OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. CRISPR/Cas9 OCRL Knock-Out
2.3. Transfection of HA-ApoER2
2.4. Western Blot
2.5. Immunofluorescence
2.6. Shell Analysis
2.7. Spreading Assay
2.8. Neurite Length Measurements
2.9. ApoER2 Surface Levels
2.10. Endocytosis/Internalization
2.11. ApoER2 Recycling
2.12. ApoER2 Half Life
2.13. Preparation of Recombinant Reelin
2.14. ApoER2/Reelin Signaling
2.15. Reelin-Induced Golgi Deployment
2.16. Microscopy and Image Analysis
2.17. Statistical Analysis
3. Results
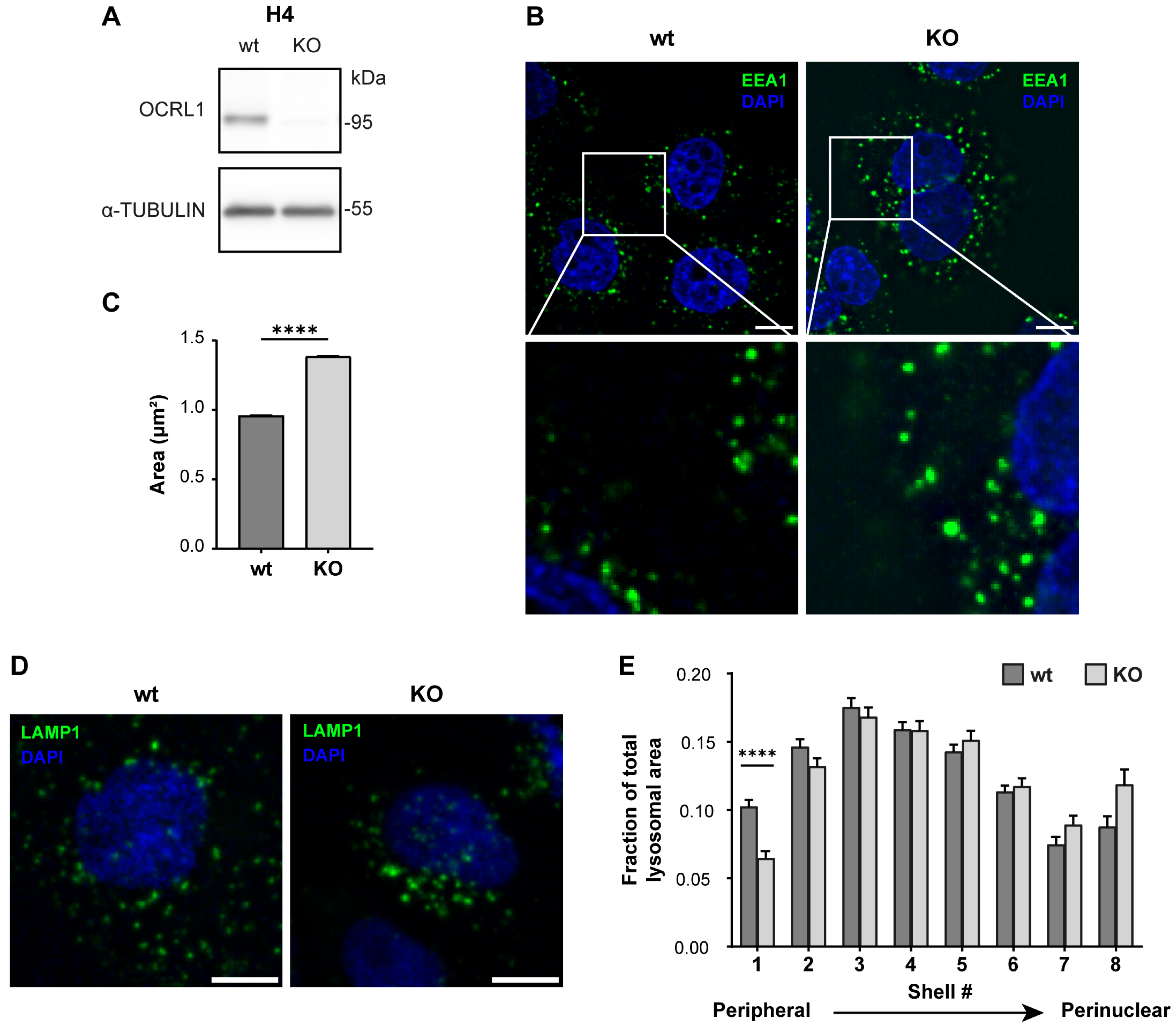
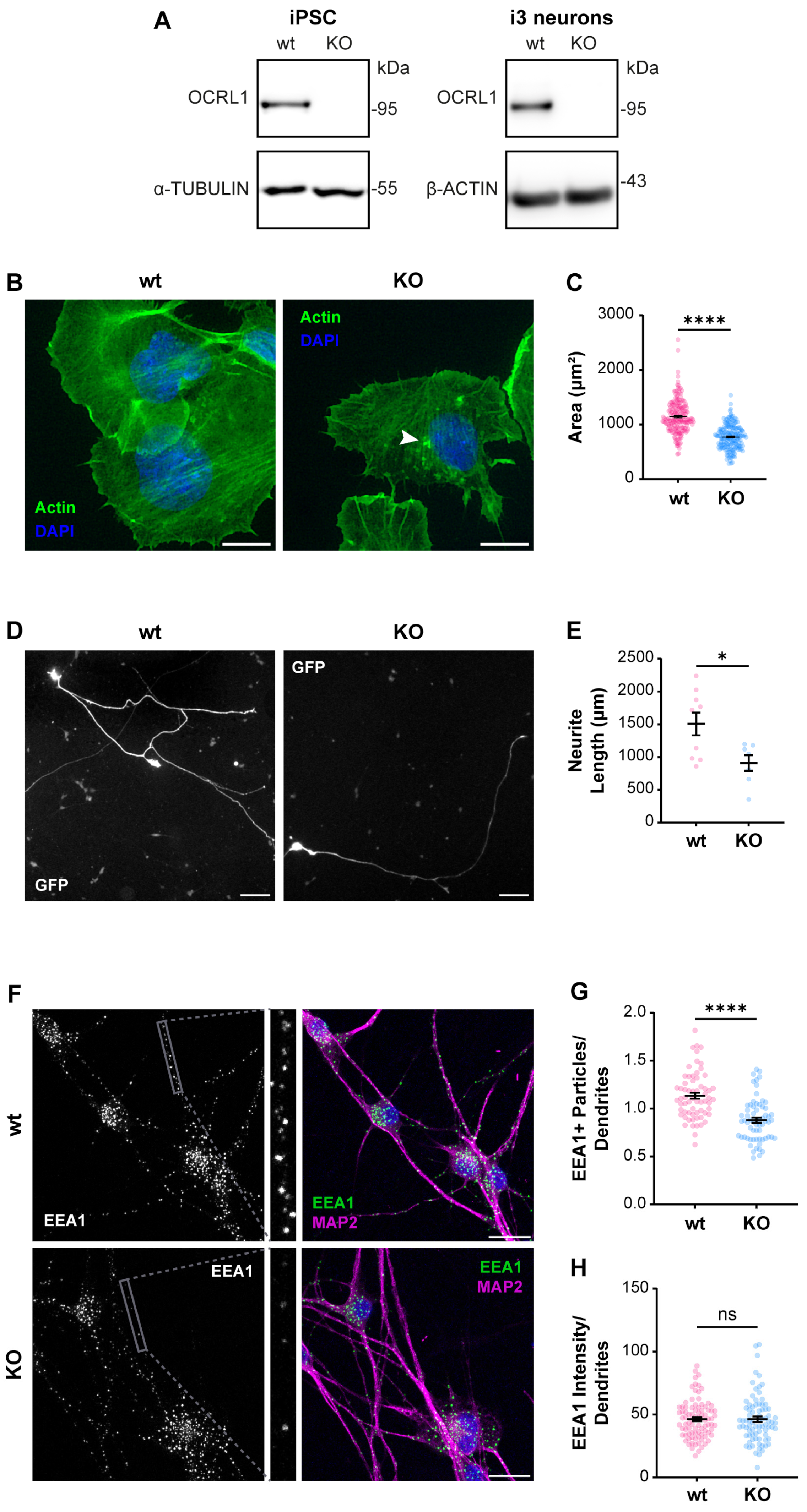
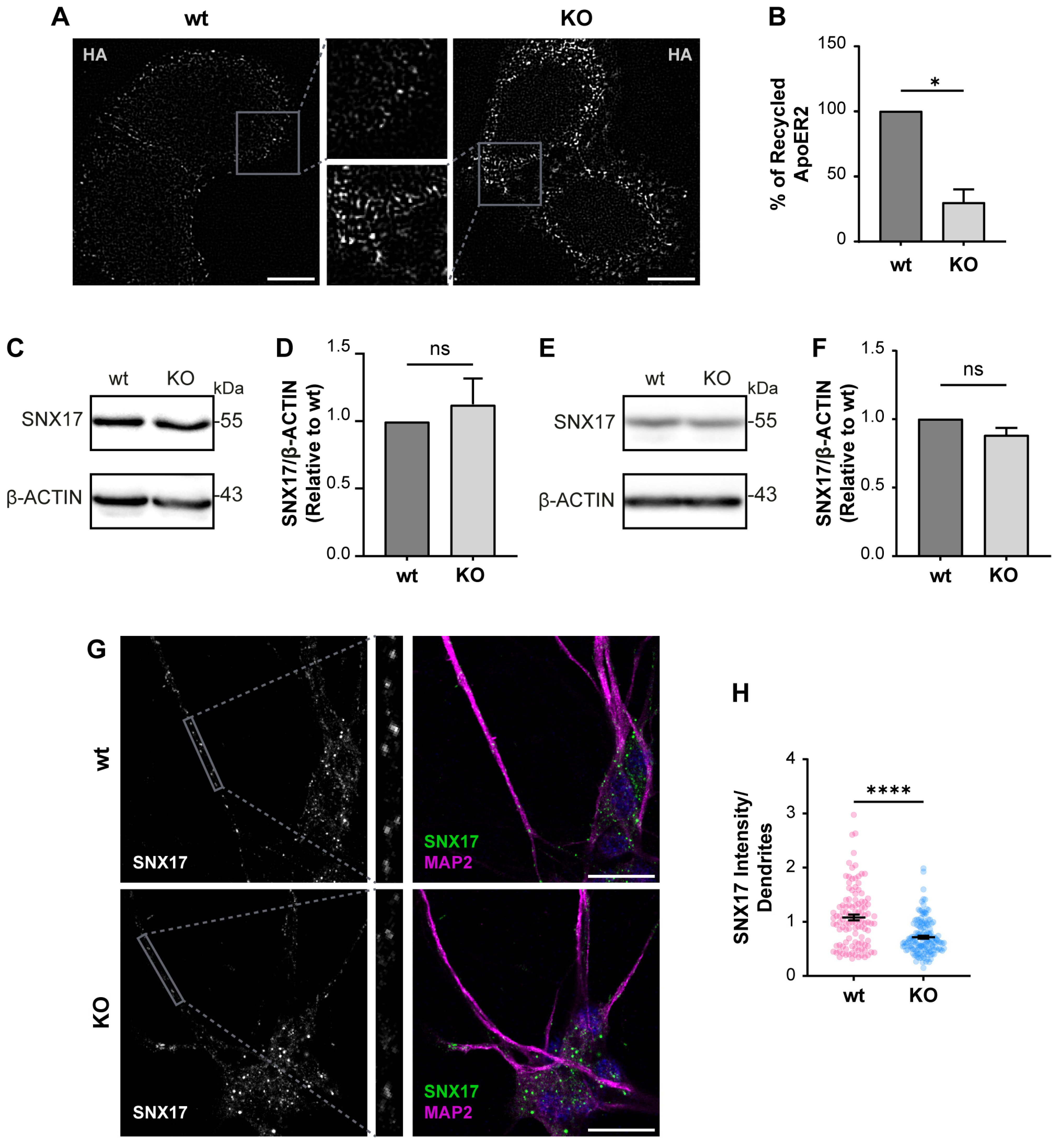
3.1. Characterization of OCRL Knock-Out Neuronal Cells
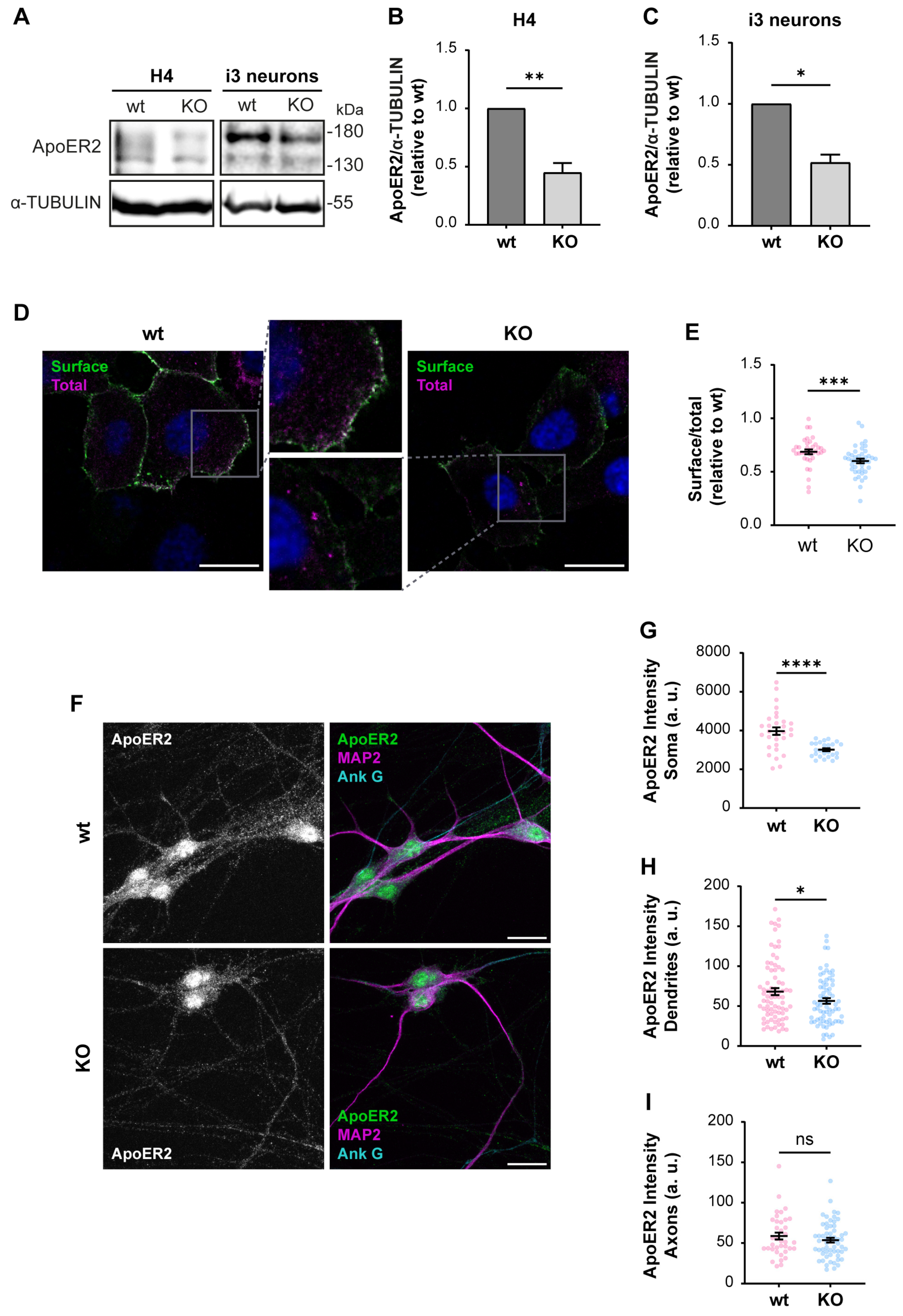
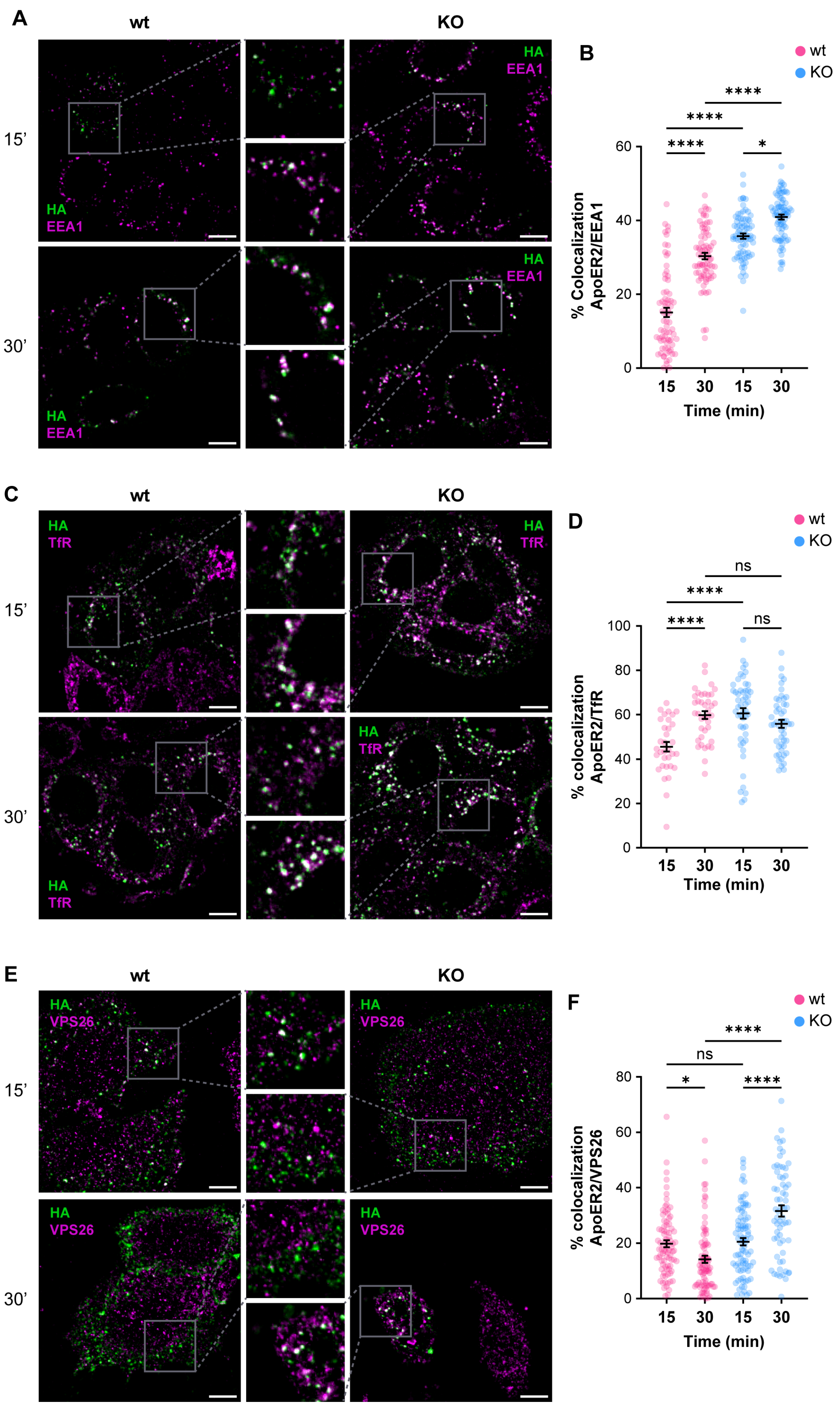
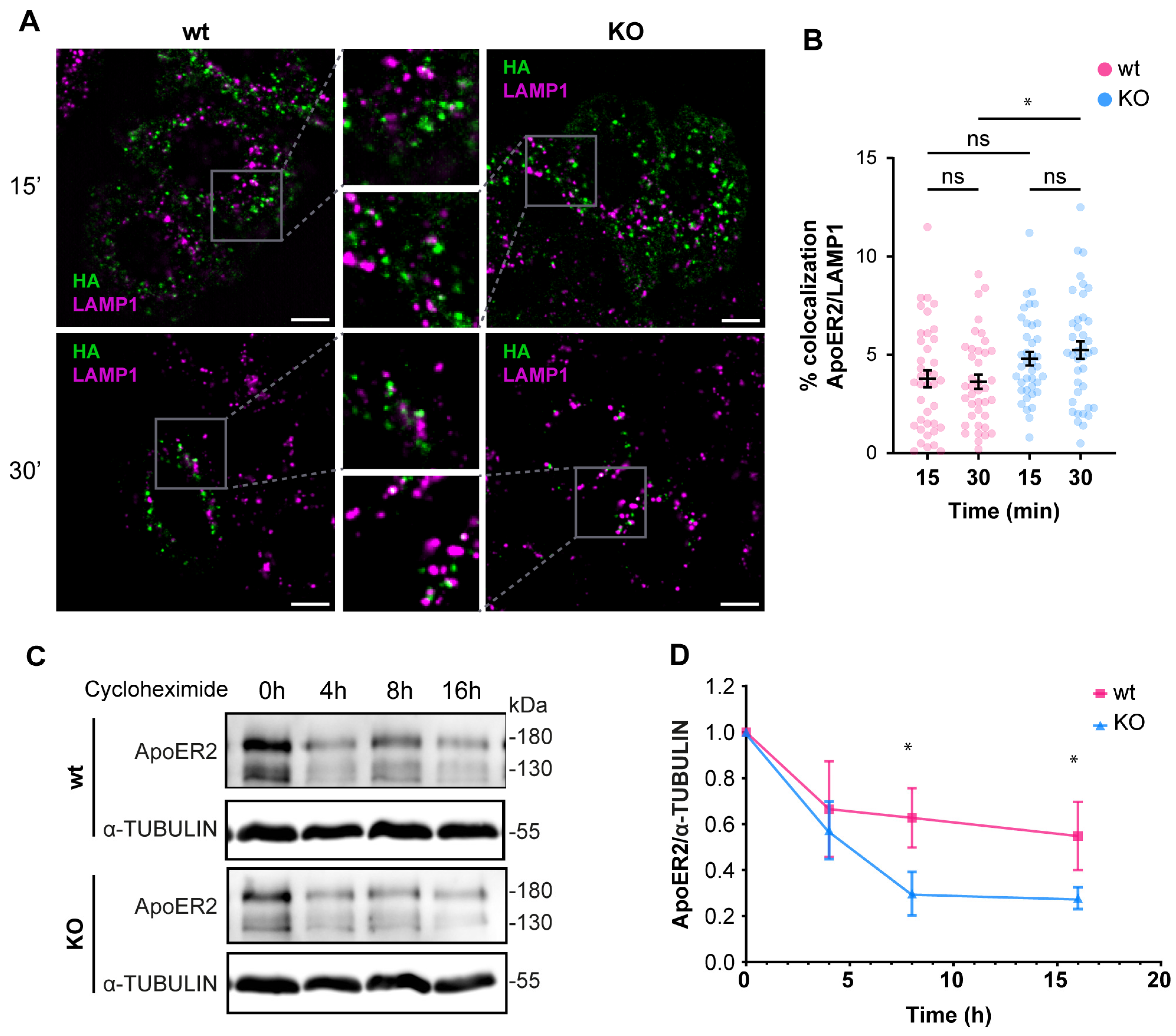
3.2. OCRL KO Cells Show Significant Alterations in ApoER2 Protein Levels and Trafficking
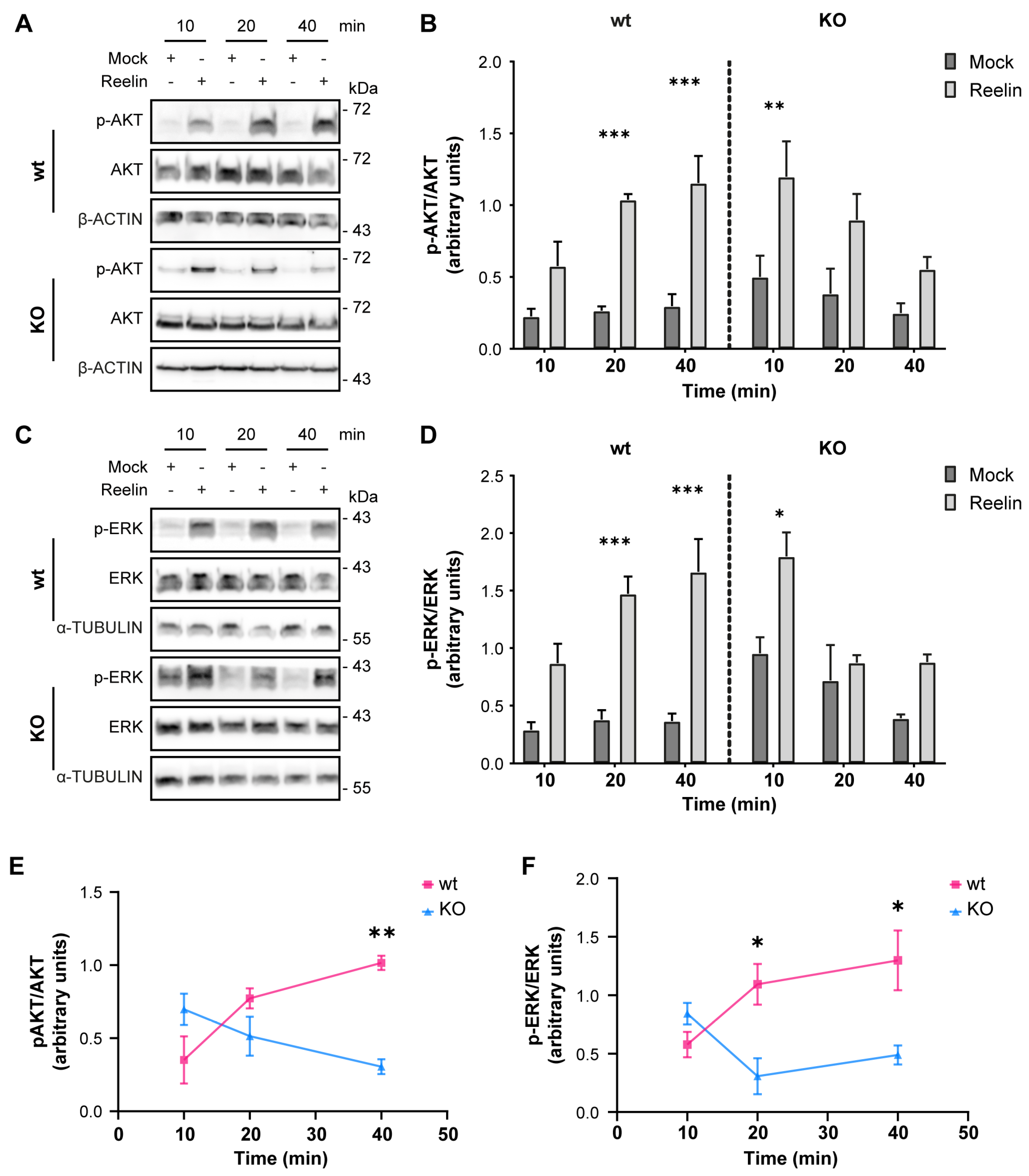
3.3. Neuronal Responses to Reelin Are Affected in OCRL KO Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, C.U.; Terrey, M.; Mac, L.E. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am. J. Dis. Child. 1952, 83, 164–184. [Google Scholar] [CrossRef]
- Reilly, D.S.; Lewis, R.A.; Nussbaum, R.L. Genetic and physical mapping of Xq24-q26 markers flanking the Lowe oculocerebrorenal syndrome. Genomics 1990, 8, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Okabe, I.; Attree, O.; Bailey, L.C.; Nelson, D.L.; Nussbaum, R.L. Isolation of cDNA sequences around the chromosomal breakpoint in a female with Lowe syndrome by direct screening of cDNA libraries with yeast artificial chromosomes. J. Inherit. Metab. Dis. 1992, 15, 526–531. [Google Scholar] [CrossRef]
- Kenworthy, L.; Park, T.; Charnas, L.R. Cognitive and behavioral profile of the oculocerebrorenal syndrome of Lowe. Am. J. Med. Genet. 1993, 46, 297–303. [Google Scholar] [CrossRef]
- Mehta, Z.B.; Pietka, G.; Lowe, M. The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic 2014, 15, 471–487. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, M.A.; Staiano, L.; Emma, F.; Devuyst, O. The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2. Nat. Rev. Nephrol. 2017, 13, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.; Iannello, G.; Skowronski, A.A.; Dannheim, K.; Cheung, L.; Agrawal, P.B.; Hirschhorn, J.N.; Zeitler, P.; LeDuc, C.A.; Stratigopoulos, G.; et al. Endocrine and behavioural features of Lowe syndrome and their potential molecular mechanisms. J. Med. Genet. 2022, 59, 1171–1178. [Google Scholar] [CrossRef]
- Festa, B.P.; Berquez, M.; Gassama, A.; Amrein, I.; Ismail, H.M.; Samardzija, M.; Staiano, L.; Luciani, A.; Grimm, C.; Nussbaum, R.L.; et al. OCRL deficiency impairs endolysosomal function in a humanized mouse model for Lowe syndrome and Dent disease. Hum. Mol. Genet. 2019, 28, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.; Naylor, R.W.; Stewart, G.; Bierzynska, A.; Saleem, M.A.; Lowe, M.; Lennon, R. A role for OCRL in glomerular function and disease. Pediatr. Nephrol. 2020, 35, 641–648. [Google Scholar] [CrossRef]
- Jänne, P.A.; Suchy, S.F.; Bernard, D.; MacDonald, M.; Crawley, J.; Grinberg, A.; Wynshaw-Boris, A.; Westphal, H.; Nussbaum, R.L. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J. Clin. Investig. 1998, 101, 2042–2053. [Google Scholar] [CrossRef]
- Bothwell, S.P.; Chan, E.; Bernardini, I.M.; Kuo, Y.M.; Gahl, W.A.; Nussbaum, R.L. Mouse model for Lowe syndrome/Dent Disease 2 renal tubulopathy. J. Am. Soc. Nephrol. 2011, 22, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Orrison, B.M.; Jänne, P.A.; Charnas, L.; Chinault, A.C. Physical mapping and genomic structure of the Lowe syndrome gene OCRL1. Hum. Genet. 1997, 99, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Attree, O.; Olivos, I.M.; Okabe, I.; Bailey, L.C.; Nelson, D.L.; Lewis, R.A.; McInnes, R.R.; Nussbaum, R.L. The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 1992, 358, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Leahey, A.M.; Charnas, L.R.; Nussbaum, R.L. Nonsense mutations in the OCRL-1 gene in patients with the oculocerebrorenal syndrome of Lowe. Hum. Mol. Genet. 1993, 2, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Orrison, B.M.; Leahey, A.M.; Suchy, S.F.; Bernard, D.J.; Lewis, R.A.; Nussbaum, R.L. Spectrum of mutations in the OCRL1 gene in the Lowe oculocerebrorenal syndrome. Am. J. Hum. Genet. 1997, 60, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- McCrea, H.J.; Paradise, S.; Tomasini, L.; Addis, M.; Melis, M.A.; De Matteis, M.A.; De Camilli, P. All known patient mutations in the ASH-RhoGAP domains of OCRL affect targeting and APPL1 binding. Biochem. Biophys. Res. Commun. 2008, 369, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ramadesikan, S.; Black, A.F.; Christoffer, C.; Pacheco, A.F.P.; Subramanian, S.; Hanna, C.B.; Barth, G.; Stauffacher, C.V.; Kihara, D.; et al. Heterogeneity in Lowe Syndrome: Mutations Affecting the Phosphatase Domain of OCRL1 Differ in Impact on Enzymatic Activity and Severity of Cellular Phenotypes. Biomolecules 2023, 13, 615. [Google Scholar] [CrossRef]
- Ramadesikan, S.; Skiba, L.; Lee, J.; Madhivanan, K.; Sarkar, D.; De La Fuente, A.; Hanna, C.B.; Terashi, G.; Hazbun, T.; Kihara, D.; et al. Genotype & phenotype in Lowe Syndrome: Specific OCRL1 patient mutations differentially impact cellular phenotypes. Hum. Mol. Genet. 2021, 30, 198–212. [Google Scholar] [CrossRef]
- Olivos-Glander, I.M.; Jänne, P.A.; Nussbaum, R.L. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am. J. Hum. Genet. 1995, 57, 817–823. [Google Scholar]
- Lichter-Konecki, U.; Farber, L.W.; Cronin, J.S.; Suchy, S.F.; Nussbaum, R.L. The effect of missense mutations in the RhoGAP-homology domain on ocrl1 function. Mol. Genet. Metab. 2006, 89, 121–128. [Google Scholar] [CrossRef]
- Vicinanza, M.; Di Campli, A.; Polishchuk, E.; Santoro, M.; Di Tullio, G.; Godi, A.; Levtchenko, E.; De Leo, M.G.; Polishchuk, R.; Sandoval, L.; et al. OCRL controls trafficking through early endosomes via PtdIns4,5P₂-dependent regulation of endosomal actin. EMBO J. 2011, 30, 4970–4985. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, L.; Fuentealba, L.M.; Marzolo, M.-P. Participation of OCRL1, and APPL1, in the expression, proteolysis, phosphorylation and endosomal trafficking of megalin: Implications for Lowe Syndrome. Front. Cell Dev. Biol. 2022, 10, 911664. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.G.; Staiano, L.; Vicinanza, M.; Luciani, A.; Carissimo, A.; Mutarelli, M.; Di Campli, A.; Polishchuk, E.; Di Tullio, G.; Morra, V.; et al. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat. Cell Biol. 2016, 18, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-C.; Ramadesikan, S.; Fekete, D.; Aguilar, R.C. Kidney-differentiated cells derived from Lowe Syndrome patient’s iPSCs show ciliogenesis defects and Six2 retention at the Golgi complex. PLoS ONE 2018, 13, e0192635. [Google Scholar] [CrossRef] [PubMed]
- Coon, B.G.; Hernandez, V.; Madhivanan, K.; Mukherjee, D.; Hanna, C.B.; Barinaga-Rementeria Ramirez, I.; Lowe, M.; Beales, P.L.; Aguilar, R.C. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum. Mol. Genet. 2012, 21, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Coon, B.G.; Mukherjee, D.; Hanna, C.B.; Riese, D.J.; Lowe, M.; Aguilar, R.C. Lowe syndrome patient fibroblasts display Ocrl1-specific cell migration defects that cannot be rescued by the homologous Inpp5b phosphatase. Hum. Mol. Genet. 2009, 18, 4478–4491. [Google Scholar] [CrossRef] [PubMed]
- van Rahden, V.A.; Brand, K.; Najm, J.; Heeren, J.; Pfeffer, S.R.; Braulke, T.; Kutsche, K. The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module. Hum. Mol. Genet. 2012, 21, 5019–5038. [Google Scholar] [CrossRef]
- Madhivanan, K.; Ramadesikan, S.; Hsieh, W.C.; Aguilar, M.C.; Hanna, C.B.; Bacallao, R.L.; Aguilar, R.C. Lowe syndrome patient cells display mTOR- and RhoGTPase-dependent phenotypes alleviated by rapamycin and statins. Hum. Mol. Genet. 2020, 29, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, B.M.; Bhatia, P.; Acharya, S.; Sharma, S.; Sharma, Y.; Bhuvanendran Nair Suseela Devi, A.; Ganapathy, K.; Vasudevan, A.; Raghu, P. A human stem cell resource to decipher the biochemical and cellular basis of neurodevelopmental defects in Lowe syndrome. Biol. Open 2022, 11, bio059066. [Google Scholar] [CrossRef]
- Suchy, S.F.; Nussbaum, R.L. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am. J. Hum. Genet. 2002, 71, 1420–1427. [Google Scholar] [CrossRef]
- Kühbacher, A.; Dambournet, D.; Echard, A.; Cossart, P.; Pizarro-Cerdá, J. Phosphatidylinositol 5-phosphatase oculocerebrorenal syndrome of Lowe protein (OCRL) controls actin dynamics during early steps of Listeria monocytogenes infection. J. Biol. Chem. 2012, 287, 13128–13136. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Salas, F.; Mokhtari, R.; Dolstra, H.; Pedrosa, E.; Lachman, H.M. Modeling the neuropsychiatric manifestations of Lowe syndrome using induced pluripotent stem cells: Defective F-actin polymerization and WAVE-1 expression in neuronal cells. Mol. Autism 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, K.; Mukherjee, D.; Aguilar, R.C. Lowe syndrome. Commun. Integr. Biol. 2012, 5, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, W.; Prosseda, P.P.; Li, L.; Kowal, T.J.; Alvarado, J.A.; Wang, Q.; Hu, Y.; Sun, Y. OCRL regulates lysosome positioning and mTORC1 activity through SSX2IP-mediated microtubule anchoring. EMBO Rep. 2021, 22, e52173. [Google Scholar] [CrossRef] [PubMed]
- Larios, J.A.; Marzolo, M.-P. Novel aspects of the apolipoprotein-E receptor family: Regulation and functional role of their proteolytic processing. Front. Biol. 2012, 7, 113–143. [Google Scholar] [CrossRef]
- Kim, D.H.; Iijima, H.; Goto, K.; Sakai, J.; Ishii, H.; Kim, H.J.; Suzuki, H.; Kondo, H.; Saeki, S.; Yamamoto, T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996, 271, 8373–8380. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.; Hiesberger, T.; Schneider, W.J.; Nimpf, J. A New Low Density Lipoprotein Receptor Homologue with 8 Ligand Binding Repeats in Brain of Chicken and Mouse. J. Biol. Chem. 1996, 271, 11732–11736. [Google Scholar] [CrossRef] [PubMed]
- Pasten, C.; Cerda, J.; Jausoro, I.; Court, F.A.; Cáceres, A.; Marzolo, M.P. ApoER2 and Reelin are expressed in regenerating peripheral nerve and regulate Schwann cell migration by activating the Rac1 GEF protein, Tiam1. Mol. Cell Neurosci. 2015, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Passarella, D.; Ciampi, S.; Di Liberto, V.; Zuccarini, M.; Ronci, M.; Medoro, A.; Foderà, E.; Frinchi, M.; Mignogna, D.; Russo, C.; et al. Low-Density Lipoprotein Receptor-Related Protein 8 at the Crossroad between Cancer and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 8921. [Google Scholar] [CrossRef]
- Cuitino, L.; Matute, R.; Retamal, C.; Bu, G.; Inestrosa, N.C.; Marzolo, M.P. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its Rafts’ association. Traffic 2005, 6, 820–838. [Google Scholar] [CrossRef]
- Sotelo, P.; Farfán, P.; Benitez, M.L.; Bu, G.; Marzolo, M.P. Sorting nexin 17 regulates ApoER2 recycling and reelin signaling. PLoS ONE 2014, 9, e93672. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Herz, J.; Calvier, L. Reelin through the years: From brain development to inflammation. Cell Rep. 2023, 42, 112669. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.H.; Herz, J. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 2003, 13, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Morfini, G.; Bock, H.H.; Reyna, H.; Brady, S.T.; Herz, J. Reelin-mediated Signaling Locally Regulates Protein Kinase B/Akt and Glycogen Synthase Kinase 3β. J. Biol. Chem. 2002, 277, 49958–49964. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.H.; Jossin, Y.; Liu, P.; Förster, E.; May, P.; Goffinet, A.M.; Herz, J. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 2003, 278, 38772–38779. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, J.; Bock, H.H. Reelin modulates cytoskeletal organization by regulating Rho GTPases. Commun. Integr. Biol. 2011, 4, 254–257. [Google Scholar] [CrossRef][Green Version]
- O’Dell, R.S.; Ustine, C.J.; Cameron, D.A.; Lawless, S.M.; Williams, R.M.; Zipfel, W.R.; Olson, E.C. Layer 6 cortical neurons require Reelin-Dab1 signaling for cellular orientation, Golgi deployment, and directed neurite growth into the marginal zone. Neural Dev. 2012, 7, 25. [Google Scholar] [CrossRef]
- Meseke, M.; Rosenberger, G.; Förster, E. Reelin and the Cdc42/Rac1 guanine nucleotide exchange factor αPIX/Arhgef6 promote dendritic Golgi translocation in hippocampal neurons. Eur. J. Neurosci. 2013, 37, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Jossin, Y.; Goffinet, A.M. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef]
- Telese, F.; Ma, Q.; Perez, P.M.; Notani, D.; Oh, S.; Li, W.; Comoletti, D.; Ohgi, K.A.; Taylor, H.; Rosenfeld, M.G. LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 2015, 86, 696–710. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.H.; Trotter, J.H.; Maher, J.N.; Keenoy, K.E.; Jang, Y.M.; Lee, Y.; Kim, J.I.; Weeber, E.J.; Hoe, H.S. Reelin and APP Cooperatively Modulate Dendritic Spine Formation. Exp. Neurobiol. 2023, 32, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Wasser, C.R.; Werthmann, G.C.; Hall, E.M.; Kuhbandner, K.; Wong, C.H.; Durakoglugil, M.S.; Herz, J. Regulation of the hippocampal translatome by Apoer2-ICD release. Mol. Neurodegener. 2023, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Omuro, K.C.; Gallo, C.M.; Scrandis, L.; Ho, A.; Beffert, U. Human APOER2 Isoforms Have Differential Cleavage Events and Synaptic Properties. J. Neurosci. 2022, 42, 4054–4068. [Google Scholar] [CrossRef] [PubMed]
- Brosda, J.; Dietz, F.; Koch, M. Impairment of cognitive performance after reelin knockdown in the medial prefrontal cortex of pubertal or adult rats. Neurobiol. Dis. 2011, 44, 239–247. [Google Scholar] [CrossRef]
- Eastwood, S.L.; Harrison, P.J. Cellular basis of reduced cortical reelin expression in schizophrenia. Am. J. Psychiatry 2006, 163, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, S.L.; Harrison, P.J. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: Towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol. Psychiatry 2003, 8, 769–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiesberger, T.; Trommsdorff, M.; Howell, B.W.; Goffinet, A.; Mumby, M.C.; Cooper, J.A.; Herz, J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 1999, 24, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Rusiana, I.; Trotter, J.; Zhao, L.; Donaldson, E.; Pak, D.T.; Babus, L.W.; Peters, M.; Banko, J.L.; Chavis, P.; et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 2011, 18, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Zhao, L.; Trotter, J.H.; Rusiana, I.; Peters, M.M.; Li, Q.; Donaldson, E.; Banko, J.L.; Keenoy, K.E.; Rebeck, G.W.; et al. Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J. Psychopharmacol. 2013, 27, 386–395. [Google Scholar] [CrossRef]
- Hethorn, W.R.; Ciarlone, S.L.; Filonova, I.; Rogers, J.T.; Aguirre, D.; Ramirez, R.A.; Grieco, J.C.; Peters, M.M.; Gulick, D.; Anderson, A.E.; et al. Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome. Eur. J. Neurosci. 2015, 41, 1372–1380. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Philips, G.T.; Wasser, C.R.; Durakoglugil, M.S.; Masiulis, I.; Upadhaya, A.; Pohlkamp, T.; Coskun, C.; Kotti, T.; Steller, L.; et al. Reelin protects against amyloid β toxicity in vivo. Sci. Signal 2015, 8, ra67. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Pohlkamp, T.; Durakoglugil, M.S.; Wong, C.H.; Beck, J.K.; Lane-Donovan, C.; Plattner, F.; Herz, J. Reversal of ApoE4-induced recycling block as a novel prevention approach for Alzheimer’s disease. Elife 2018, 7, e40048. [Google Scholar] [CrossRef] [PubMed]
- Durakoglugil, M.S.; Chen, Y.; White, C.L.; Kavalali, E.T.; Herz, J. Reelin signaling antagonizes β-amyloid at the synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.E.; Shugart, Y.Y.; Huang, D.T.; Shahwan, S.A.; Grant, P.E.; Hourihane, J.O.; Martin, N.D.; Walsh, C.A. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 2000, 26, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, L.; Charnas, L. Evidence for a discrete behavioral phenotype in the oculocerebrorenal syndrome of Lowe. Am. J. Med. Genet. 1995, 59, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Chan, A.H.; Judge, L.M.; Yoo, J.; Huang, M.; Nguyen, T.D.; Lizarraga, P.P.; So, P.-L.; Conklin, B.R. Isolation of single-base genome-edited human iPS cells without antibiotic selection. Nat. Methods 2014, 11, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ward, M.E.; Chen, R.; Liu, K.; Tracy, T.E.; Chen, X.; Xie, M.; Sohn, P.D.; Ludwig, C.; Meyer-Franke, A.; et al. Scalable Production of iPSC-Derived Human Neurons to Identify Tau-Lowering Compounds by High-Content Screening. Stem Cell Rep. 2017, 9, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Fernandopulle, M.S.; Prestil, R.; Grunseich, C.; Wang, C.; Gan, L.; Ward, M.E. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Curr. Protoc. Cell Biol. 2018, 79, e51. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef]
- Labun, K.; Montague, T.G.; Gagnon, J.A.; Thyme, S.B.; Valen, E. CHOPCHOP v2: A web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016, 44, W272–W276. [Google Scholar] [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.D.; Guardia, C.M.; De Pace, R.; Bonifacino, J.S.; Saric, A. Measurement of Lysosome Positioning by Shell Analysis and Line Scan. Methods Mol. Biol. 2022, 2473, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Caracci, M.O.; Pizarro, H.; Alarcón-Godoy, C.; Fuentealba, L.M.; Farfán, P.; De Pace, R.; Santibañez, N.; Cavieres, V.A.; Pástor, T.P.; Bonifacino, J.S.; et al. The Reelin receptor ApoER2 is a cargo for the adaptor protein complex AP-4: Implications for Hereditary Spastic Paraplegia. Prog. Neurobiol. 2024, 234, 102575. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, H.; Aguet, F.; Sage, D.; Unser, M. 3-D PSF fitting for fluorescence microscopy: Implementation and localization application. J. Microsc. 2013, 249, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Sage, D.; Donati, L.; Soulez, F.; Fortun, D.; Schmit, G.; Seitz, A.; Guiet, R.; Vonesch, C.; Unser, M. DeconvolutionLab2: An open-source software for deconvolution microscopy. Methods 2017, 115, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Pirruccello, M.; Nandez, R.; Idevall-Hagren, O.; Alcazar-Roman, A.; Abriola, L.; Berwick, S.A.; Lucast, L.; Morel, D.; De Camilli, P. Identification of inhibitors of inositol 5-phosphatases through multiple screening strategies. ACS Chem. Biol. 2014, 9, 1359–1368. [Google Scholar] [CrossRef]
- Oltrabella, F.; Pietka, G.; Ramirez, I.B.; Mironov, A.; Starborg, T.; Drummond, I.A.; Hinchliffe, K.A.; Lowe, M. The Lowe syndrome protein OCRL1 is required for endocytosis in the zebrafish pronephric tubule. PLoS Genet. 2015, 11, e1005058. [Google Scholar] [CrossRef]
- Santana, J.; Marzolo, M.P. The functions of Reelin in membrane trafficking and cytoskeletal dynamics: Implications for neuronal migration, polarization and differentiation. Biochem. J. 2017, 474, 3137–3165. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.N.J.; Michael Mccaffery, J.; Emr, S.D. A Membrane Coat Complex Essential for Endosome-to-Golgi Retrograde Transport in Yeast. J. Cell Biol. 1998, 142, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.E.; Healy, M.D.; Collins, B.M. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Bugarcic, A.; Zhe, Y.; Kerr, M.C.; Griffin, J.; Collins, B.M.; Teasdale, R.D. Vps26A and Vps26B subunits define distinct retromer complexes. Traffic 2011, 12, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.M.; Norwood, S.J.; Kerr, M.C.; Mahony, D.; Seaman, M.N.; Teasdale, R.D.; Owen, D.J. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 2008, 9, 366–379. [Google Scholar] [CrossRef]
- Williams, D.M.; Gungordu, L.; Jackson-Crawford, A.; Lowe, M. Assessment of endocytic traffic and Ocrl function in the developing zebrafish neuroepithelium. J. Cell Sci. 2022, 135, jcs260339. [Google Scholar] [CrossRef]
- Zhu, S.; Dai, J.; Liu, H.; Cong, X.; Chen, Y.; Wu, Y.; Hu, H.; Heng, B.C.; Ouyang, H.W.; Zhou, Y. Down-regulation of Rac GTPase-activating protein OCRL1 causes aberrant activation of Rac1 in osteoarthritis development. Arthritis Rheumatol. 2015, 67, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, R.V.; Scott, J.L.; Frick, C.T. Cellular and molecular interactions of phosphoinositides and peripheral proteins. Chem. Phys. Lipids 2014, 182, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, J.R.; Al-Maawali, A.; Smith, L.; Al-Hashim, A.; Brill, J.A.; Dowling, J.J. The expanding spectrum of neurological disorders of phosphoinositide metabolism. Dis. Models Mech. 2019, 12, dmm038174. [Google Scholar] [CrossRef]
- Mcnally, K.E.; Faulkner, R.; Steinberg, F.; Gallon, M.; Ghai, R.; Pim, D.; Langton, P.; Pearson, N.; Danson, C.M.; Nägele, H.; et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017, 19, 1214–1225. [Google Scholar] [CrossRef]
- Donoso, M.; Cancino, J.; Lee, J.; van Kerkhof, P.; Retamal, C.; Bu, G.; Gonzalez, A.; Cáceres, A.; Marzolo, M.P. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol. Biol. Cell 2009, 20, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhof, P.; Lee, J.; Mccormick, L.; Tetrault, E.; Lu, W.; Schoenfish, M.; Oorschot, V.; Strous, G.J.; Klumperman, J.; Bu, G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005, 24, 2851–2861. [Google Scholar] [CrossRef]
- Ghai, R.; Bugarcic, A.; Liu, H.; Norwood, S.J.; Skeldal, S.; Coulson, E.J.; Li, S.S.; Teasdale, R.D.; Collins, B.M. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E643–E652. [Google Scholar] [CrossRef]
- Farfán, P.; Lee, J.; Larios, J.; Sotelo, P.; Bu, G.; Marzolo, M.P. A Sorting Nexin 17-Binding Domain Within the <scp>LRP1</scp> Cytoplasmic Tail Mediates Receptor Recycling Through the Basolateral Sorting Endosome. Traffic 2013, 14, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, W.; Sailler, B.; Strasser, V.; Recheis, B.; Fasching, D.; Kahr, L.; Schneider, W.J.; Nimpf, J. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002, 21, 4259–4267. [Google Scholar] [CrossRef]
- Lee, J.; Retamal, C.; Cuitiño, L.; Caruano-Yzermans, A.; Shin, J.-E.; Van Kerkhof, P.; Marzolo, M.-P.; Bu, G. Adaptor Protein Sorting Nexin 17 Regulates Amyloid Precursor Protein Trafficking and Processing in the Early Endosomes. J. Biol. Chem. 2008, 283, 11501–11508. [Google Scholar] [CrossRef]
- McCarthy, R.A.; Barth, J.L.; Chintalapudi, M.R.; Knaak, C.; Argraves, W.S. Megalin functions as an endocytic sonic hedgehog receptor. J. Biol. Chem. 2002, 277, 25660–25667. [Google Scholar] [CrossRef] [PubMed]
- Spoelgen, R.; Hammes, A.; Anzenberger, U.; Zechner, D.; Andersen, O.M.; Jerchow, B.; Willnow, T.E. LRP2/megalin is required for patterning of the ventral telencephalon. Development 2005, 132, 405–414. [Google Scholar] [CrossRef]
- Willnow, T.E.; Hilpert, J.; Armstrong, S.A.; Rohlmann, A.; Hammer, R.E.; Burns, D.K.; Herz, J. Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. USA 1996, 93, 8460–8464. [Google Scholar] [CrossRef]
- Steinberg, F.; Gallon, M.; Winfield, M.; Thomas, E.C.; Bell, A.J.; Heesom, K.J.; Tavaré, J.M.; Cullen, P.J. A global analysis of SNX27–retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013, 15, 461–471. [Google Scholar] [CrossRef]
- Gallon, M.; Clairfeuille, T.; Steinberg, F.; Mas, C.; Ghai, R.; Sessions, R.B.; Teasdale, R.D.; Collins, B.M.; Cullen, P.J. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. USA 2014, 111, E3604–E3613. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Tello-Lafoz, M.; Norwood, S.J.; Yang, Z.; Clairfeuille, T.; Teasdale, R.D.; Mérida, I.; Collins, B.M. Phosphoinositide binding by the SNX27 FERM domain regulates localisation at the immune synapse of activated T-cells. J. Cell Sci. 2014, 128, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Mobli, M.; Collins, B.M. Measuring interactions of FERM domain-containing sorting Nexin proteins with endosomal lipids and cargo molecules. Methods Enzymol. 2014, 534, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, B.; Guo, Q.; Giménez-Andrés, M.; Chen, K.-E.; Moody, E.R.R.; Evans, A.J.; Chandra, M.; Danson, C.M.; Williams, T.A.; Collins, B.M.; et al. SNX27–Retromer directly binds ESCPE-1 to transfer cargo proteins during endosomal recycling. PLoS Biol. 2022, 20, e3001601. [Google Scholar] [CrossRef]
- Ramirez, I.B.; Pietka, G.; Jones, D.R.; Divecha, N.; Alia, A.; Baraban, S.C.; Hurlstone, A.F.; Lowe, M. Impaired neural development in a zebrafish model for Lowe syndrome. Hum. Mol. Genet. 2012, 21, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Barco, A. CREB’s control of intrinsic and synaptic plasticity: Implications for CREB-dependent memory models. Trends Neurosci. 2010, 33, 230–240. [Google Scholar] [CrossRef]
- Wasser, C.R.; Herz, J. Reelin: Neurodevelopmental Architect and Homeostatic Regulator of Excitatory Synapses. J. Biol. Chem. 2017, 292, 1330–1338. [Google Scholar] [CrossRef]
- Wang, J.; Daniszewski, M.; Hao, M.M.; Hernández, D.; Pébay, A.; Gleeson, P.A.; Fourriere, L. Organelle mapping in dendrites of human iPSC-derived neurons reveals dynamic functional dendritic Golgi structures. Cell Rep. 2023, 42, 112709. [Google Scholar] [CrossRef]
- Caracci, M.O.; Fuentealba, L.M.; Marzolo, M.P. Golgi Complex Dynamics and Its Implication in Prevalent Neurological Disorders. Front. Cell Dev. Biol. 2019, 7, 75. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Markiewicz, R.; Markiewicz-Gospodarek, A.; Borowski, B.; Trubalski, M.; Łoza, B. Reelin Signaling and Synaptic Plasticity in Schizophrenia. Brain Sci. 2023, 13, 1704. [Google Scholar] [CrossRef] [PubMed]
- Joly-Amado, A.; Kulkarni, N.; Nash, K.R. Reelin Signaling in Neurodevelopmental Disorders and Neurodegenerative Diseases. Brain Sci. 2023, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, A.C.; Martín-Cuevas, C.; Crespo-Facorro, B.; Garrido-Torres, N. Reelin Alterations, Behavioral Phenotypes, and Brain Anomalies in Schizophrenia: A Systematic Review of Insights From Rodent Models. Front. Neuroanat. 2022, 16, 844737. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Delaby, E.; Merico, D.; Barbosa, M.; Merikangas, A.; Klei, L.; Thiruvahindrapuram, B.; Xu, X.; Ziman, R.; Wang, Z.; et al. Convergence of Genes and Cellular Pathways Dysregulated in Autism Spectrum Disorders. Am. J. Hum. Genet. 2014, 94, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.C.; Anderson, R.C.; McDermott, K.W. Reelin: Diverse roles in central nervous system development, health and disease. Int. J. Biochem. Cell Biol. 2019, 112, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.; Grasso, E.A.; Di Cara, G.; Riva, A.; Striano, P.; Verrotti, A. The Pathophysiological Link Between Reelin and Autism: Overview and New Insights. Front. Genet. 2022, 13, 869002. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Massaro, C.M.; Palop, J.J.; Thwin, M.T.; Yu, G.Q.; Bien-Ly, N.; Bender, A.; Mucke, L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J. Neurosci. 2007, 27, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Herring, A.; Donath, A.; Steiner, K.M.; Widera, M.P.; Hamzehian, S.; Kanakis, D.; Kölble, K.; ElAli, A.; Hermann, D.M.; Paulus, W.; et al. Reelin depletion is an early phenomenon of Alzheimer’s pathology. J. Alzheimers Dis. 2012, 30, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.N.; Tan, M.S.; Yu, J.T.; Xie, A.M.; Tan, L. The Role of Reelin Signaling in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5692–5700. [Google Scholar] [CrossRef]
- Lopera, F.; Marino, C.; Chandrahas, A.S.; O’Hare, M.; Villalba-Moreno, N.D.; Aguillon, D.; Baena, A.; Sanchez, J.S.; Vila-Castelar, C.; Ramirez Gomez, L.; et al. Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Nat. Med. 2023, 29, 1243–1252. [Google Scholar] [CrossRef]
- Dlugosz, P.; Nimpf, J. The Reelin Receptors Apolipoprotein E receptor 2 (ApoER2) and VLDL Receptor. Int. J. Mol. Sci. 2018, 19, 3090. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Liraz, O.; Ovadia, M.; Michaelson, D. The Role of Impaired Receptor Trafficking in Mediating the Pathological Effects of APOE4 in Alzheimer’s Disease. J. Alzheimers Dis. 2024, 97, 753–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Durakoglugil, M.S.; Xian, X.; Herz, J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. USA 2010, 107, 12011–12016. [Google Scholar] [CrossRef] [PubMed]
- Strasser, V.; Fasching, D.; Hauser, C.; Mayer, H.; Bock, H.H.; Hiesberger, T.; Herz, J.; Weeber, E.J.; Sweatt, J.D.; Pramatarova, A.; et al. Receptor clustering is involved in Reelin signaling. Mol. Cell Biol. 2004, 24, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Weeber, E.J.; Durudas, A.; Qiu, S.; Masiulis, I.; Sweatt, J.D.; Li, W.P.; Adelmann, G.; Frotscher, M.; Hammer, R.E.; et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 2005, 47, 567–579. [Google Scholar] [CrossRef]
- Herz, J.; Chen, Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006, 7, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Cui, D.; Li, Y.; Shi, J.; Xiang, L.; Bian, H.; Ma, Z.; Xia, W.; Wei, G. Carnosic Acid Reverses the Inhibition of ApoE4 on Cell Surface Level of ApoER2 and Reelin Signaling Pathway. J. Alzheimers Dis. 2020, 73, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Serajee, F.J.; Zhong, H.; Mahbubul Huq, A.H. Association of Reelin gene polymorphisms with autism. Genomics 2006, 87, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Konrad, C.; Suslow, T.; Domschke, K.; Birosova, E.; Sehlmeyer, C.; Beste, C. The Reelin (RELN) gene is associated with executive function in healthy individuals. Neurobiol. Learn. Mem. 2010, 94, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Marzan, S.; Aziz, M.A.; Islam, M.S. Association Between REELIN Gene Polymorphisms (rs7341475 and rs262355) and Risk of Schizophrenia: An Updated Meta-analysis. J. Mol. Neurosci. 2021, 71, 675–690. [Google Scholar] [CrossRef]
- Antoniades, D.; Katopodi, T.; Pappa, S.; Lampropoulos, A.; Konsta, V.; Frydas, E.; Mpalogiannis, S.; Hatzistilianou, M. The role of reelin gene polymorphisms in the pathogenesis of Alzheimer’s disease in a Greek population. J. Biol. Regul. Homeost. Agents 2011, 25, 351–358. [Google Scholar] [PubMed]
- Liu, H.; Barnes, J.; Pedrosa, E.; Herman, N.S.; Salas, F.; Wang, P.; Zheng, D.; Lachman, H.M. Transcriptome analysis of neural progenitor cells derived from Lowe syndrome induced pluripotent stem cells: Identification of candidate genes for the neurodevelopmental and eye manifestations. J. Neurodev. Disord. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Balmaceda, V.; Cuchillo-Ibáñez, I.; Pujadas, L.; García-Ayllón, M.S.; Saura, C.A.; Nimpf, J.; Soriano, E.; Sáez-Valero, J. ApoER2 processing by presenilin-1 modulates reelin expression. FASEB J. 2014, 28, 1543–1554. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentealba, L.M.; Pizarro, H.; Marzolo, M.-P. OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses. Biomolecules 2024, 14, 799. https://doi.org/10.3390/biom14070799
Fuentealba LM, Pizarro H, Marzolo M-P. OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses. Biomolecules. 2024; 14(7):799. https://doi.org/10.3390/biom14070799
Chicago/Turabian StyleFuentealba, Luz M., Héctor Pizarro, and María-Paz Marzolo. 2024. "OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses" Biomolecules 14, no. 7: 799. https://doi.org/10.3390/biom14070799
APA StyleFuentealba, L. M., Pizarro, H., & Marzolo, M.-P. (2024). OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses. Biomolecules, 14(7), 799. https://doi.org/10.3390/biom14070799






