Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation
2.2. Human Full-Thickness 3D Skin Culture
2.3. Collagen Type I Immunofluorescence Staining in Skin Tissues
2.4. Hyaluronic Acid and IL-6 Secretion in Tissue Culture Mediums
2.5. ROS and 8-OHdG Levels in Tissue Culture Mediums
2.6. In Vitro Skin Irritation Tests Using Reconstructed Human Epidermis Skin (OECD Guidelines No. 439)
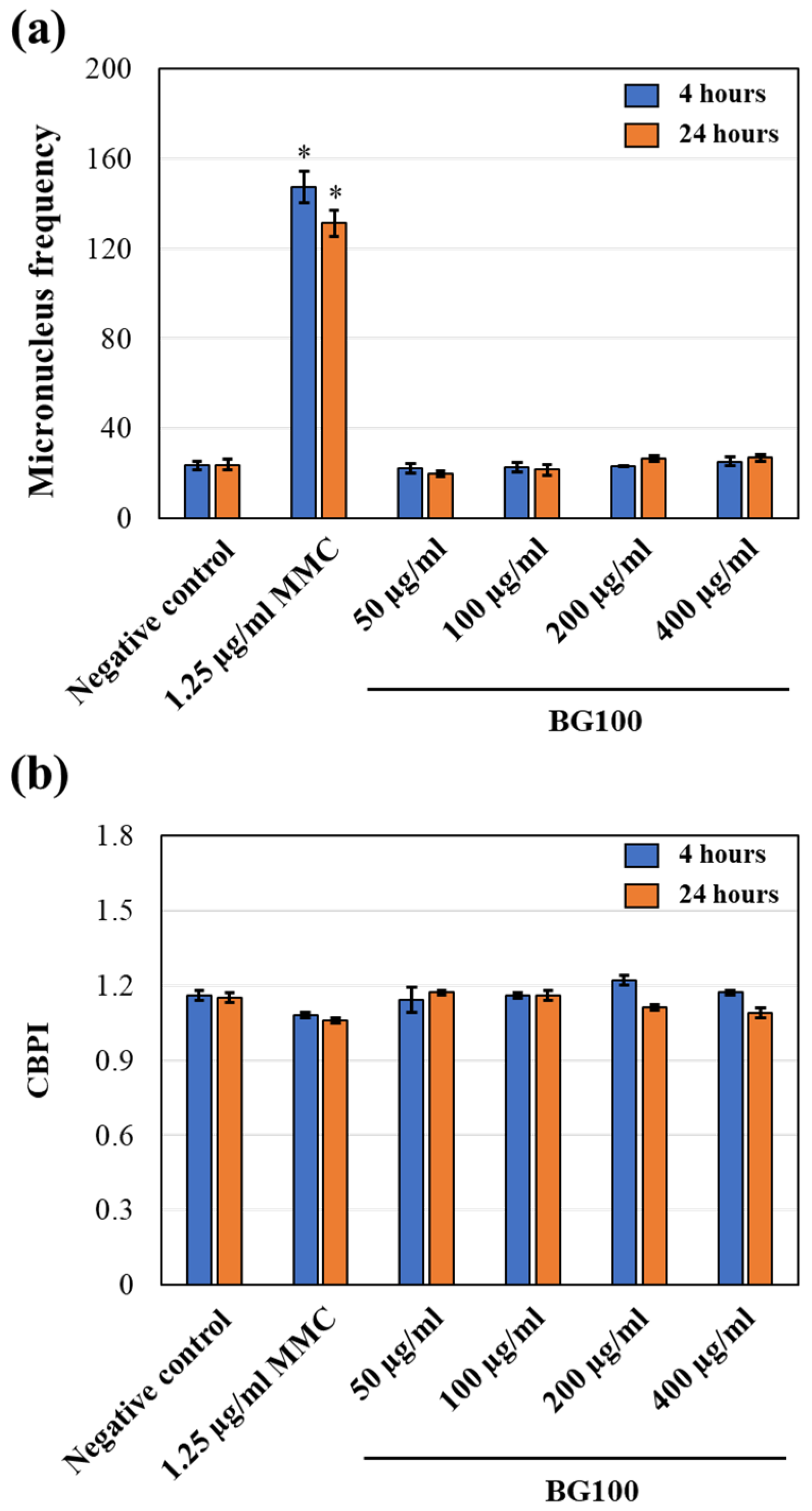
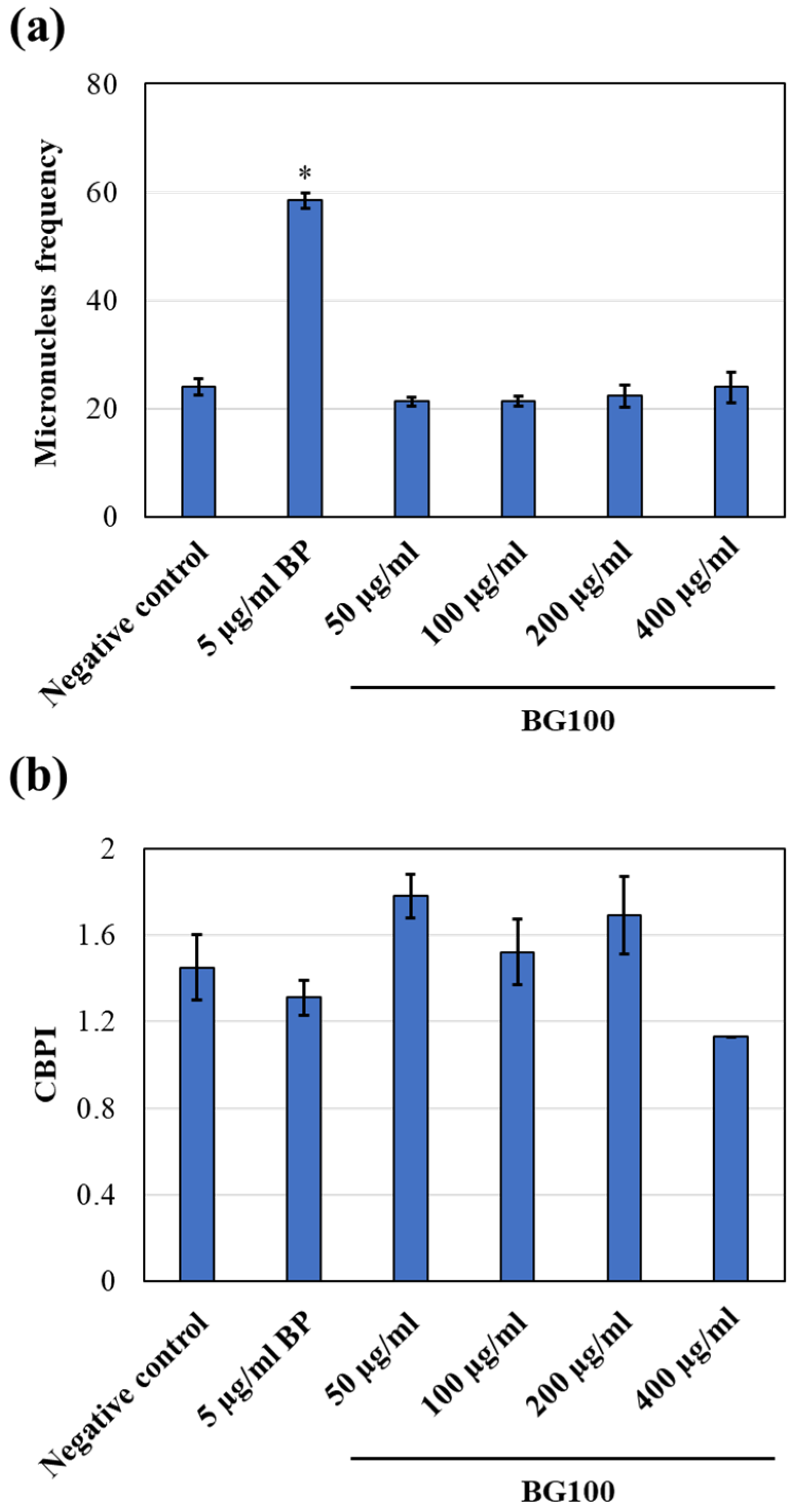
2.7. In Vitro Mammalian Cell Micronucleus Test (OECD Guidelines No. 487)
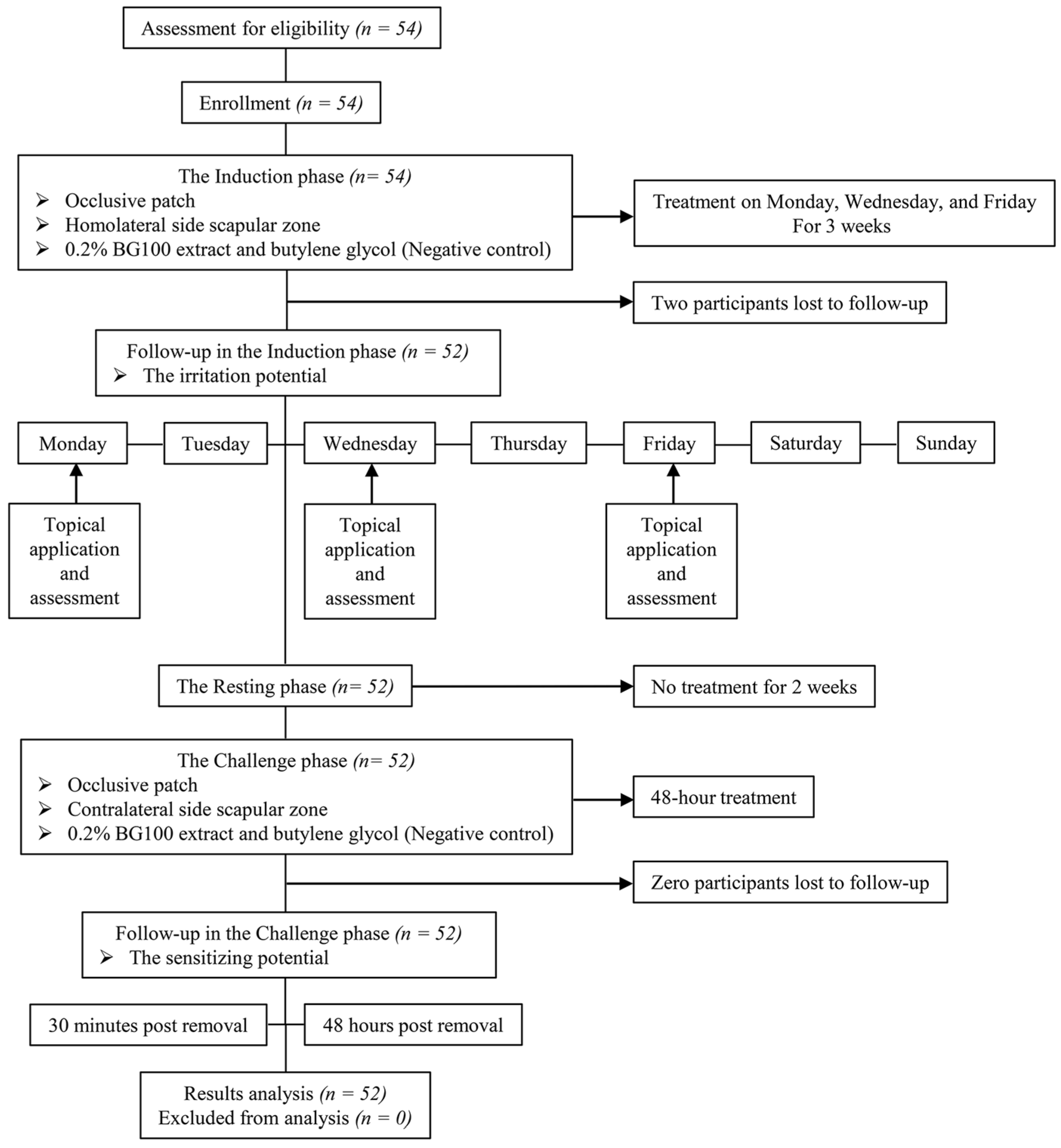
2.8. Human Volunteer Skin Irritation and Sensitization Tests
2.9. Statistical Analysis
3. Results
3.1. Polymethoxyflavone Composition in BG100 Extract
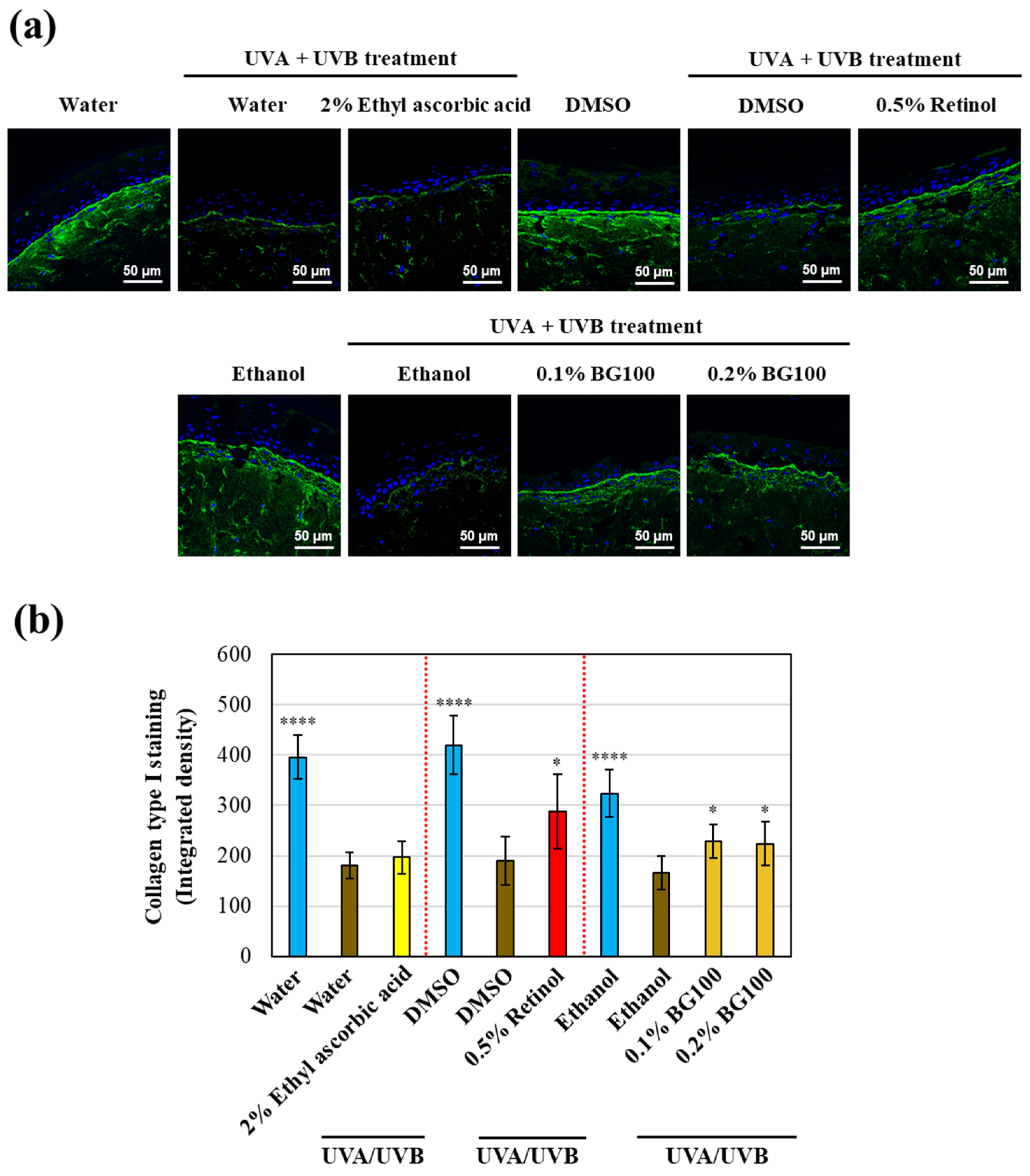
3.2. BG100 Extract-Induced Collagen Type I Stimulation in UV-Exposed Human Full-Thickness 3D Skin Tissues
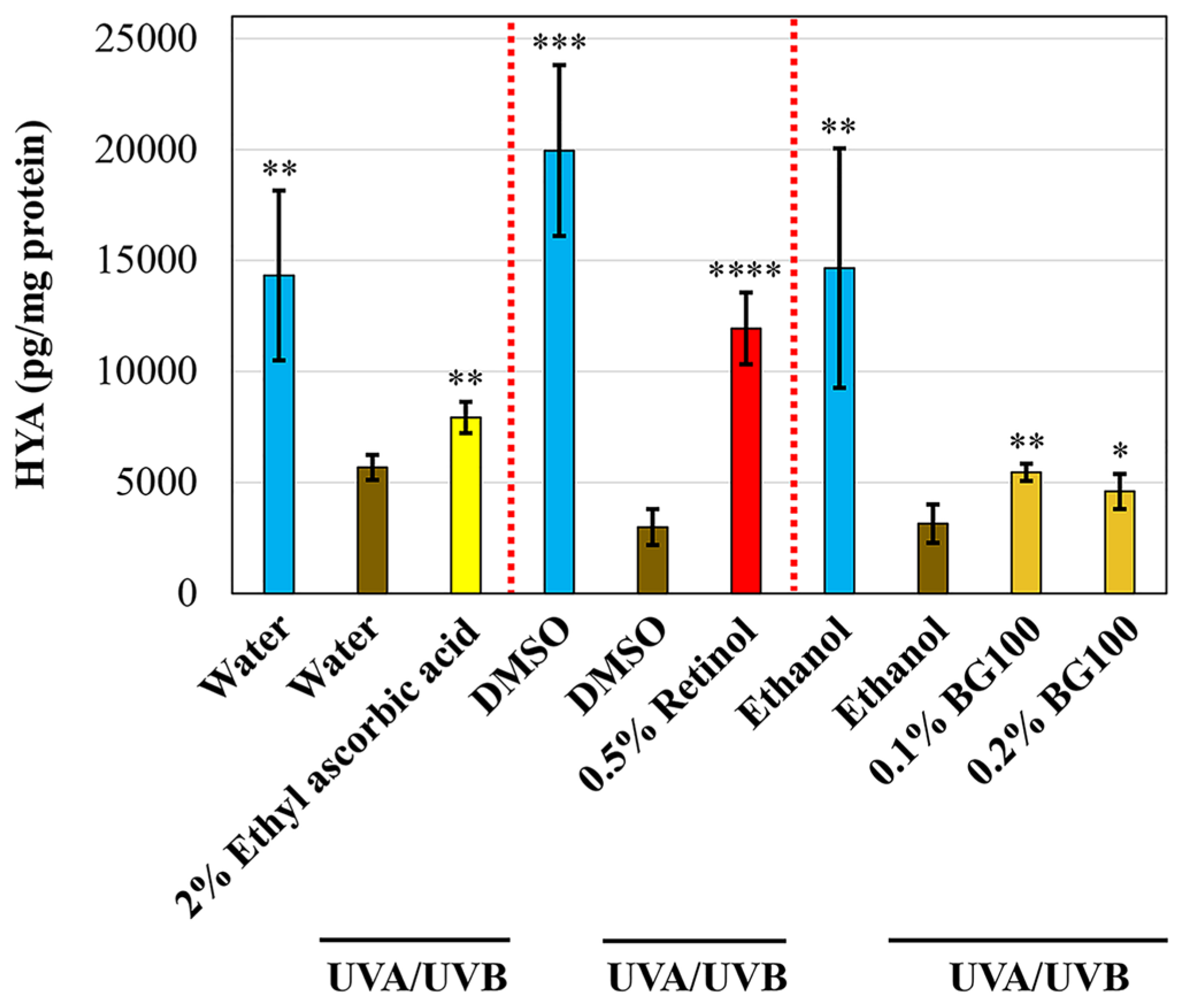
3.3. BG100 Extract-Stimulated Hyaluronic Acid Secretion in UV-Exposed Human Full-Thickness 3D Skin Tissues
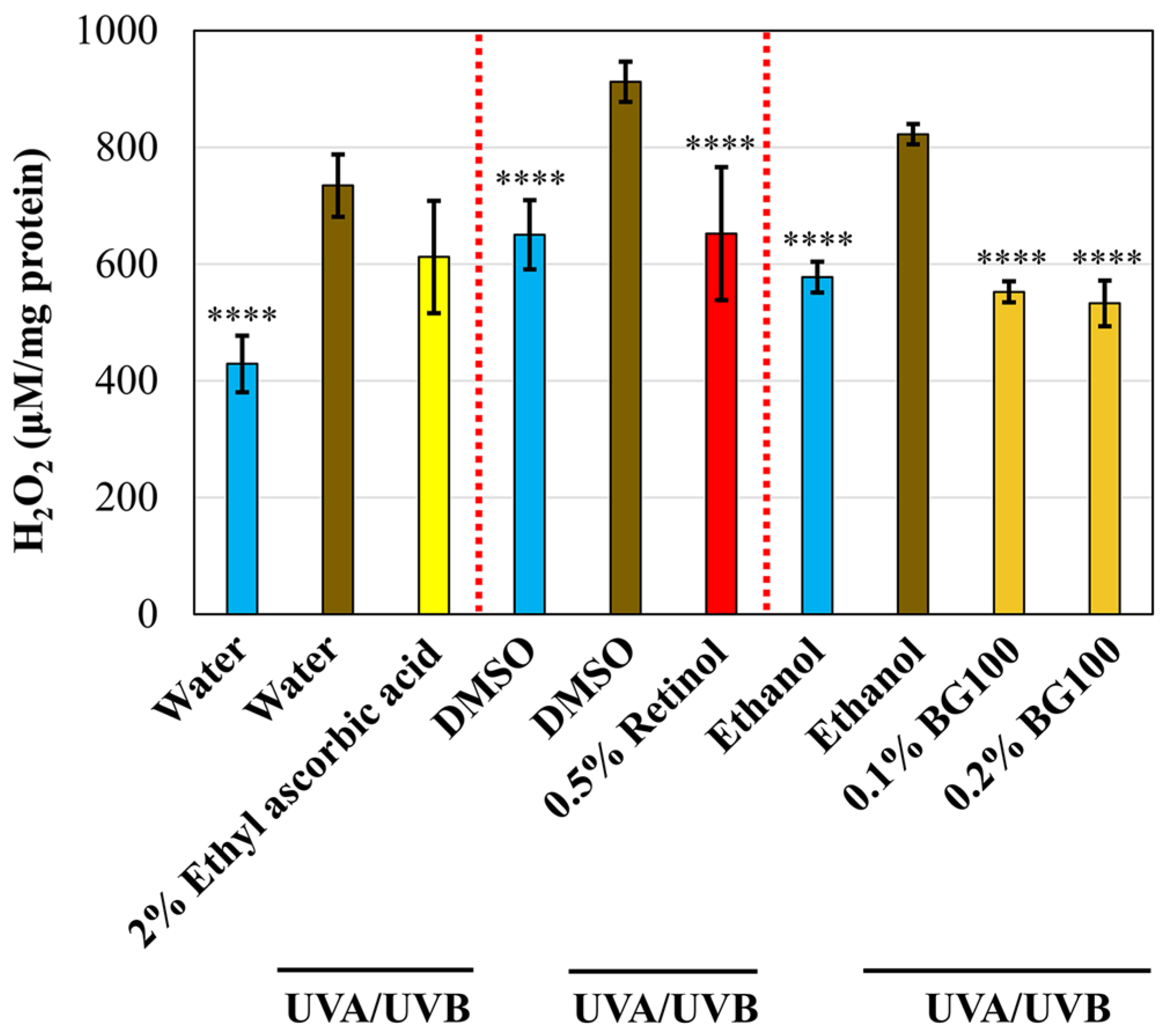
3.4. BG100 Extract-Mediated ROS Inhibition in UV-Exposed Human Full-Thickness 3D Skin Tissues
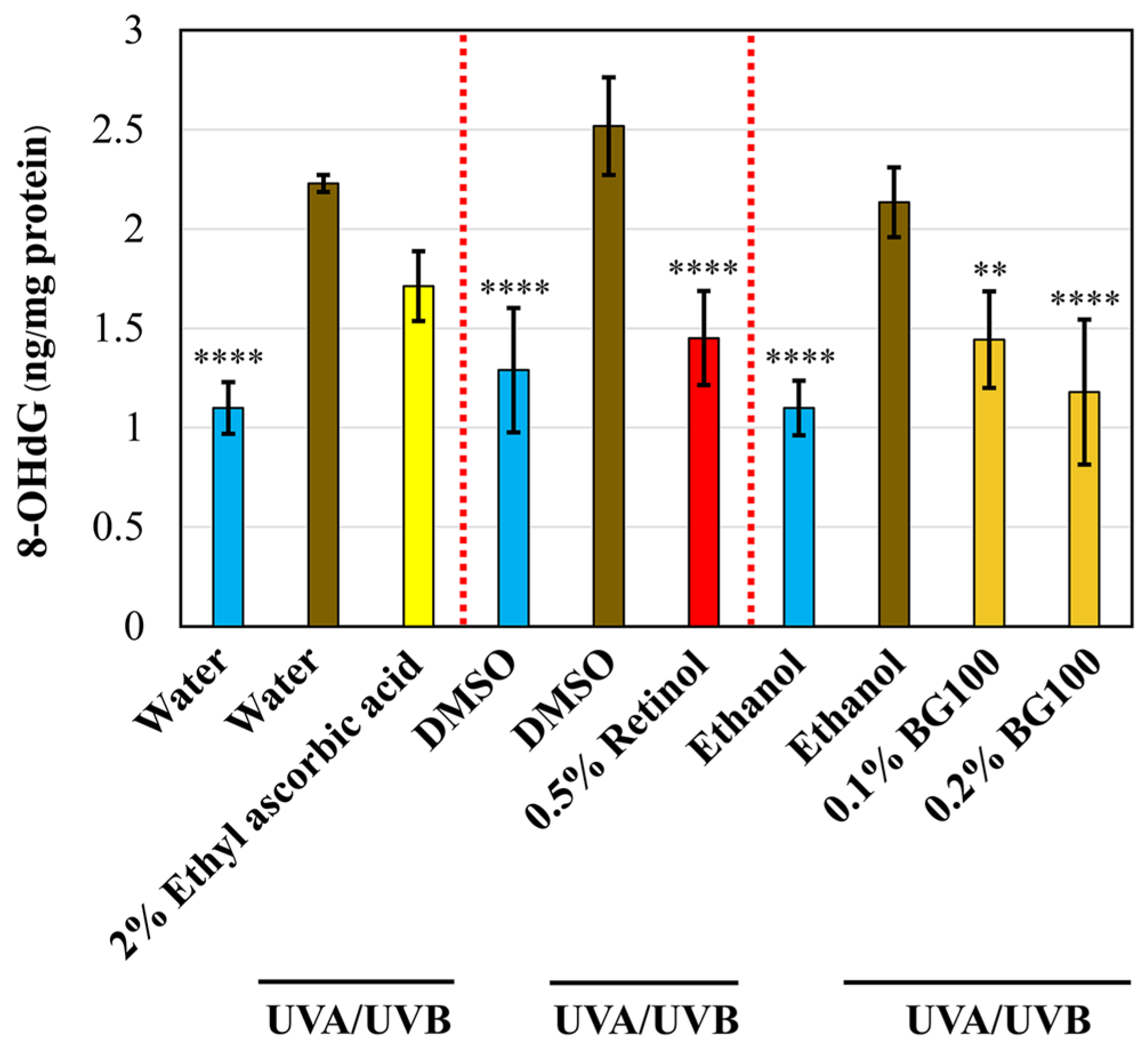
3.5. BG100 Extract-Mediated Attenuation of DNA Damage in UV-Exposed Human Full-Thickness 3D Skin Tissues
3.6. Absence of Irritation by 0.2% BG100 Extract on In Vitro Reconstructed 3D Human Skin Tissues
3.7. Non-Genotoxicity of BG100 Extract on V79-4 Cells
3.8. Non-Irritating and Non-Sensitizing Properties of 0.2% BG100 Extract, as Demonstrated in A Clinical Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2010, 29, 3–14. [Google Scholar] [CrossRef]
- Krutmann, J.; Schroeder, P. Role of Mitochondria in Photoaging of Human Skin: The Defective Powerhouse Model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Saokaew, S.; Wilairat, P.; Raktanyakan, P.; Dilokthornsakul, P.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Clinical Effects of Krachaidum (Kaempferia parviflora): A Systematic Review. J. Evid. Based Complement. Altern. Med. 2016, 22, 413–428. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Sato, K.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ichinose, K.; Aburada, M.; Ochiai, W.; Sugiyama, K.; et al. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018, 72, 136–144. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid. Based Complement. Altern. Med. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Nakata, A.; Koike, Y.; Matsui, H.; Shimada, T.; Aburada, M.; Yang, J. Potent SIRT1 Enzyme-stimulating and Anti-glycation Activities of Polymethoxyflavonoids from Kaempferia parviflora. Nat. Prod. Commun. 2014, 9, 1291–1294. [Google Scholar] [CrossRef]
- Lee, S.; Jang, T.; Kim, K.H.; Kang, K.S. Improvement of Damage in Human Dermal Fibroblasts by 3,5,7-Trimethoxyflavone from Black Ginger (Kaempferia parviflora). Antioxidants 2022, 11, 425. [Google Scholar] [CrossRef]
- Lert-Amornpat, T.; Maketon, C.; Fungfuang, W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia 2017, 49, e12770. [Google Scholar] [CrossRef]
- Kim, J.K.; Mun, S.; Kim, M.; Kim, M.; Sa, B.; Hwang, J. 5,7-Dimethoxyflavone, an activator of PPARα/γ, inhibits UVB-induced MMP expression in human skin fibroblast cells. Exp. Dermatol. 2011, 21, 211–216. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Choi, E.-J.; Choi, Y.; Hwang, J.-K. 5,7-Dimethoxyflavone induces melanogenesis in B16F10 melanoma cells through cAMP-dependent signalling. Exp. Dermatol. 2011, 20, 445–447. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.-K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Tansakul, P.; Tewtrakul, S. Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J. Ethnopharmacol. 2009, 124, 576–580. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimada, T.; Horikawa, T.; Kinoshita, K.; Koyama, K.; Ichinose, K.; Aburada, M.; Takahashi, K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine 2014, 21, 800–806. [Google Scholar] [CrossRef]
- Youn, K.; Lee, J.; Ho, C.-T.; Jun, M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods 2016, 20, 567–574. [Google Scholar] [CrossRef]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2003, 75, 89–92. [Google Scholar] [CrossRef]
- Park, J.-E.; Woo, S.W.; Kim, M.-B.; Kim, C.; Hwang, J.-K. Standardized Kaempferia parviflora Extract Inhibits Intrinsic Aging Process in Human Dermal Fibroblasts and Hairless Mice by Inhibiting Cellular Senescence and Mitochondrial Dysfunction. Evid. Based Complement. Altern. Med. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Park, J.; Pyun, H.; Woo, S.W.; Jeong, J.; Hwang, J. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef]
- Tonsomboon, A.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans. Biology 2021, 10, 264. [Google Scholar] [CrossRef]
- Klinngam, W.; Rungkamoltip, P.; Thongin, S.; Joothamongkhon, J.; Khumkhrong, P.; Khongkow, M.; Namdee, K.; Tepaamorndech, S.; Chaikul, P.; Kanlayavattanakul, M.; et al. Polymethoxyflavones from Kaempferia parviflora ameliorate skin aging in primary human dermal fibroblasts and ex vivo human skin. Biomed. Pharmacother. 2021, 145, 112461. [Google Scholar] [CrossRef]
- Joothamongkhon, J.; Susantikarn, P.; Kongkachana, W.; Ketngamkum, Y.; Batthong, S.; Jomchai, N.; Yingyong, P.; Asawapirom, U.; Tangphatsornruang, S.; Paemanee, A.; et al. Quantitative analysis of methoxyflavones discriminates between the two types of Kaempferia parviflora. Phytochem. Anal. 2022, 33, 670–677. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2021. [Google Scholar]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Walker, A.; Basketter, D.; Baverel, M.; Diembeck, W.; Matthies, W.; Mougin, D.; Paye, M.; Röthlisberger, R.; Dupuis, J. Test guidelines for assessment of skin compatibility of cosmetic finished products in man. Food Chem. Toxicol. 1996, 34, 651–660. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Edwards, C.; Pearse, A.; Marks, R.; Nishimori, Y.; Matsumoto, K.; Kawai, M. Degenerative Alterations of Dermal Collagen Fiber Bundles in Photodamaged Human Skin and UV-Irradiated Hairless Mouse Skin: Possible Effect on Decreasing Skin Mechanical Properties and Appearance of Wrinkles. J. Investig. Dermatol. 2001, 117, 1458–1463. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.; Wang, Z.; Li, X.-Y.; Quan, T.; Chung, J.H.; Kang, S.; Voorhees, J.J. c-Jun–dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Investig. 2000, 106, 663–670. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential Regulation of Hyaluronan Metabolism in the Epidermal and Dermal Compartments of Human Skin by UVB Irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Yasui, H.; Sakurai, H. Chemiluminescent Detection and Imaging of Reactive Oxygen Species in Live Mouse Skin Exposed to UVA. Biochem. Biophys. Res. Commun. 2000, 269, 131–136. [Google Scholar] [CrossRef]
- Brenneisen, P.; Wenk, J.; Klotz, L.O.; Wlaschek, M.; Briviba, K.; Krieg, T.; Sies, H.; Scharffetter-Kochanek, K. Central Role of Ferrous/Ferric Iron in the Ultraviolet B Irradiation-mediated Signaling Pathway Leading to Increased Interstitial Collagenase (Matrix-degrading Metalloprotease (MMP)-1) and Stromelysin-1 (MMP-3) mRNA Levels in Cultured Human Dermal Fibroblasts. J. Biol. Chem. 1998, 273, 5279–5287. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Xiong, Z.-M.; O’donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Hoffmann, S. Skin Irritation Validation Study Phase II: Analysis of the primary endpoint MTT and the secondary endpoint IL1-α. IHCP-Inst. Health Consum. Prot. 2006. [Google Scholar] [CrossRef]
- Ma, X.; Wang, F.; Wang, B. Application of an in vitro reconstructed human skin on cosmetics in skin irritation tests. J. Cosmet. Dermatol. 2021, 20, 1933–1941. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. ANTI-AGING Med. 2009, 6, 46–59. [Google Scholar] [CrossRef]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic Ultraviolet B Irradiation Causes Loss of Hyaluronic Acid from Mouse Dermis because of Down-Regulation of Hyaluronic Acid Synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Wang, P.-W.; Hung, Y.-C.; Lin, T.-Y.; Fang, J.-Y.; Yang, P.-M.; Chen, M.-H.; Pan, T.-L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef]
- Krutmann, J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000, 23, S22–S26. [Google Scholar] [CrossRef]
- MatTek. Tissue model: EpiDermFTTM. Available online: https://www.mattek.com/mattekproduct/epidermft/ (accessed on 28 April 2024).
- Welss, T.; A Basketter, D.; Schröder, K.R. In vitro skin irritation: Facts and future. State of the art review of mechanisms and models. Toxicol. Vitr. 2004, 18, 231–243. [Google Scholar] [CrossRef]
- Bernhofer, L.; Seiberg, M.; Martin, K. The Influence of the Response of Skin Equivalent Systems to Topically Applied Consumer Products by Epithelial—Mesenchymal Interactions. Toxicol. Vitr. 1999, 13, 219–229. [Google Scholar] [CrossRef]
- Kirsch-Volders, M. Towards a validation of the micronucleus test. Mutat. Res. Toxicol. Environ. Mutagen. 1997, 392, 1–4. [Google Scholar] [CrossRef]
- EFSA Scientific Committee (SC); More, S.J.; Bampidis, V.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on aneugenicity assessment. EFSA J. 2021, 19, e06770. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Sofuni, T.; Aardema, M.; Albertini, S.; Eastmond, D.; Fenech, M.; Ishidate, M.; Kirchner, S.; Lorge, E.; Morita, T.; et al. Report from the in vitro micronucleus assay working group. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 540, 153–163. [Google Scholar] [CrossRef]
- Zijno, A.; Leopardi, P.; Marcon, F.; Crebelli, R. Analysis of chromosome segregation by means of fluorescence in situ hybridization: Application to cytokinesis-blocked human lymphocytes. Mutat. Res. Mol. Mech. Mutagen. 1996, 372, 211–219. [Google Scholar] [CrossRef]
- Politano, V.T.; Api, A.M. The Research Institute for Fragrance Materials’ human repeated insult patch test protocol. Regul. Toxicol. Pharmacol. 2008, 52, 35–38. [Google Scholar] [CrossRef]

| Score | Quotation | Criteria | |
|---|---|---|---|
| Erythema (E) | Edema (O) | ||
| 0 | Absent | No erythema | No edema |
| 0.5 | Very slight | Barely perceptible: pinkish coloration of one part of tested area | Palpable, barely visible |
| 1 | Slight | Pinkish coloration of the complete tested area or rather visible on one part of the tested area | Palpable, visible |
| 2 | Obvious | Obvious erythema covering the whole tested area | Obvious edema (thickness < 1 mm) with or without blister(s) or vesicle(s) |
| 3 | Important | Severe erythema covering all the tested area or obvious erythema diffusing outside the tested area | Severe edema (thickness ≥ 1 mm or diffusing outside the tested area) with or without blister(s) or vesicle(s) |
| M.C.I.I. | Class |
|---|---|
| M.C.I.I. < 0.25 | Non-irritating |
| 0.25 ≤ M.C.I.I. < 0.50 | Very slightly irritating |
| 0.5 ≤ M.C.I.I. < 1 | Slightly irritating |
| 1 ≤ M.C.I.I. < 2 | Moderate irritating |
| M.C.I.I. ≥ 2 | Irritating |
| Criterion | ICDRG Quotation | Numeric Score Quotation |
|---|---|---|
| No reaction | 0 | 0 |
| Doubtful reaction | ? | 0.5 |
| Erythema and edema | + | 1 |
| Erythema, edema, and vesicles | ++ | 2 |
| Severe reaction with blisters or post-blister ulcerations | +++ | 3 |
| Reading Zone | Criterion | Score | Day 37 | Day 39 | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Contralateral | T+ | 1 | 1.9% | 0 | 0.0% | |
| No reaction | 0 | 43 | 82.7% | 51 | 98.1% | |
| Doubtful reaction | 0.5 (?) | 8 | 15.4% | 1 | 1.9% | |
| Erythema and edema | 1 (+) | 0 | 0.0% | 0 | 0.0% | |
| Erythema, edema, and vesicles | 2 (++) | 0 | 0.0% | 0 | 0.0% | |
| Severe reaction with blisters or post-blister ulcerations | 3 (+++) | 0 | 0.0% | 0 | 0.0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinngam, W.; Rungkamoltip, P.; Wongwanakul, R.; Joothamongkhon, J.; Du-a-man, S.; Khongkow, M.; Asawapirom, U.; Iempridee, T.; Ruktanonchai, U. Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial. Biomolecules 2024, 14, 776. https://doi.org/10.3390/biom14070776
Klinngam W, Rungkamoltip P, Wongwanakul R, Joothamongkhon J, Du-a-man S, Khongkow M, Asawapirom U, Iempridee T, Ruktanonchai U. Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial. Biomolecules. 2024; 14(7):776. https://doi.org/10.3390/biom14070776
Chicago/Turabian StyleKlinngam, Wannita, Phetploy Rungkamoltip, Ratjika Wongwanakul, Jaruwan Joothamongkhon, Sakkarin Du-a-man, Mattaka Khongkow, Udom Asawapirom, Tawin Iempridee, and Uracha Ruktanonchai. 2024. "Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial" Biomolecules 14, no. 7: 776. https://doi.org/10.3390/biom14070776
APA StyleKlinngam, W., Rungkamoltip, P., Wongwanakul, R., Joothamongkhon, J., Du-a-man, S., Khongkow, M., Asawapirom, U., Iempridee, T., & Ruktanonchai, U. (2024). Skin Rejuvenation Efficacy and Safety Evaluation of Kaempferia parviflora Standardized Extract (BG100) in Human 3D Skin Models and Clinical Trial. Biomolecules, 14(7), 776. https://doi.org/10.3390/biom14070776


_Kwok.png)






