CrossRef]
- Petkidis, A.; Andriasyan, V.; Greber, U.F. Machine learning for cross-scale microscopy of viruses. Cell Rep. Methods 2023, 3, 100557. [Google Scholar] [CrossRef]
- Pethe, M.A.; Rubenstein, A.B.; Khare, S.D. Data-driven supervised learning of a viral protease specificity landscape from deep sequencing and molecular simulations. Proc. Natl. Acad. Sci. USA 2019, 116, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Shiaelis, N.; Tometzki, A.; Peto, L.; McMahon, A.; Hepp, C.; Bickerton, E.; Favard, C.; Muriaux, D.; Andersson, M.; Oakley, S.; et al. Virus Detection and Identification in Minutes Using Single-Particle Imaging and Deep Learning. ACS Nano 2023, 17, 697–710. [Google Scholar] [CrossRef]
- Nadkarni, P.M.; Ohno-Machado, L.; Chapman, W.W. Natural language processing: An introduction. J. Am. Med. Inform. Assoc. 2011, 18, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lo-Ciganic, W.H.; Huang, J.; Wu, Y.; Henry, L.; Peter, J.; Sulkowski, M.; Nelson, D.R. Machine learning algorithms for predicting direct-acting antiviral treatment failure in chronic hepatitis C: An HCV-TARGET analysis. Hepatology 2022, 76, 483–491. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Forum on Microbial Threats. The National Academies Collection: Reports funded by National Institutes of Health. In Big Data and Analytics for Infectious Disease Research, Operations, and Policy: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Corsi, A.; de Souza, F.F.; Pagani, R.N.; Kovaleski, J.L. Big data analytics as a tool for fighting pandemics: A systematic review of literature. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 9163–9180. [Google Scholar] [CrossRef] [PubMed]
- Pun, F.W.; Ozerov, I.V.; Zhavoronkov, A. AI-powered therapeutic target discovery. Trends Pharmacol. Sci. 2023, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Rajput, A.; Rastogi, A.; Thakur, A.; Kumar, M. Targeting non-structural proteins of Hepatitis C virus for predicting repurposed drugs using QSAR and machine learning approaches. Comput. Struct. Biotechnol. J. 2022, 20, 3422–3438. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi Arshadi, A.; Webb, J.; Salem, M.; Cruz, E.; Calad-Thomson, S.; Ghadirian, N.; Collins, J.; Diez-Cecilia, E.; Kelly, B.; Goodarzi, H.; et al. Artificial Intelligence for COVID-19 Drug Discovery and Vaccine Development. Front. Artif. Intell. 2020, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.H.; Webber, J.; Mehbodniya, A. Decision tree based ensemble machine learning model for the prediction of Zika virus T-cell epitopes as potential vaccine candidates. Sci. Rep. 2022, 12, 7810. [Google Scholar] [CrossRef]
- Bzhalava, Z.; Tampuu, A.; Bala, P.; Vicente, R.; Dillner, J. Machine Learning for detection of viral sequences in human metagenomic datasets. BMC Bioinform. 2018, 19, 336. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Wang, K.; Gao, Y.; Li, G.; Baptista-Hon, D.T.; Yang, X.H.; Xue, K.; Tai, W.H.; Jiang, Z.; et al. Deep-learning-enabled protein-protein interaction analysis for prediction of SARS-CoV-2 infectivity and variant evolution. Nat. Med. 2023, 29, 2007–2018. [Google Scholar] [CrossRef]
- Pillai, N.; Ramkumar, M.; Nanduri, B. Artificial Intelligence Models for Zoonotic Pathogens: A Survey. Microorganisms 2022, 10, 1911. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Speiser, J.L.; Miller, M.E.; Tooze, J.; Ip, E. A Comparison of Random Forest Variable Selection Methods for Classification Prediction Modeling. Expert Syst. Appl. 2019, 134, 93–101. [Google Scholar] [CrossRef]
- Montomoli, J.; Romeo, L.; Moccia, S.; Bernardini, M.; Migliorelli, L.; Berardini, D.; Donati, A.; Carsetti, A.; Bocci, M.G.; Wendel Garcia, P.D.; et al. Machine learning using the extreme gradient boosting (XGBoost) algorithm predicts 5-day delta of SOFA score at ICU admission in COVID-19 patients. J. Intensive Med. 2021, 1, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Petkidis, A.; Andriasyan, V.; Murer, L.; Volle, R.; Greber, U.F. A versatile automated pipeline for quantifying virus infectivity by label-free light microscopy and artificial intelligence. Nat. Commun. 2024, 15, 5112. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, R.; Zhang, X.; Sun, Y.; Zhang, H.; Zhao, Z.; Zheng, Y.; Luo, J.; Zhang, J.; Wu, H.; et al. A deep learning model and human-machine fusion for prediction of EBV-associated gastric cancer from histopathology. Nat. Commun. 2022, 13, 2790. [Google Scholar] [CrossRef]
- Lee, J.S.; Yun, J.; Ham, S.; Park, H.; Lee, H.; Kim, J.; Byeon, J.S.; Jung, H.Y.; Kim, N.; Kim, D.H. Machine learning approach for differentiating cytomegalovirus esophagitis from herpes simplex virus esophagitis. Sci. Rep. 2021, 11, 3672. [Google Scholar] [CrossRef]
- Natarajan, R.; Matai, H.D.; Raman, S.; Kumar, S.; Ravichandran, S.; Swaminathan, S.; Rani Alex, J.S. Advances in the diagnosis of herpes simplex stromal necrotising keratitis: A feasibility study on deep learning approach. Indian J. Ophthalmol. 2022, 70, 3279–3283. [Google Scholar] [CrossRef]
- Dong, L.; He, W.; Zhang, R.; Ge, Z.; Wang, Y.X.; Zhou, J.; Xu, J.; Shao, L.; Wang, Q.; Yan, Y.; et al. Artificial Intelligence for Screening of Multiple Retinal and Optic Nerve Diseases. JAMA Netw. Open 2022, 5, e229960. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Blazes, M.S.; Lee, C.S. Imaging Amyloid and Tau in the Retina: Current Research and Future Directions. J. Neuroophthalmol. 2023, 43, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Andriasyan, V.; Yakimovich, A.; Petkidis, A.; Georgi, F.; Witte, R.; Puntener, D.; Greber, U.F. Microscopy deep learning predicts virus infections and reveals mechanics of lytic-infected cells. iScience 2021, 24, 102543. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Barratt, J.L.N.; Kennedy, P.J.; Kaufer, A.; Calarco, L.; Ellis, J.T. Machine learning and applications in microbiology. FEMS Microbiol. Rev. 2021, 45, fuab015. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, J.; Huang, D.; Liu, Y.; Li, D.D. Machine Learning Advances in Microbiology: A Review of Methods and Applications. Front. Microbiol. 2022, 13, 925454. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ye, G.; Jiang, Y.; Wang, Z.; Yu, H.; Yang, M. Artificial Intelligence in battling infectious diseases: A transformative role. J. Med. Virol. 2024, 96, e29355. [Google Scholar] [CrossRef]
- Colubri, A.; Silver, T.; Fradet, T.; Retzepi, K.; Fry, B.; Sabeti, P. Transforming Clinical Data into Actionable Prognosis Models: Machine-Learning Framework and Field-Deployable App to Predict Outcome of Ebola Patients. PLoS Neglected Trop. Dis. 2016, 10, e0004549. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Adams, J.; Broni, E.; Enninful, K.S.; Agoni, C.; Soliman, M.E.S.; Wilson, M.D. Artificial Intelligence, Machine Learning, and Big Data for Ebola Virus Drug Discovery. Pharmaceuticals 2023, 16, 332. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Malik, A.A.; Chotpatiwetchkul, W.; Phanus-Umporn, C.; Nantasenamat, C.; Charoenkwan, P.; Shoombuatong, W. StackHCV: A web-based integrative machine-learning framework for large-scale identification of hepatitis C virus NS5B inhibitors. J. Comput. Aided Mol. Des. 2021, 35, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hao, J.; Peng, L.; Duan, H.; Luo, Q.; Yan, H.; Wan, H.; Hu, Y.; Liang, L.; Xie, Z.; et al. Classification and Design of HIV-1 Integrase Inhibitors Based on Machine Learning. Comput. Math. Methods Med. 2021, 2021, 5559338. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Du, J.; Fujimoto, K.; Li, F.; Schneider, J.; Tao, C. Application of artificial intelligence and machine learning for HIV prevention interventions. Lancet HIV 2022, 9, e54–e62. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.M.; Nikolaev, G.I.; Shuldov, N.A.; Bosko, I.P.; Anischenko, A.I.; Tuzikov, A.V. Application of deep learning and molecular modeling to identify small drug-like compounds as potential HIV-1 entry inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 7555–7573. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Zemgulyte, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science 2023, 381, eadg7492. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, H.; Ma, L.; Xu, Y.; Gan, J.; Fan, Z.; Yang, F.; Ma, K.; Yang, J.; Bai, S.; et al. Advancing COVID-19 Diagnosis with Privacy-Preserving Collaboration in Artificial Intelligence. Nat. Mach. Intell. 2021, 3, 1081–1089. [Google Scholar] [CrossRef]
- Chadaga, K.; Prabhu, S.; Sampathila, N.; Chadaga, R.; Umakanth, S.; Bhat, D.; GS, S.K. Explainable artificial intelligence approaches for COVID-19 prognosis prediction using clinical markers. Sci. Rep. 2024, 14, 1783. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, J. UniBind: A novel artificial intelligence-based prediction model for SARS-CoV-2 infectivity and variant evolution. Signal Transduct. Target. Ther. 2023, 8, 464. [Google Scholar] [CrossRef]
- Li, F.; He, H. Assessing the Accuracy of Diagnostic Tests. Shanghai Arch. Psychiatry 2018, 30, 207–212. [Google Scholar] [CrossRef]
- Stebbing, J.; Krishnan, V.; de Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020, 12, e12697. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Chan, M.; Vijay, S.; McNevin, J.; McElrath, M.J.; Holland, E.C.; Gujral, T.S. Machine learning identifies molecular regulators and therapeutics for targeting SARS-CoV2-induced cytokine release. Mol. Syst. Biol. 2021, 17, e10426. [Google Scholar] [CrossRef]
- Kao, H.J.; Weng, T.H.; Chen, C.H.; Chen, Y.C.; Huang, K.Y.; Weng, S.L. iDVEIP: A computer-aided approach for the prediction of viral entry inhibitory peptides. Proteomics 2024, 24, e2300257. [Google Scholar] [CrossRef]
- Dey, L.; Chakraborty, S.; Mukhopadhyay, A. Machine learning techniques for sequence-based prediction of viral-host interactions between SARS-CoV-2 and human proteins. Biomed. J. 2020, 43, 438–450. [Google Scholar] [CrossRef]
- Elend, L.; Jacobsen, L.; Cofala, T.; Prellberg, J.; Teusch, T.; Kramer, O.; Solov’yov, I.A. Design of SARS-CoV-2 Main Protease Inhibitors Using Artificial Intelligence and Molecular Dynamic Simulations. Molecules 2022, 27, 4020. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Topol, E. Solving the puzzle of Long Covid. Science 2024, 383, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, S.; Gao, G.F.; Shi, W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature 2021, 600, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Nachega, J.B.; Nsanzimana, S.; Rawat, A.; Wilson, L.A.; Rosenthal, P.J.; Siedner, M.J.; Varma, J.K.; Kilmarx, P.H.; Mutesa, L.; Tanner, M.; et al. Advancing detection and response capacities for emerging and re-emerging pathogens in Africa. Lancet Infect. Dis. 2023, 23, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Rader, B.; Astley, C.M.; Tian, H. Advances in Artificial Intelligence for Infectious-Disease Surveillance. N. Engl. J. Med. 2023, 388, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020, 177, 104759. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Beer, J.C.; Sankaranarayanan, N.V.; Swanson-Mungerson, M.; Desai, U.R. Discovering small-molecule therapeutics against SARS-CoV-2. Drug Discov. Today 2020, 25, 1535–1544. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Thadani, N.N.; Gurev, S.; Notin, P.; Youssef, N.; Rollins, N.J.; Ritter, D.; Sander, C.; Gal, Y.; Marks, D.S. Learning from prepandemic data to forecast viral escape. Nature 2023, 622, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Adesola, R.O.; Miranda, A.V.; Tran, Y.S.J.; Idris, I.; Lin, X.; Kouwenhoven, M.B.N.; Lucero-Prisno, D.E., 3rd. Langya virus outbreak: Current challenges and lesson learned from previous henipavirus outbreaks in China, Australia, and Southeast Asia. Bull. Natl. Res. Cent. 2023, 47, 87. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Mensink, M.; Begeman, L.; Embregts, C.; de Vrijer, M.; De Baerdemaeker, A.; Scheuer, R.; Vuong, O.; Fouchier, R.A.M.; Kuiken, T. Highly Pathogenic Avian Influenza Virus (H5n8) Outbreak in a Wild Bird Rescue Center, the Netherlands: Consequences and Recommendations. J. Zoo Wildl. Med. 2022, 53, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah virus encephalitis reemergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef]
- Makoni, M. Ebola outbreak in DR Congo. Lancet 2022, 399, 1766. [Google Scholar] [CrossRef]
- Saba Villarroel, P.M.; Gumpangseth, N.; Songhong, T.; Yainoy, S.; Monteil, A.; Leaungwutiwong, P.; Misse, D.; Wichit, S. Emerging and re-emerging zoonotic viral diseases in Southeast Asia: One Health challenge. Front. Public Health 2023, 11, 1141483. [Google Scholar] [CrossRef]
- Fischhoff, I.R.; Castellanos, A.A.; Rodrigues, J.; Varsani, A.; Han, B.A. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proc. Biol. Sci. 2021, 288, 20211651. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Amelio, A.; Merla, A.; Scozzari, F. A survey on the role of artificial intelligence in managing Long COVID. Front. Artif. Intell. 2023, 6, 1292466. [Google Scholar] [CrossRef]
- Cau, R.; Faa, G.; Nardi, V.; Balestrieri, A.; Puig, J.; Suri, J.S.; SanFilippo, R.; Saba, L. Long-COVID diagnosis: From diagnostic to advanced AI-driven models. Eur. J. Radiol. 2022, 148, 110164. [Google Scholar] [CrossRef]
- Frey, L.J.; Talbert, D.A. Artificial Intelligence Pipeline to Bridge the Gap between Bench Researchers and Clinical Researchers in Precision Medicine. Med. One 2020, 5. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Wong, F.; de la Fuente-Nunez, C.; Collins, J.J. Leveraging artificial intelligence in the fight against infectious diseases. Science 2023, 381, 164–170. [Google Scholar] [CrossRef] [PubMed]




| AI Applications in the Field of Virus-Host Cell Interactions | References |
|---|---|
| Understanding and predicting key components of viral proteins facilitating cell entry | [52,92,93,94,95,96,97,98,99] |
| Predictions about the commonality and diversity among the viral entry receptors | [44] |
| Glycan immunogenicity and pathogenicity, and glycan-mediated immune evasion | [27,28,29] |
| Glycan motifs involved in virus-host cell interactions | [20,27,28,29,43] |
| Understanding viral tropism, pathogenesis, and cross-species transmissibility | [44,61,62,63,114,115,168] |
| Designing of novel antiviral drugs targeting viral entry and vaccine design | [100,110,145] |
| Identifying molecular regulators and therapeutics for targeting virus induced cytokine release | [144] |
| Laboratory diagnostics, drug screening, serum neutralization | [120] |
| Predictions for treatment failure with the antiviral drugs | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elste, J.; Saini, A.; Mejia-Alvarez, R.; Mejía, A.; Millán-Pacheco, C.; Swanson-Mungerson, M.; Tiwari, V. Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions. Biomolecules 2024, 14, 911. https://doi.org/10.3390/biom14080911
Elste J, Saini A, Mejia-Alvarez R, Mejía A, Millán-Pacheco C, Swanson-Mungerson M, Tiwari V. Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions. Biomolecules. 2024; 14(8):911. https://doi.org/10.3390/biom14080911
Chicago/Turabian StyleElste, James, Akash Saini, Rafael Mejia-Alvarez, Armando Mejía, Cesar Millán-Pacheco, Michelle Swanson-Mungerson, and Vaibhav Tiwari. 2024. "Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions" Biomolecules 14, no. 8: 911. https://doi.org/10.3390/biom14080911
APA StyleElste, J., Saini, A., Mejia-Alvarez, R., Mejía, A., Millán-Pacheco, C., Swanson-Mungerson, M., & Tiwari, V. (2024). Significance of Artificial Intelligence in the Study of Virus–Host Cell Interactions. Biomolecules, 14(8), 911. https://doi.org/10.3390/biom14080911






