Complete Plastid Genome Sequences of Four Salsoleae s.l. Species: Comparative and Phylogenetic Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and DNA Extraction
2.2. Library Construction and De Novo Sequencing
2.3. Genome Assembly and Annotation
2.4. Simple Sequence Repeats (SSRs) and Long Repetitive Sequence Analysis
2.5. Nucleotide Diversity and Ka/Ks Ratio Analysis
2.6. Phylogenetic Analyses
3. Results
3.1. The Features of the S. foliosa, S. tragus, P. affinis, and X. richteri Plastid Genomes
3.2. Repeat Sequences Analysis
3.3. Sliding Window Analysis
3.4. Substitution Rate of Protein-Coding Genes of Salsoleae s.l. Species
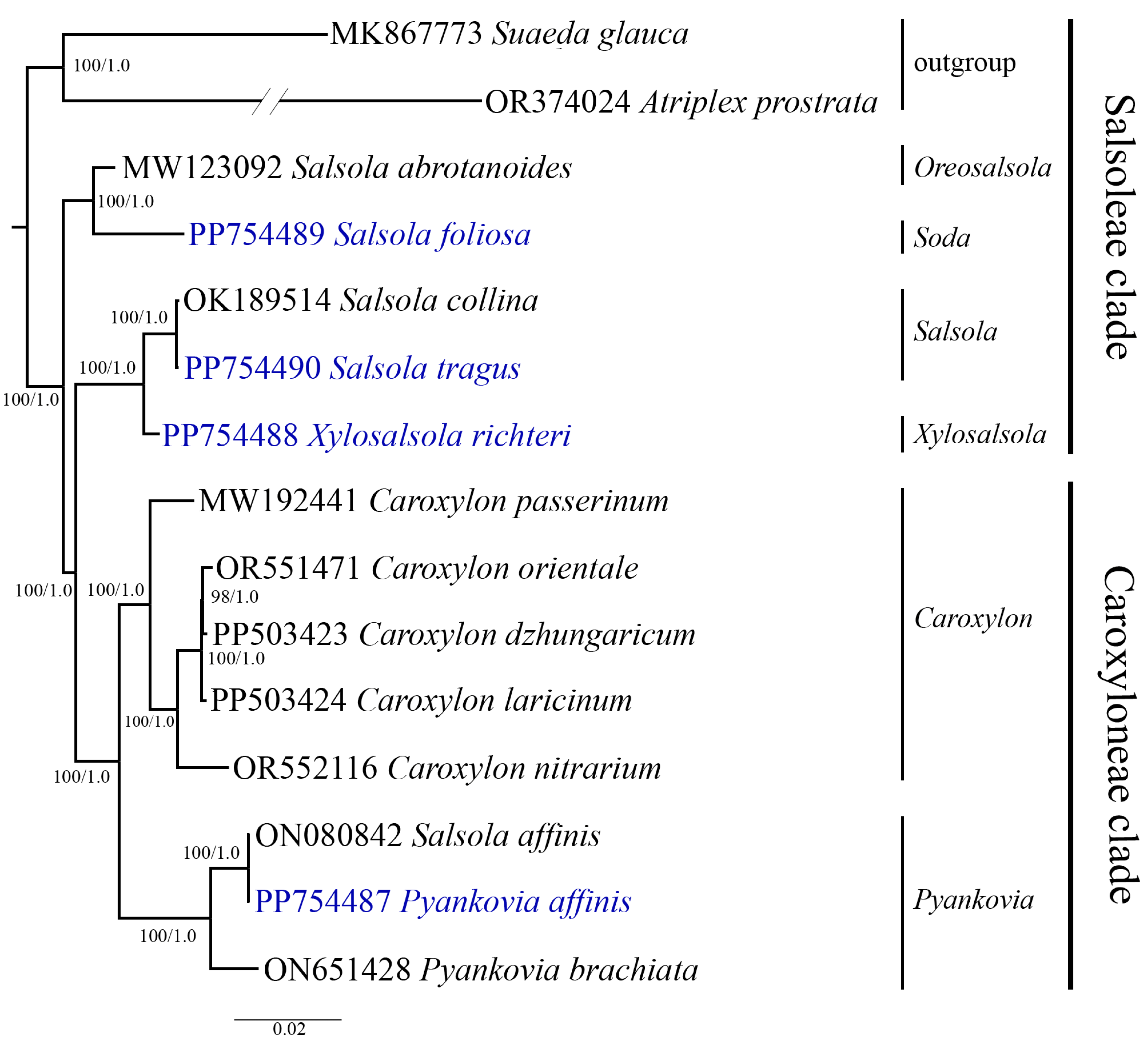
3.5. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadereit, G.; Borsch, T.; Weising, K.; Freitag, H. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int. J. Plant Sci. 2003, 164, 959–986. [Google Scholar] [CrossRef]
- Latałowa, M.; Kupryjanowicz, M.; Nalepka, D. Chenopodiaceae–Goosefoot family. In Late Glacial and Holocene History of Vegetation in Poland Based on Isopollen Maps; Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2004; pp. 273–281. [Google Scholar]
- Mucina, L. Caroxylon (Chenopodiaceae s. str.) in continental southern Africa and Madagascar: A preliminary nomenclatural synopsis and biogeographical considerations. Phytotaxa 2017, 312, 151–178. [Google Scholar] [CrossRef]
- Bochantsev, V.P. The genus Salsola L., a brief history of its development and settlement. Bot. J. 1969, 54, 989–1001. (In Russian) [Google Scholar]
- Kühn, U.; Bittrich, V.; Carolin, R.; Freitag, H.; Hedge, I.C.; Uotila, P.; Wilson, P.G. Chenopodiaceae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 1993; pp. 253–281. [Google Scholar]
- Freitag, H. Salsola L. (Chenopodiaceae). In Flora Iranica; Rechinger, K.N., Ed.; Akadesche Druck-u. Verlagsanstalt: Graz, Austria, 1997; Volume 173, pp. 154–255. [Google Scholar]
- Willis, J.C.; Airy Shaw, H.G. A Dictionary of the Flowering Plants and Ferns, 8th ed.; Cambridge University Press: Cambridge, UK, 1973; p. 209. [Google Scholar]
- Abtahi, M.; Zandi Esfahan, E. Effects of phenological stage on forage quality of halophyte species Salsola arbuscula Pall. in the central desert of Iran. Appl. Ecol. Environ. Res. 2017, 15, 3. [Google Scholar] [CrossRef]
- Murshid, S.S.; Atoum, D.; Abou-Hussein, D.R.; Abdallah, H.M.; Hareeri, R.H.; Almukadi, H.; Edrada-Ebel, R. Genus Salsola: Chemistry, biological activities and future prospective—A review. Plants 2022, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Akhani, H.; Edwards, G.; Roalson, E.H. Diversification of the old world Salsoleae sl (Chenopodiaceae): Molecular phylogenetic analysis of nuclear and chloroplast data sets and a revised classification. Int. J. Plant Sci. 2007, 168, 931–956. [Google Scholar] [CrossRef]
- Wen, Z.B.; Zhang, M.L.; Zhu, G.L.; Sanderson, S.C. Phylogeny of Salsoleae sl (Chenopodiaceae) based on DNA sequence data from ITS, psbB–psbH, and rbcL, with emphasis on taxa of northwestern China. Plant Syst. Evol. 2010, 288, 25–42. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 11 May 2024).
- Wei, Y.; Dong, M.; Huang, Z.; Tan, D. Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 134–140. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Mirhosseini, A.; Akram, N.A.; Hasanuzzaman, M. Forage potential of Salsola species in arid-saline rangelands. Turk. J. Bot. 2021, 45, 203–215. [Google Scholar] [CrossRef]
- Dianati Tilaki, G.; Haidarian Aghakhani, M.; Filehkesh, E.; Naghipour Borj, A.A. Investigation on the effects of phenological stages on forage quality and soluble carbohydrates in Salsola arbuscula and Salsola richteri species in saline rangelands of sabzevar. Iran. J. Range Desert Res. 2012, 18, 652–661. [Google Scholar]
- Altay, V.; Ozturk, M. The genera Salsola and Suaeda (Amaranthaceae) and their value as fodder. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–12. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Passalacqua, N.G.; Peruzzi, L.; Menichini, F. In vitro angiotensin converting enzyme inhibiting activity of Salsola oppositifolia Desf., Salsola soda L. and Salsola tragus L. Nat. Prod. Res. 2007, 21, 846–851. [Google Scholar] [CrossRef] [PubMed]
- ElNaggar, M.H.; Eldehna, W.M.; Abourehab, M.A.; Abdel Bar, F.M. The old world Salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: A review. J. Enzym. Inhib. Med. Chem. 2022, 37, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Pyankov, V.I.; Artyusheva, E.G.; Edwards, G.E.; Black, C.C., Jr.; Soltis, P.S. Phylogenetic analysis of tribe Salsoleae (Chenopodiaceae) based on ribosomal ITS sequences: Implications for the evolution of photosynthesis types. Am. J. Bot. 2001, 88, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Borger, C.P.; Yan, G.; Scott, J.K.; Walsh, M.J.; Powles, S.B. Salsola tragus or S. australis (Chenopodiaceae) in Australia—Untangling taxonomic confusion through molecular and cytological analyses. Aust. J. Bot. 2008, 56, 600–608. [Google Scholar] [CrossRef]
- Saeed, A.S.; Saeidi, H.; Baghestani, M.N.; Mirhosseini, A. The taxonomy of the genus Salsola (Chenopodiaceae) in Yazd province. J. Taxon. Biosist. 2016, 8, 51–60. [Google Scholar]
- Mosyakin, S.L. Taxonomic and nomenclatural notes on Pontic-Mediterranean coastal and some Australasian taxa of Salsola (Chenopodiaceae). Ukr. Bot. J. 2017, 74, 521–531. [Google Scholar] [CrossRef]
- Gao, T.P.; Gao, H.N.; Zhang, Y.; Xu, Y.; An, L.Z. Genetic diversity of Salsola passerina populations in northwestern China based on inter-simple sequence repeat (ISSR). J. Lanzhou Univ. Nat. Sci. 2009, 45, 66–74. [Google Scholar]
- Alotaibi, M.O.; Abd-Elgawad, M.E. ISSR and SCoT for evaluation of hereditary differences of 29 wild plants in Al Jubail Saudi Arabian. Saudi J. Biol. Sci. 2022, 29, 3223–3231. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M. Characterization of four Salsola species and their genetic relationship by AFLP. Pak. J. Bot. 2016, 48, 1183–1187. [Google Scholar]
- McGray, H.G.; Ayres, D.R.; Sloop, C.M.; Lee, A.K. Beta SSR loci cross-amplify in five Salsola taxa. Mol. Ecol. Resour. 2008, 8, 608–611. [Google Scholar] [CrossRef]
- Almerekova, S.; Favarisova, N.; Turuspekov, Y.; Abugalieva, S. Cross-Genera Transferability of Microsatellite Markers and Phylogenetic Assessment of Three Salsola Species from Western Kazakhstan. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2020, 74, 325–334. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Raubeson, L.A.; Boore, J.L.; DePamphilis, C.W.; Chumley, T.W.; Haberle, R.C.; Wyman, S.K.; Alverson, A.J.; Peery, R.; Herman, S.J.; et al. Methods for obtaining and analyzing whole chloroplast genome sequences. Methods Enzymol. 2005, 395, 348–384. [Google Scholar]
- Yu, X.Q.; Drew, B.T.; Yang, J.B.; Gao, L.M.; Li, D.Z. Comparative chloroplast genomes of eleven Schima (Theaceae) species: Insights into DNA barcoding and phylogeny. PLoS ONE 2017, 12, e0178026. [Google Scholar] [CrossRef]
- Ahmed, I.; Matthews, P.J.; Biggs, P.J.; Naeem, M.; McLenachan, P.A.; Lockhart, P.J. Identification of chloroplast genome loci suitable for high-resolution phylogeographic studies of Colocasia esculenta (L.) Schott (Araceae) and closely related taxa. Mol. Ecol. Resour. 2013, 13, 929–937. [Google Scholar] [CrossRef]
- Bilgen, B.B.; Kaya, N. Genetic diversity among Pinus sylvestris L. populations and its implications for genetic conservation: Comparison of nuclear and chloroplast microsatellite markers. Fresenius Environ. Bull. 2017, 26, 6873–6881. [Google Scholar]
- Jayaswall, K.; Sharma, H.; Jayaswal, D.; Sagar, R.; Bhandawat, A.; Kumar, A.; Singh, M. Development of chloroplast derived SSR markers for genus Allium and their characterization in the allies for genetic improvement of Alliums. S. Afr. J. Bot. 2023, 162, 304–313. [Google Scholar] [CrossRef]
- Ginwal, H.S.; Chauhan, P.; Barthwal, S.; Sharma, A.; Sharma, R. Short Note: Cross-Species Amplification and Characterization of Chloroplast Microsatellite Markers in Roxb. Silvae Genet. 2011, 60, 65–69. [Google Scholar] [CrossRef][Green Version]
- Żukowska, W.B.; Wójkiewicz, B.; Litkowiec, M.; Wachowiak, W. Cross-amplification and multiplexing of cpSSRs and nSSRs in two closely related pine species (Pinus sylvestris L. and P. mugo Turra). Dendrobiology 2017, 77, 59–64. [Google Scholar] [CrossRef]
- Sharma, H.; Hyvönen, J.; Poczai, P. Development of chloroplast microsatellite markers for giant ragweed (Ambrosia trifida). Appl. Plant Sci. 2020, 8, 11313. [Google Scholar] [CrossRef]
- Terakami, S.; Matsumura, Y.; Kurita, K.; Kanamori, H.; Katayose, Y.; Yamamoto, T.; Katayama, H. Complete sequence of the chloroplast genome from pear (Pyrus pyrifolia): Genome structure and comparative analysis. Tree Genet. Genomes 2012, 8, 841–854. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Duo, J.; Yang, Y.; Ju, X.; Duan, R.; Xiong, H. The complete chloroplast genome of Salsola abrotanoides (Chenopodiaceae), a desert halophyte shrub in China. Mitochondrial DNA Part B 2021, 6, 1152–1153. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, C.; Wang, Y.; Zhang, Y. The complete chloroplast genome of Caroxylon passerinum (Chenopodiaceae), an annual desert plant. Mitochondrial DNA Part B 2022, 7, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Munch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. Reputer: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.; Kim, Y.; Chung, Y. The comparative analyses of six complete chloroplast genomes of morphologically diverse Chenopodium album L. (Amaranthaceae) collected in Korea. Int. J. Genom. 2021, 2021, 6643444. [Google Scholar] [CrossRef] [PubMed]
- Wariss, H.M.; Qu, X.J. The complete chloroplast genome of Chenopodium acuminatum Willd. (Amaranthaceae). Mitochondrial DNA Part B 2021, 6, 174–175. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, F.; Ding, H.; Liu, W.; Yang, B.; Geng, J.; Guo, S. Comparative Analysis of Six Chloroplast Genomes in Chenopodium and Its Related Genera (Amaranthaceae): New Insights into Phylogenetic Relationships and the Development of Species-Specific Molecular Markers. Genes 2023, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tao, M.; Shan, X.; Pan, Y.; Sun, C.; Song, L.; Dai, Z. Characterization of the complete chloroplast genome of Brassica oleracea var. italica and phylogenetic relationships in Brassicaceae. PLoS ONE 2022, 17, e0263310. [Google Scholar]
- Feng, L.; Zhao, G.; An, M.; Wang, C.; Yin, Y. Complete chloroplast genome sequences of the ornamental plant Prunus cistena and comparative and phylogenetic analyses with its closely related species. BMC Genom. 2023, 24, 739. [Google Scholar] [CrossRef]
- He, L.; Qian, J.; Li, X.; Sun, Z.; Xu, X.; Chen, S. Complete chloroplast genome of medicinal plant Lonicera japonica: Genome rearrangement, intron gain and loss, and implications for phylogenetic studies. Molecules 2017, 22, 249. [Google Scholar] [CrossRef]
- Claude, S.J.; Park, S.; Park, S. Gene loss, genome rearrangement, and accelerated substitution rates in plastid genome of Hypericum ascyron (Hypericaceae). BMC Plant Biol. 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef]
- Fu, P.C.; Zhang, Y.Z.; Geng, H.M.; Chen, S.L. The complete chloroplast genome sequence of Gentiana lawrencei var. farreri (Gentianaceae) and comparative analysis with its congeneric species. PeerJ 2016, 4, e2540. [Google Scholar]
- Gao, B.; Yuan, L.; Tang, T.; Hou, J.; Pan, K.; Wei, N. The complete chloroplast genome sequence of Alpinia oxyphylla Miq. and comparison analysis within the Zingiberaceae family. PLoS ONE 2019, 14, e0218817. [Google Scholar] [CrossRef]
- Ebert, D.; Peakall, R. Chloroplast simple sequence repeats (cpSSRs): Technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef]
- Makrickiene, E.; Danusevičius, D.; Brazaitis, G.; Manton, M. Morphological and genetic differentiation of wolf trees in Scots pine stands based on chloroplast microsatellite markers. Eur. J. For. Res. 2019, 138, 527–537. [Google Scholar] [CrossRef]
- Lācis, G.; Kārkliņa, K.; Bartulsons, T.; Stalažs, A.; Jundzis, M.; Baļķe, I.; Strautiņa, S. Genetic structure of a Ribes genetic resource collection: Inter-and intra-specific diversity revealed by chloroplast DNA simple sequence repeats (cpSSRs). Sci. Hortic. 2022, 304, 111285. [Google Scholar] [CrossRef]
- Guo, Q.; Xue, X.; Wang, D.; Zhang, L.; Liu, W.; Wang, E.; Hou, X. Genetic diversity and population genetic structure of Paeonia suffruticosa by chloroplast DNA simple sequence repeats (cpSSRs). Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Mehmetoğlu, E.; Kaymaz, Y.; Ateş, D.; Kahraman, A.; Tanyolaç, M.B. The complete chloroplast genome of Cicer reticulatum and comparative analysis against relative Cicer species. Sci. Rep. 2023, 13, 17871. [Google Scholar] [CrossRef]
- Jin, G.; Li, W.; Song, F.; Yang, L.; Wen, Z.; Feng, Y. Comparative analysis of complete Artemisia subgenus Seriphidium (Asteraceae: Anthemideae) chloroplast genomes: Insights into structural divergence and phylogenetic relationships. BMC Plant Biol. 2023, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Rono, P.C.; Dong, X.; Kirika, P.M.; Hu, G.W.; Wang, Q.F. Initial complete chloroplast genomes of Alchemilla (Rosaceae): Comparative analysis and phylogenetic relationships. Front. Genet. 2020, 11, 560368. [Google Scholar] [CrossRef] [PubMed]
- Yermagambetova, M.; Abugalieva, S.; Turuspekov, Y.; Almerekova, S. Illumina sequencing data of the complete chloroplast genome of rare species Juniperus seravschanica (Cupressaceae) from Kazakhstan. Data Brief 2023, 46, 108866. [Google Scholar] [CrossRef] [PubMed]
- Almerekova, S.; Yermagambetova, M.; Jumanov, S.; Abugalieva, S.; Turuspekov, Y. Comparative analysis of chloroplast genomes of seven Juniperus species from Kazakhstan. PLoS ONE 2024, 19, e0295550. [Google Scholar] [CrossRef] [PubMed]
- Almerekova, S.; Yermagambetova, M.; Osmonali, B.; Vesselova, P.; Abugalieva, S.; Turuspekov, Y. Characterization of the Plastid Genomes of Four Caroxylon Thunb. Species from Kazakhstan. Plants 2024, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Safiul Azam, F.M.; Akter, M.L.; Ao, L.; Zou, Y.; Qian, Y. The first complete chloroplast genome of Thalictrum fargesii: Insights into phylogeny and species identification. Front. Plant Sci. 2024, 15, 1356912. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Q.; Zhai, J.; Wu, K.; Fang, L.; Li, M.; Li, S. Comparative chloroplast genomics of 24 species shed light on the genome evolution and phylogeny of subtribe Coelogyninae (Orchidaceae). BMC Plant Biol. 2024, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Akhani, H.; Khoshravesh, R.; Malekmohammadi, M. Taxonomic novelties from Irano-Turanian region and NE Iran: Oreosalsola, a new segregate from Salsola s.l., two new species in Anabasis and Salvia, and two new combinations in Caroxylon and Seseli. Phytotaxa 2016, 249, 159–180. [Google Scholar] [CrossRef][Green Version]
- Rudov, A.; Mashkour, M.; Djamali, M.; Akhani, H. A review of C4 plants in southwest Asia: An ecological, geographical and taxonomical analysis of a region with high diversity of C4 eudicots. Front. Plant Sci. 2020, 11, 546518. [Google Scholar] [CrossRef]



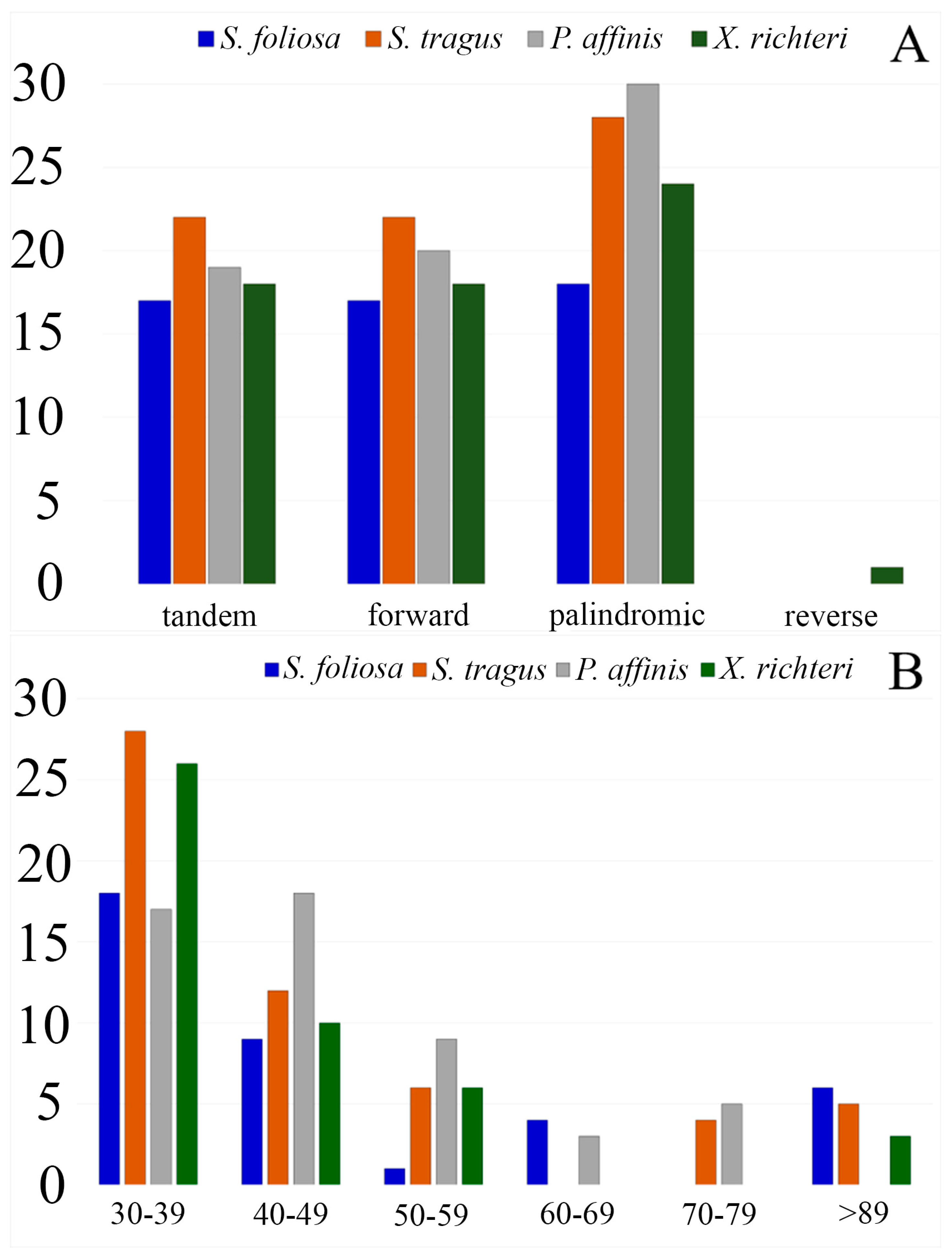


| Species | S. foliosa | S. tragus | P. affinis | X. richteri |
|---|---|---|---|---|
| Collected place | West Kazakhstan region, Borly district | Kyzylorda region, Zhalagash district | Zhetysu region, Panfilov district | Kyzylorda region, Zhanakorgan district |
| GPS coordinates | 51.29 | 45.08 | 44.17 | 44.33 |
| 53.36 | 64.78 | 79.53 | 66.21 | |
| 82 m a.s.l. | 110 m a.s.l. | 890 m a.s.l. | 150 m a.s.l. |
| S. foliosa | S. tragus | P. affinis | X. richteri | |
|---|---|---|---|---|
| GenBank numbers | PP754489 | PP754490 | PP754487 | PP754488 |
| Genome size (bp) | 151,577 | 152,969 | 151,239 | 151,177 |
| LSC (bp) | 83,871 | 83,993 | 83,324 | 84,136 |
| SSC (bp) | 18,962 | 18,576 | 18,739 | 20,087 |
| IR (bp) | 48,744 | 50,400 | 49,176 | 46,954 |
| Number of total genes | 133 | 133 | 133 | 133 |
| Protein-coding genes | 80 | 80 | 80 | 80 |
| tRNAs | 30 | 30 | 30 | 30 |
| rRNAs | 4 | 4 | 4 | 4 |
| Total GC content (%) | 36.55 | 36.60 | 36.26 | 36.60 |
| LSC GC content (%) | 34.45 | 34.60 | 34.10 | 34.57 |
| SSC GC content (%) | 29.59 | 29.38 | 28.75 | 29.76 |
| IR GC content (%) | 42.88 | 42.58 | 42.77 | 43.17 |
| Category | Group of Genes | Name of Genes |
|---|---|---|
| Self-replication | Ribosomal RNA | rrn4.5 (2), rrn5 (2), rrn16 (2), rrn23 (2) |
| Transfer RNA | trnA-UGC * (2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC *, trnG-UCC, trnH-GUG, trnI-CAU (2), trnI-GAU * (2), trnK-UUU *, trnL-CAA (2), trnL-UAA *, trnL-UAG, trnM-CAU, trnN-GUU (2), trnP-UGG, trnQ-UUG, trnR-ACG (2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC (2), trnV-UAC *, trnW-CCA, trnY-GUA | |
| Small subunit of ribosome | rps2, rps3, rps4, rps7 (2), rps8, rps11, rps12 * (2), rps14, rps15, rps16 *, rps18, rps19 | |
| Large subunit of ribosome | rpl2 (2), rpl14, rpl16 *, rpl20, rpl22, rpl23 (2), rpl32, rpl33, rpl36 | |
| RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 | |
| Translation initiation factor | infA | |
| Photosynthesis | ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI |
| NADH dehydrogenase | ndhA *, ndhB * (2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome | petA, petB *, petD *, petG, petL, petN | |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ | |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Rubisco | rbcL | |
| Other genes | Maturase | matK |
| Protease | clpP ** | |
| Envelope membrane protein | cemA | |
| Subunit of acetyl-CoA-carboxylase | accD | |
| C-type cytochrome synthesis gene | ccsA | |
| Genes of unknown function | Hypothetical chloroplast reading frames | ycf1 (2), ycf2 (2), ycf3 **, ycf4, ycf15 (2) |
| Type | Repeats | S. foliosa | S. tragus | P. affinis | X. richteri | Total | % |
|---|---|---|---|---|---|---|---|
| Mono | A/T | 180 | 207 | 160 | 197 | 744 | 77.20 |
| C/G | 3 | 6 | 5 | 4 | 18 | ||
| Di | AC/GT | 2 | 1 | 4 | 1 | 8 | 16.72 |
| AG/CT | 16 | 14 | 14 | 16 | 60 | ||
| AT/AT | 28 | 24 | 18 | 27 | 97 | ||
| Tri | AAG/CTT | 1 | 1 | 2 | 1 | 5 | 2.33 |
| AAT/ATT | 1 | 12 | 2 | 3 | 18 | ||
| Tetra | AAAC/GTTT | 1 | 1 | 0 | 1 | 3 | 3.34 |
| AAAG/CTTT | 1 | 1 | 2 | 1 | 5 | ||
| AAAT/ATTT | 1 | 0 | 1 | 1 | 3 | ||
| AAGG/CCTT | 0 | 1 | 0 | 1 | 2 | ||
| AATG/ATTC | 0 | 1 | 0 | 1 | 2 | ||
| AATT/AATT | 3 | 2 | 3 | 2 | 10 | ||
| ACCT/AGGT | 2 | 2 | 2 | 2 | 8 | ||
| Penta | AAATG/ATTTC | 0 | 0 | 1 | 1 | 2 | 0.20 |
| Hexa | AAAATT/AATTTT | 0 | 1 | 0 | 0 | 1 | 0.20 |
| AATCCG/ATTCGG | 0 | 0 | 1 | 0 | 1 | ||
| Total | 239 | 274 | 215 | 259 | 987 | 100 | |
| Variable Region | Length | Variable Sites | Parsimony-Informative Sites | Nucleotide Diversity |
|---|---|---|---|---|
| ndhC-ndhD | 600 | 75 | 44 | 0.03947 |
| rps16-psbK | 609 | 82 | 47 | 0.03778 |
| petD | 688 | 90 | 44 | 0.03905 |
| rpoC2 | 606 | 86 | 45 | 0.04046 |
| ndhA | 864 | 83 | 45 | 0.04086 |
| petB | 744 | 98 | 43 | 0.04238 |
| clpP | 723 | 96 | 46 | 0.04262 |
| atpF | 779 | 87 | 48 | 0.04278 |
| ycf3 | 669 | 96 | 50 | 0.04564 |
| accD | 729 | 110 | 53 | 0.05114 |
| ndhF-ndhG | 606 | 106 | 63 | 0.05288 |
| matK | 606 | 106 | 65 | 0.05478 |
| rpl20-rpl22 | 606 | 133 | 58 | 0.06000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almerekova, S.; Yermagambetova, M.; Osmonali, B.; Vesselova, P.; Turuspekov, Y.; Abugalieva, S. Complete Plastid Genome Sequences of Four Salsoleae s.l. Species: Comparative and Phylogenetic Analyses. Biomolecules 2024, 14, 890. https://doi.org/10.3390/biom14080890
Almerekova S, Yermagambetova M, Osmonali B, Vesselova P, Turuspekov Y, Abugalieva S. Complete Plastid Genome Sequences of Four Salsoleae s.l. Species: Comparative and Phylogenetic Analyses. Biomolecules. 2024; 14(8):890. https://doi.org/10.3390/biom14080890
Chicago/Turabian StyleAlmerekova, Shyryn, Moldir Yermagambetova, Bektemir Osmonali, Polina Vesselova, Yerlan Turuspekov, and Saule Abugalieva. 2024. "Complete Plastid Genome Sequences of Four Salsoleae s.l. Species: Comparative and Phylogenetic Analyses" Biomolecules 14, no. 8: 890. https://doi.org/10.3390/biom14080890
APA StyleAlmerekova, S., Yermagambetova, M., Osmonali, B., Vesselova, P., Turuspekov, Y., & Abugalieva, S. (2024). Complete Plastid Genome Sequences of Four Salsoleae s.l. Species: Comparative and Phylogenetic Analyses. Biomolecules, 14(8), 890. https://doi.org/10.3390/biom14080890






