SKGQA, a Peptide Derived from the ANA/BTG3 Protein, Cleaves Amyloid-β with Proteolytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Peptides
2.2. Analysis of Proteolytic Activity and Determination of Cleavage Sites
2.3. Cell Experiments
2.4. Stereo-Structure Analysis
3. Results
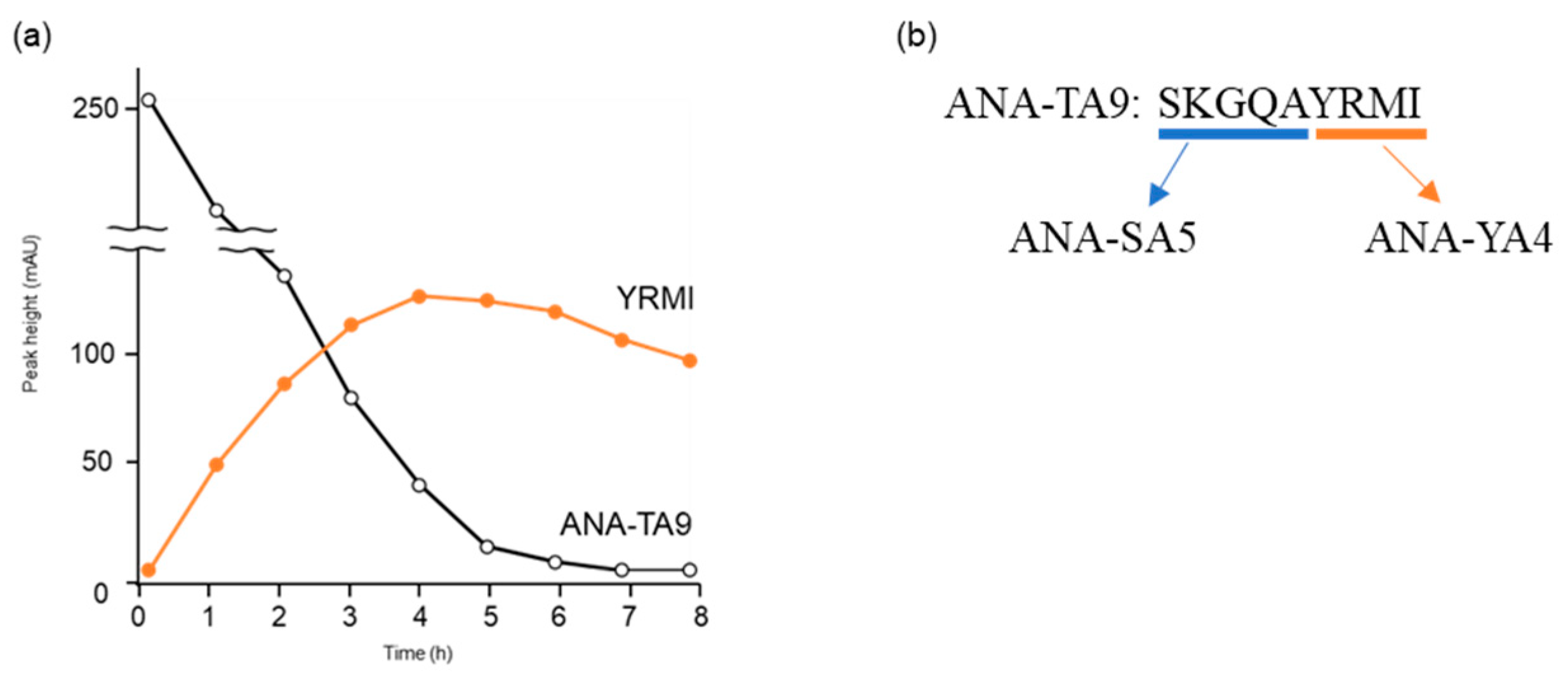
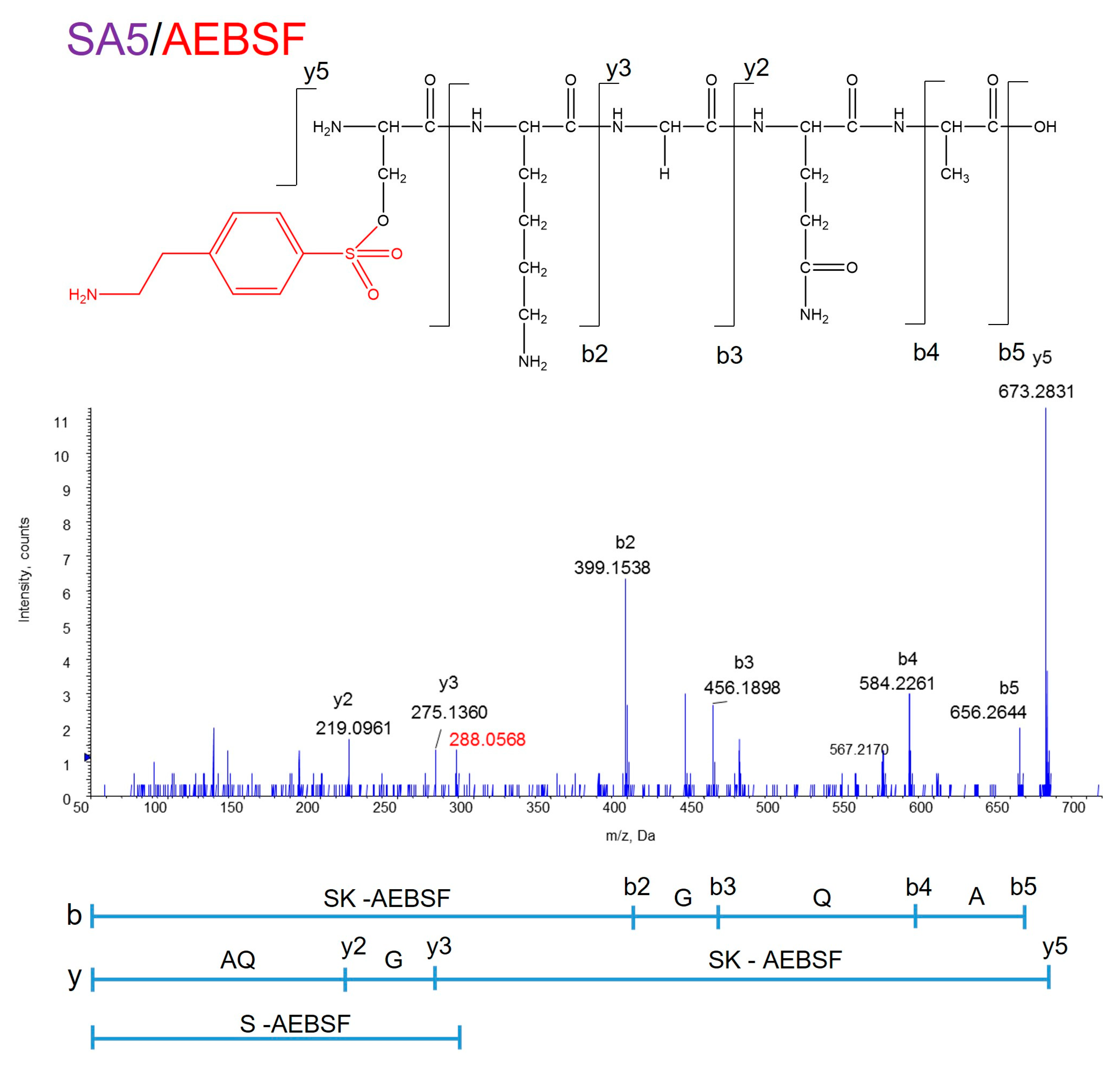
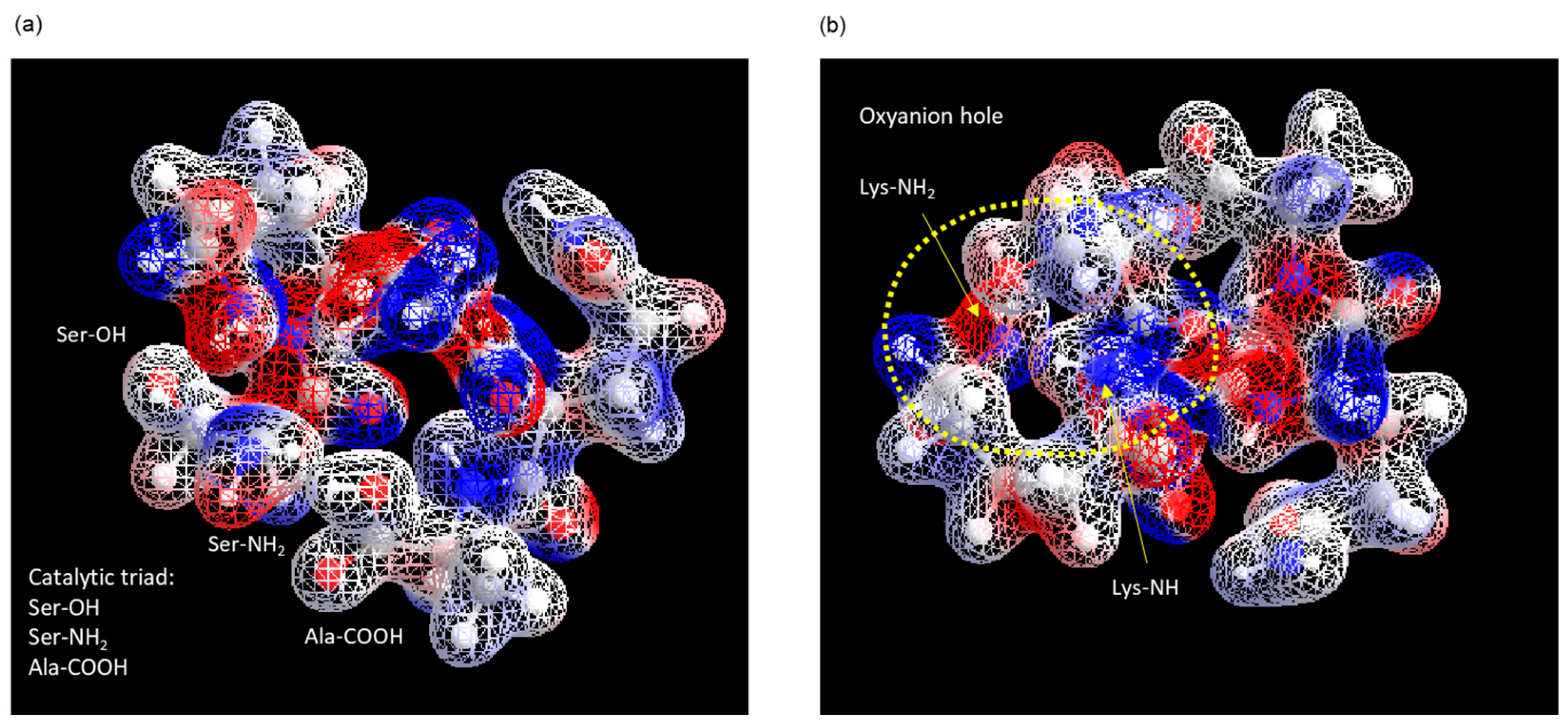
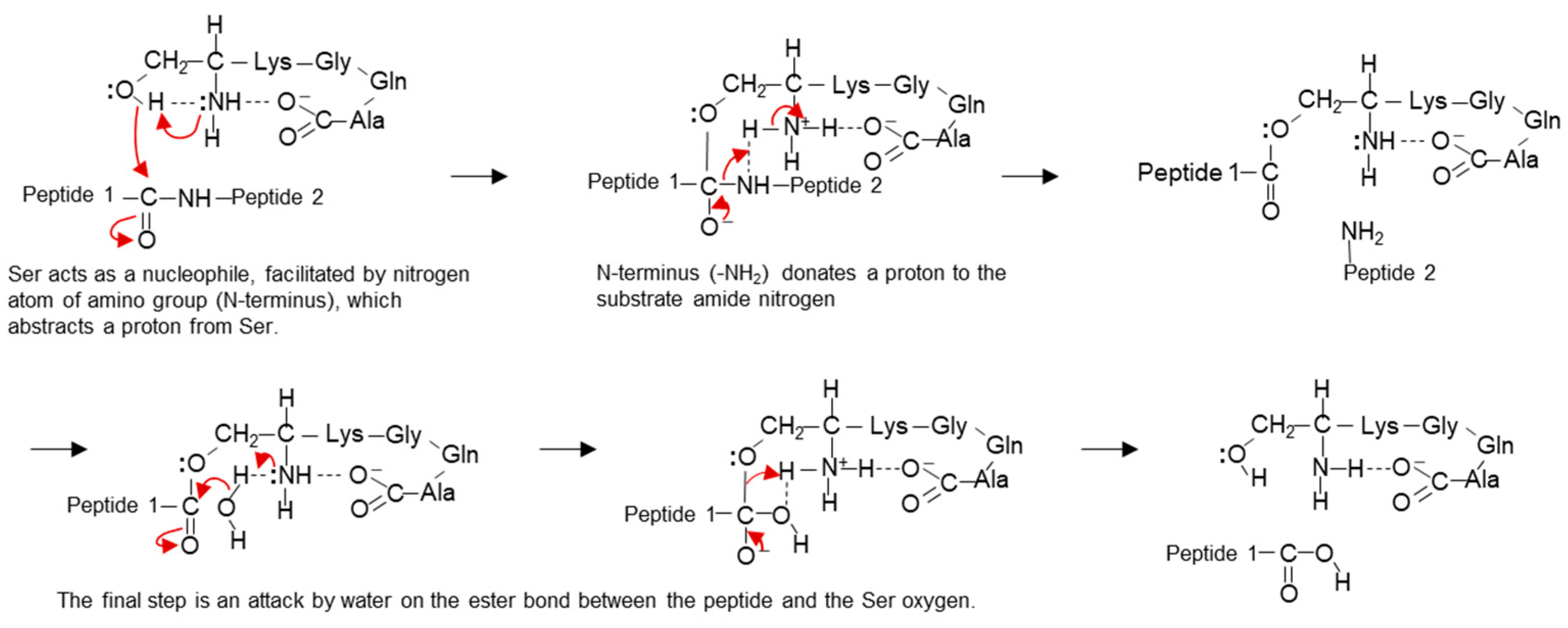
3.1. Identification of the Active Center of ANA-SA5
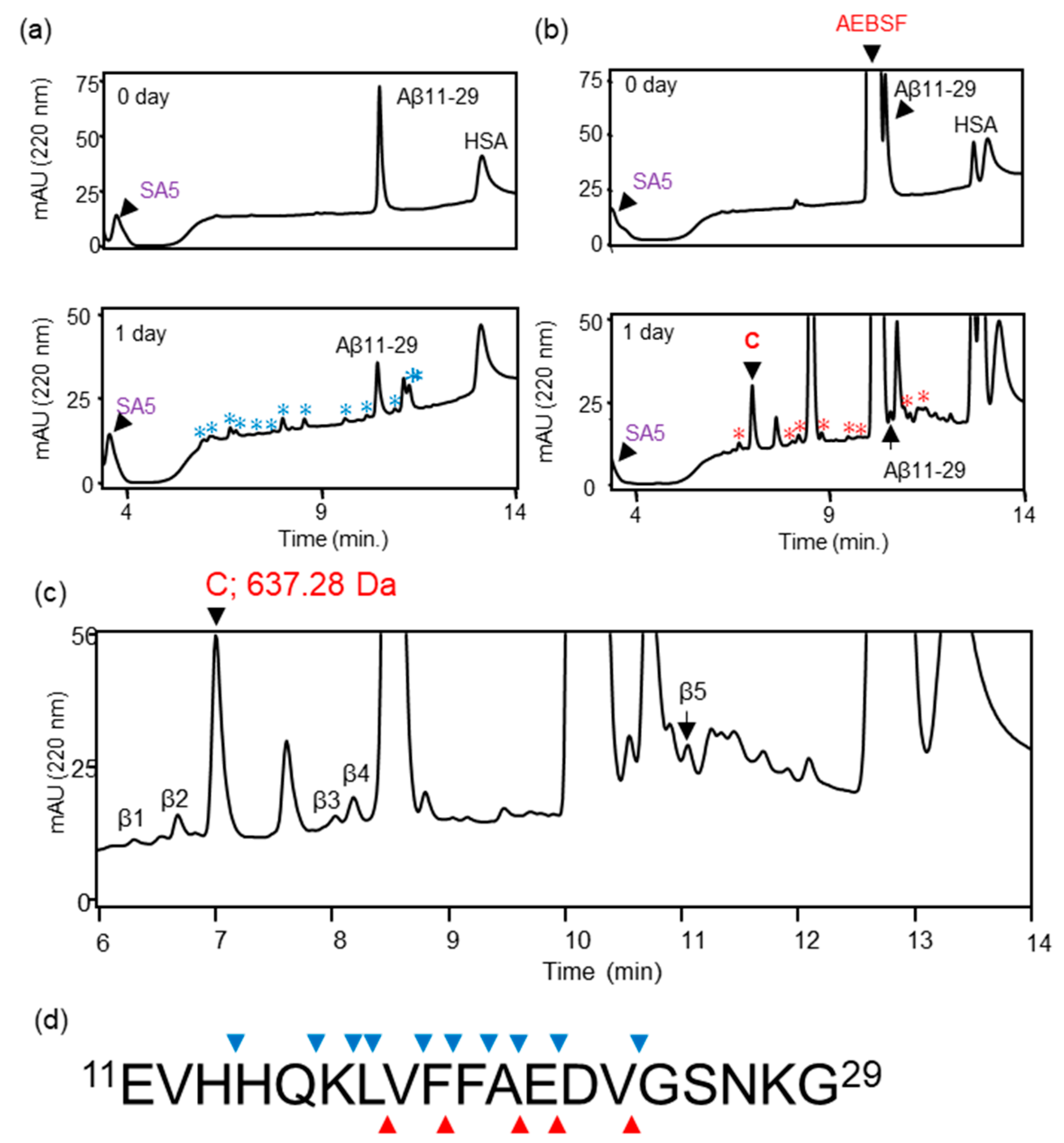
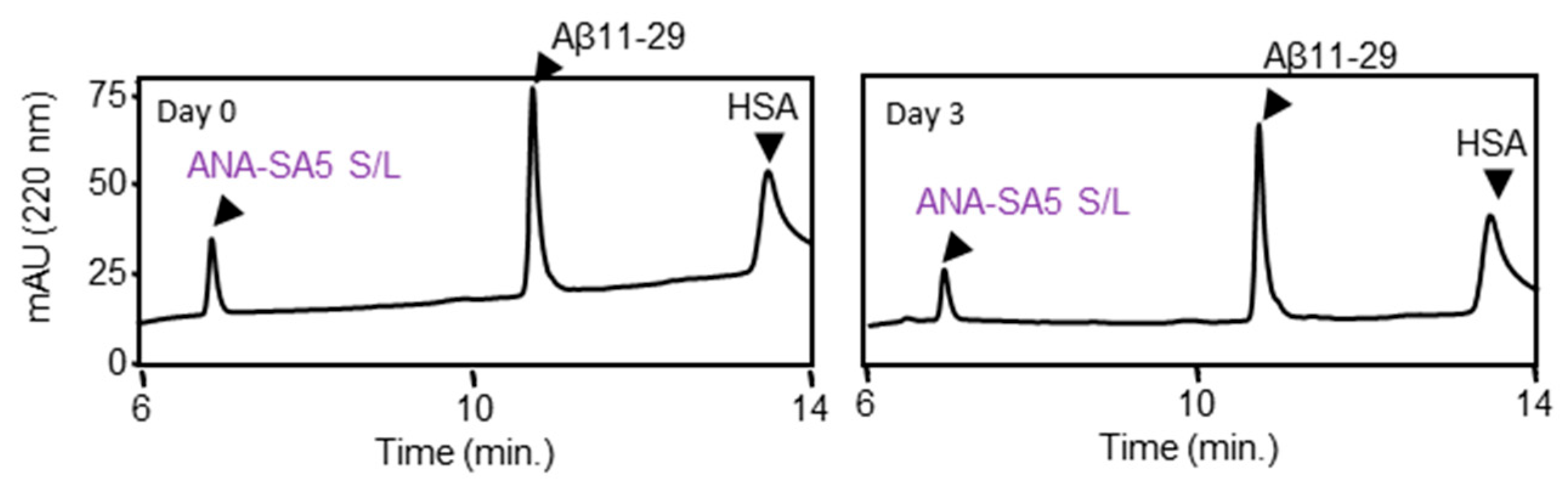
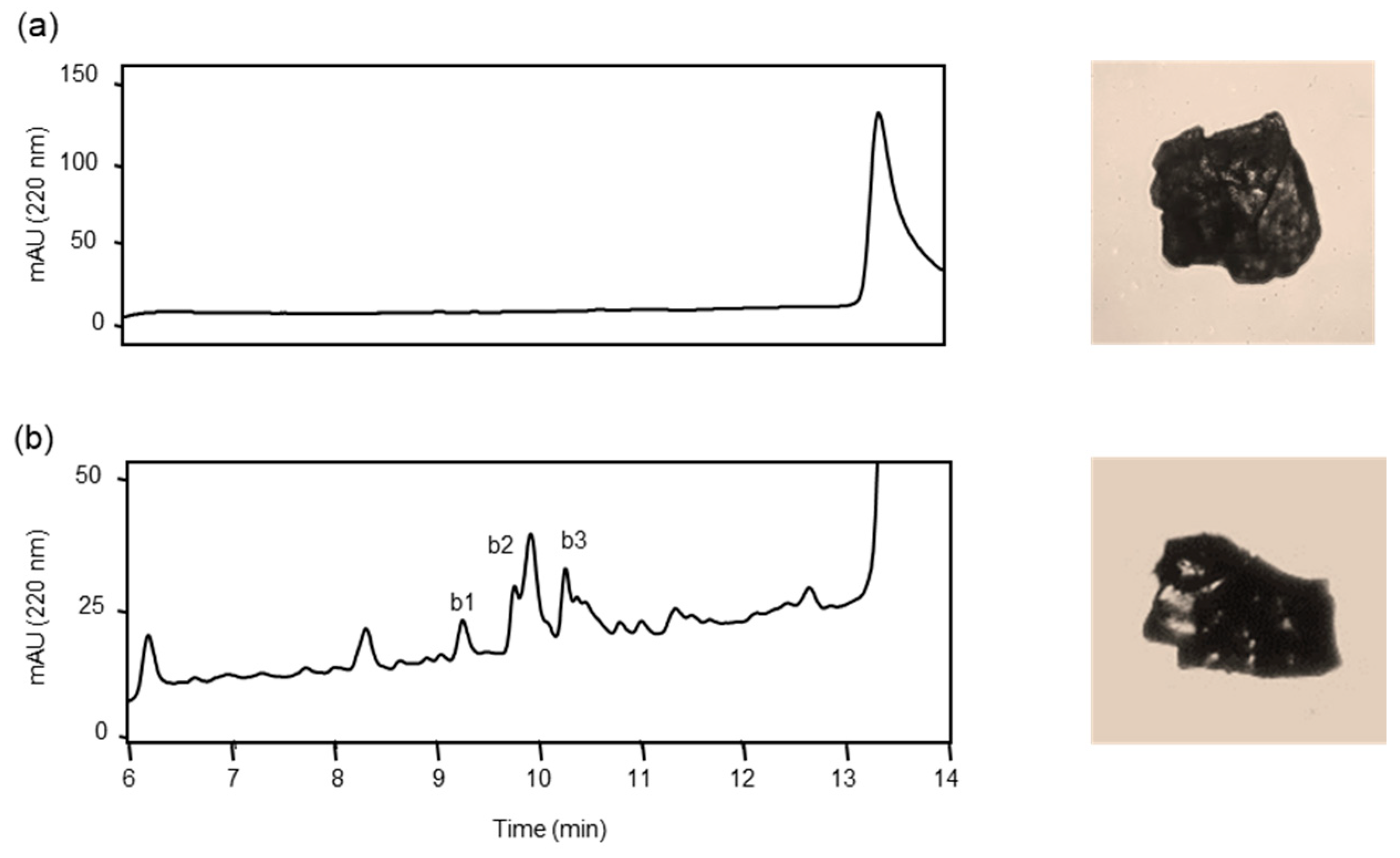
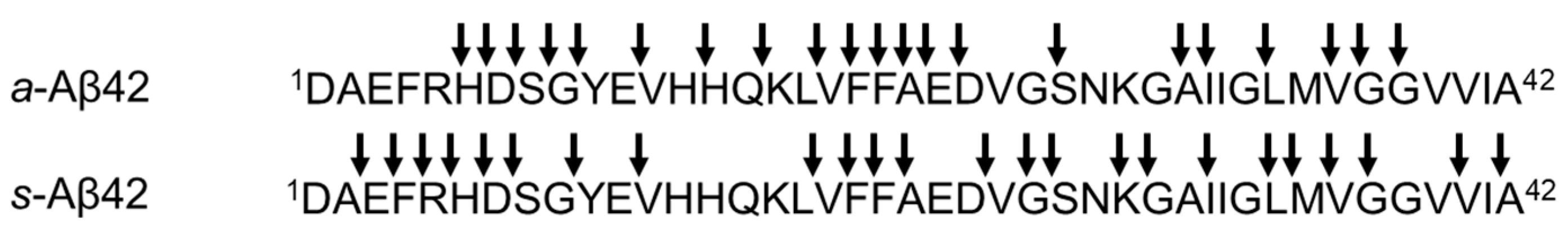
3.2. Proteolysis of Authentic Soluble Form Aβ42 (a-Aβ42) by ANA-SA5
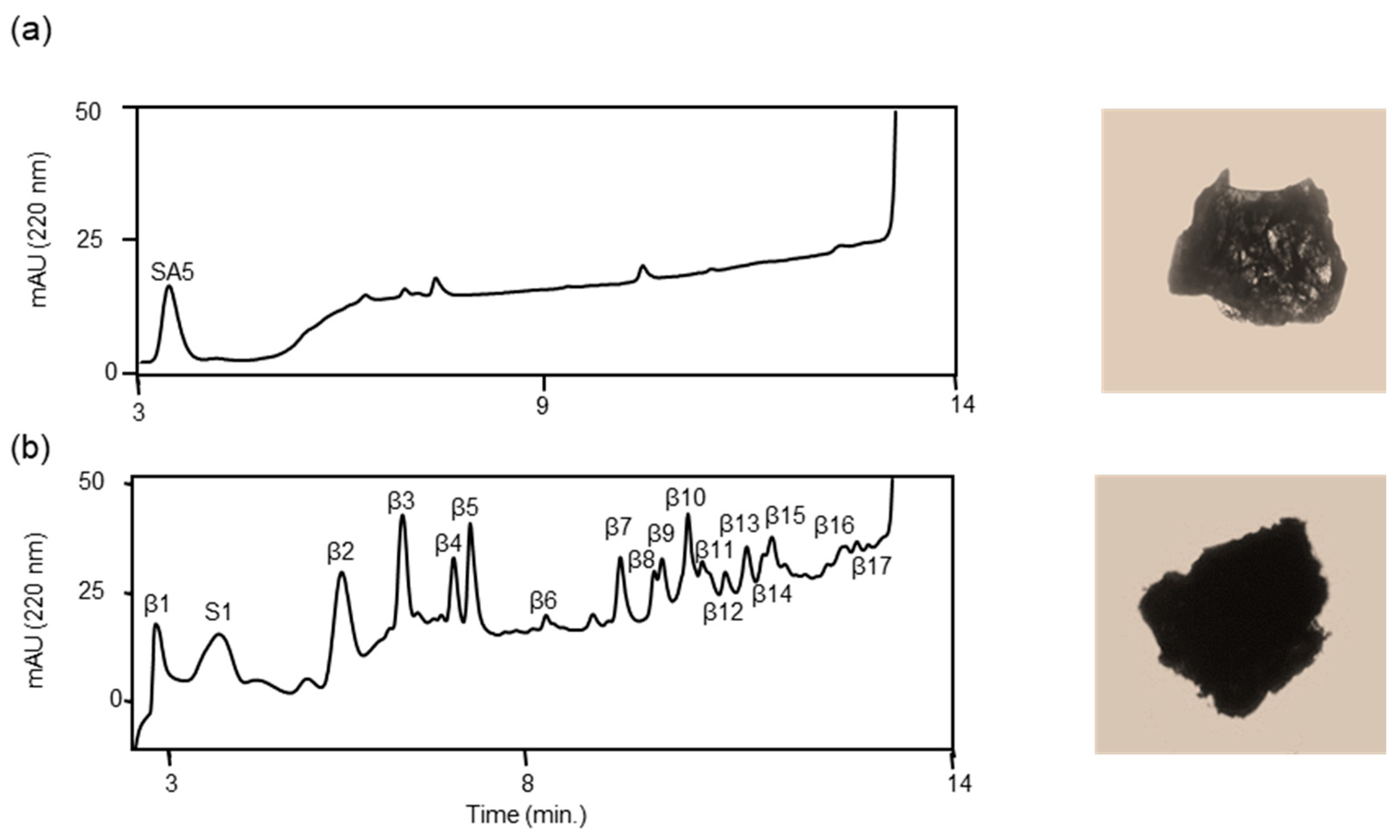
3.3. Proteolysis of Solid Form Aβ42 (s-Aβ42) by ANA-SA5
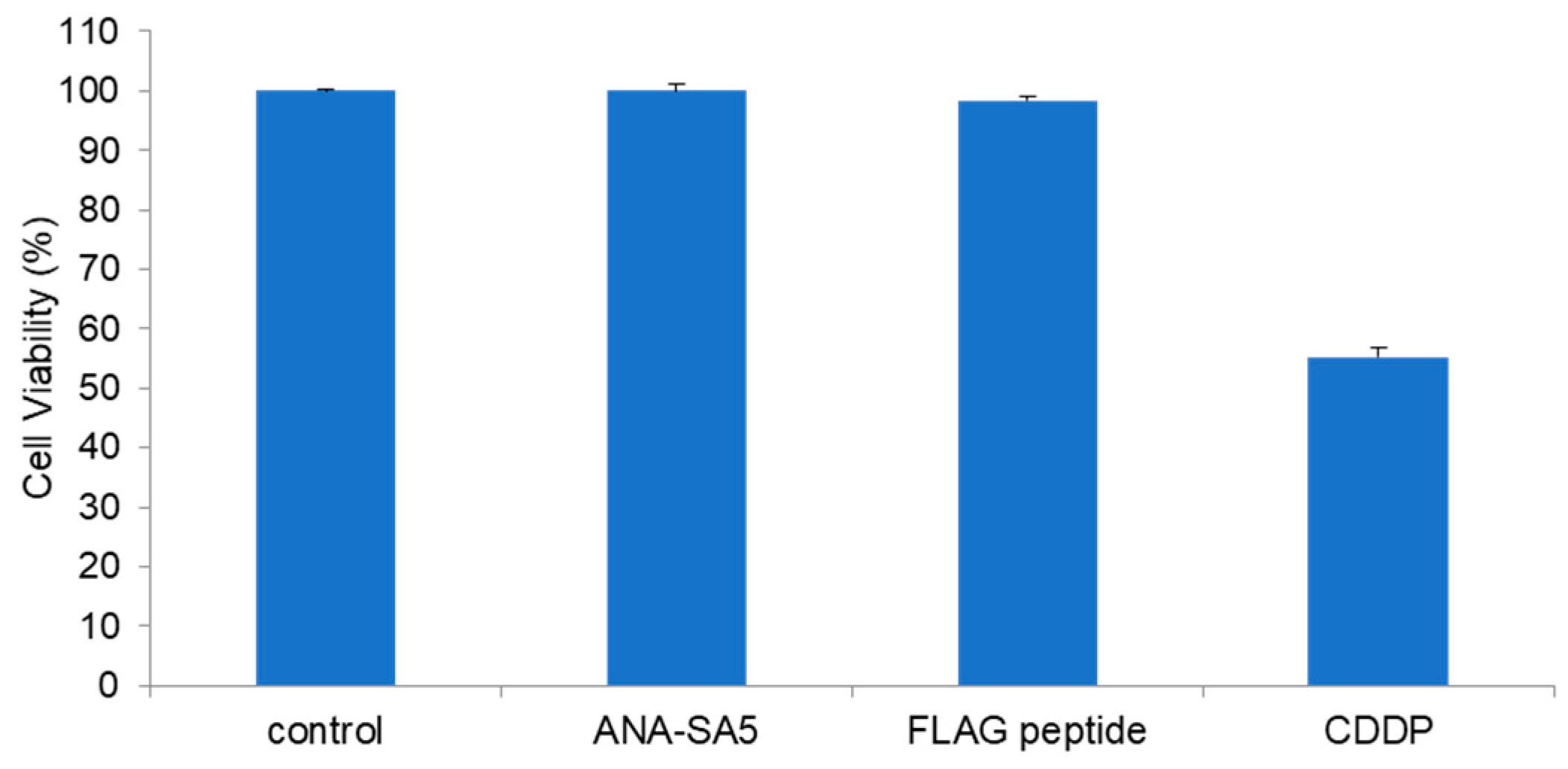
3.4. Effect of ANA-SA5 on the Growth of A549 Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, R.; Akizawa, T.; Konishi, M. Structure–Activity Relationship of 5-Mer Catalytides, GSGYR and RYGSG. Biomolecules 2022, 12, 1766. [Google Scholar] [CrossRef]
- Nakamura, R.; Konishi, M.; Higashi, Y.; Saito, M.; Akizawa, T. Five-Mer Peptides Prevent Short-Term Spatial Memory Deficits in Aβ25-35-Induced Alzheimer’s Model Mouse by Suppressing Aβ25-35 Aggregation and Resolving Its Aggregate Form. Alzheimers Res. Ther. 2023, 15, 83. [Google Scholar] [CrossRef]
- Nakamura, R.; Konishi, M.; Hatakawa, Y.; Saito, M.; Akizawa, T. The Novel Catalytic Peptide, A Synthetic Nona-Peptide (JAL-TA9) Derived from Tob1 Protein, Digests the Amyloid-β Peptide. J. R. Sci. 2019, 1, 30. [Google Scholar]
- Nakamura, R.; Konishi, M.; Higashi, Y.; Saito, M.; Akizawa, T. Comparison of the Catalytic Activities of 5-Mer Synthetic Peptides Derived from Box A Region of Tob/BTG Family Proteins against the Amyloid-Beta Fragment Peptides. Integr. Mol. Med. 2019, 6, 1–4. [Google Scholar] [CrossRef]
- Nakamura, R.; Konishi, M.; Taniguchi, M.; Hatakawa, Y.; Akizawa, T. The Discovery of Shorter Synthetic Proteolytic Peptides Derived from Tob1 Protein. Peptides 2019, 116, 71–77. [Google Scholar] [CrossRef]
- Hatakawa, Y.; Nakamura, R.; Konishi, M.; Sakane, T.; Saito, M.; Akizawa, T. Catalytides Derived from the Box A Region in the ANA/BTG3 Protein Cleave Amyloid-β Fragment Peptide. Heliyon 2019, 5, e02454. [Google Scholar] [CrossRef]
- Hatakawa, Y.; Tanaka, A.; Furubayashi, T.; Nakamura, R.; Konishi, M.; Akizawa, T.; Sakane, T. Direct Delivery of ANA-TA9, a Peptide Capable of Aβ Hydrolysis, to the Brain by Intranasal Administration. Pharmaceutics 2021, 13, 1673. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef]
- Young, L.M.; Saunders, J.C.; Mahood, R.A.; Revill, C.H.; Foster, R.J.; Tu, L.H.; Raleigh, D.P.; Radford, S.E.; Ashcroft, A.E. Screening and Classifying Small-Molecule Inhibitors of Amyloid Formation Using Ion Mobility Spectrometry-Mass Spectrometry. Nat. Chem. 2015, 7, 73–81. [Google Scholar] [CrossRef]
- Malar, D.S.; Suryanarayanan, V.; Prasanth, M.I.; Singh, S.K.; Balamurugan, K.; Devi, K.P. Vitexin Inhibits Aβ25-35 Induced Toxicity in Neuro-2a Cells by Augmenting Nrf-2/HO-1 Dependent Antioxidant Pathway and Regulating Lipid Homeostasis by the Activation of LXR-α. Toxicol. Vitr. 2018, 50, 160–171. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. Correction: A Randomized, Double-Blind, Phase 2b Proof-of-Concept Clinical Trial in Early Alzheimer’s Disease with Lecanemab, an Anti-Aβ Protofibril Antibody. Alzheimers Res. Ther. 2022, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Racke, M.M.; Boone, L.I.; Hepburn, D.L.; Parsadainian, M.; Bryan, M.T.; Ness, D.K.; Piroozi, K.S.; Jordan, W.H.; Brown, D.D.; Hoffman, W.P.; et al. Exacerbation of Cerebral Amyloid Angiopathy-Associated Microhemorrhage in Amyloid Precursor Protein Transgenic Mice by Immunotherapy Is Dependent on Antibody Recognition of Deposited Forms of Amyloid β. J. Neurosci. 2005, 25, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.; Greenberg, S.M.; Savoiardo, M.; Gardinetti, M.; Chiapparini, L.; Raicher, I.; Nitrini, R.; Sakaguchi, H.; Brioschi, M.; Billo, G.; et al. Anti-Amyloid β Autoantibodies in Cerebral Amyloid Angiopathy-Related Inflammation: Implications for Amyloid-Modifying Therapies. Ann. Neurol. 2013, 73, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Zotova, E.; Weller, R.O.; Love, S.; Neal, J.W.; Pickering, R.M.; Wilkinson, D.; Holmes, C.; Nicoll, J.A.R. Consequence of Aβ Immunization on the Vasculature of Human Alzheimer’s Disease Brain. Brain 2008, 131, 3299–3310. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Topphof, U.; Casazza, S.; Varrin-Doyer, M.; Pekarek, K.; Sobe, R.A.; Hauser, S.L.; Oksenberg, J.R.; Zamvil, S.S.; Baranzini, S.E. Tob1 Plays a Critical Role in the Activation of Encephalitogenic t Cells in Cns Autoimmunity. J. Exp. Med. 2013, 210, 1301–1309. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Wu, J.; Li, L. BTG/Tob Family Members Tob1 and Tob2 Inhibit Proliferation of Mouse Embryonic Stem Cells via Id3 MRNA Degradation. Biochem. Biophys. Res. Commun. 2015, 462, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Morita, M.; Wang, H.; Suzuki, T.; Yang, W.; Luo, Y.; Zhao, C.; Yu, Y.; Bartlam, M.; Yamamoto, T.; et al. Crystal Structures of Human BTG2 and Mouse TIS21 Involved in Suppression of CAF1 Deadenylase Activity. Nucleic Acids Res. 2008, 36, 6872–6881. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Yoshida, M.; Emi, M.; Nakamura, Y.; Onda, M.; Yoshida, Y.; Nishiyama, A.; Yamamoto, T. Tob, a Novel Protein That Interacts with P185erbB2, Is Associated with Anti-Proliferative Activity. Oncogene 1996, 12, 705–713. [Google Scholar]
- Horiuchi, M.; Takeuchi, K.; Noda, N.; Muroya, N.; Suzuki, T.; Nakamura, T.; Kawamura-Tsuzuku, J.; Takahasi, K.; Yamamoto, T.; Inagaki, F. Structural Basis for the Antiproliferative Activity of the Tob-HCaf1 Complex. J. Biol. Chem. 2009, 284, 13244–13255. [Google Scholar] [CrossRef]
- Hatakawa, Y.; Nakamura, R.; Konishi, M.; Sakane, T.; Tanaka, A.; Matsuda, A.; Saito, M.; Akizawa, T. Amyloid Beta Cleavage by ANA-TA9, a Synthetic Peptide from the ANA/BTG3 Box A Region. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12146. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yamamoto, S.; Sano, N.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct Drug Delivery of Low-Permeable Compounds to the Central Nervous System Via Intranasal Administration in Rats and Monkeys. Pharm. Res. 2019, 36, 76. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4523. [Google Scholar] [CrossRef] [PubMed]
- Carl, W.C.; Aileen, J.A. A Potential Role for Apoptosis in Neurodegeneration and Alzheimer’s Disease. Mol. Neurobiol. 1995, 10, 19–45. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Tzabazis, A.Z.; Klukinov, M.; Manering, N.A.; Yeomans, D.C. Intranasal Administration for Pain: Oxytocin and Other Polypeptides. Pharmaceutics 2021, 13, 1088. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Drug Targeting of Hypocretin-1 (Orexin-A) to the Central Nervous System. J. Pharm. Sci. 2009, 98, 2501–2515. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Liu, H.J.; Cheng, S.M.; Wang, Z.L.; Cheng, X.; Yu, H.X.; Liu, X.F. Direct Transport of VEGF from the Nasal Cavity to Brain. Neurosci. Lett. 2009, 449, 108–111. [Google Scholar] [CrossRef]
- Thorne, R.G.; Hanson, L.R.; Ross, T.M.; Tung, D.; Frey, W.H. Delivery of Interferon-β to the Monkey Nervous System Following Intranasal Administration. Neuroscience 2008, 152, 785–797. [Google Scholar] [CrossRef]
- Nonaka, N.; Farr, S.A.; Kageyama, H.; Shioda, S.; Banks, W.A. Delivery of Galanin-like Peptide to the Brain: Targeting with Intranasal Delivery and Cyclodextrins. J. Pharmacol. Exp. Ther. 2008, 325, 513–519. [Google Scholar] [CrossRef]
- Rodríguez, J.C.G.; Teste, I.S. The Nasal Route as a Potential Pathway for Delivery of Erythropoietin in the Treatment of Acute Ischemic Stroke in Humans. Sci. World J. 2009, 9, 970–981. [Google Scholar] [CrossRef]
- Zada, M.H.; Kubek, M.; Khan, W.; Kumar, A.; Domb, A. Dispersible Hydrolytically Sensitive Nanoparticles for Nasal Delivery of Thyrotropin Releasing Hormone (TRH). J. Control. Release 2019, 295, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.H.; Liu, J.; Chen, X.; Thorne, R.G.; Fawcett, J.R.; Ala, T.A.; Rahman, Y.E. Delivery of 125I-NGF to the Brain via the Olfactory Route. Drug Deliv. J. Deliv. Target. Ther. Agents 1997, 4, 87–92. [Google Scholar] [CrossRef]

| Peak | Fragment | Calculated Mass | Observed Mass |
|---|---|---|---|
| β1 | DVGSNKG | 676.3260 | 676.3221 |
| GSNKG | 461.2307 | 461.2234 | |
| β2 | EDVGSNKG | 805.3686 | 805.3601 |
| β3 | EVHHQKL | 890.4842 | 890.5015 |
| β4 | FAEDVGSNKG | 1023.4741 | 1023.4890 |
| β5 | EVHHQKLVFFAE | 1482.76 | 1482.7860 |
| Length (Å) | |
|---|---|
| Ser (-OH)-Ser(-NH2) | 2.26 |
| Ala (-COOH)-Ser(NH2) | 2.03 |
| Peak | Fragment | Calculated Mass | Observed Mass |
|---|---|---|---|
| β1 | AEDVGSNKG | 876.4057 | 876.4001 |
| AEDVG | 490.2143 | 490.2314 | |
| β2 | AEDVGSNKGA | 947.4428 | 947.4432 |
| β3 | HQKL | 525.3143 | 525.3073 |
| YEVH | 547.2511 | 547.2429 | |
| F | 166.0862 | 166.0736 | |
| β4 | DAEFRH | 774.3529 | 774.3491 |
| β5 | DSGYEVH | 806.3315 | 806.3282 |
| SGYEVH | 691.3046 | 691.3023 | |
| GYEVH | 604.2725 | 604.2654 | |
| β6 | DAEFR | 637.2940 | 637.2875 |
| β7 | HQKLV | 624.3827 | 624.3879 |
| FAEDVGSNKGA | 1094.5112 | 1094.5014 | |
| β8 | GGVVIA | 515.3187 | 515.3027 |
| GVVIA | 458.2973 | 458.2883 | |
| FFAEDVGSNKGA | 1241.5796 | 1241.5872 | |
| β9 | FFAE | 513.2343 | 513.2232 |
| VHHQ | 520.2626 | 520.3233 | |
| β10 | HQKLVF | 771.4512 | 771.4414 |
| β11 | AIIG | 373.2445 | 373.2334 |
| β12 | VFFA | 483.2602 | 483.2494 |
| AEDVGSNKGAIIGLM | 1474.7570 | 1474.7415 | |
| β13 | AIIGLM | 617.3691 | 617.3527 |
| HQKLVFF | 918.5196 | 918.5002 | |
| β14 | IIGLM | 546.3320 | 546.3109 |
| β15 | Aβ42 | 4512.2768 | 4512.2800 |
| Peak | Fragment | Calculated Mass | Observed Mass |
|---|---|---|---|
| b1 | GGVVIA | 515.3187 | 515.4486 |
| VVIA | 401.2758 | 401.3619 | |
| b2 | GVVIA | 458.2973 | 458.4021 |
| b3 | VGGVVIA | 614.3871 | 614.5443 |
| Peak | Fragment | Calculated Mass | Observed Mass |
|---|---|---|---|
| S1 | SKGQA | 490.2620 | 490.2721 |
| β1 | QK | 275.1714 | 275.1445 |
| β2 | SGYE | 455.1772 | 455.0973 |
| β3 | R | 175.1189 | 175.1210 |
| β4 | IIGLM | 546.3320 | 546.3343 |
| β5 | GAII or AIIG | 373.2445 | 373.2331 |
| AII | 316.2231 | 316.2004 | |
| β6 | EFRH | 588.2888 | 588.3154 |
| β7 | KGAIIG | 558.3609 | 558.3452 |
| β8 | GGVVIA | 515.3187 | 515.3301 |
| β9 | VGSNKGAIIG | 915.5258 | 914.6014 |
| β10 | YEVHHQKL | 1053.5476 | 1053.5873 |
| FAEDVGSNK | 966.4527 | 966.5642 | |
| β11 | VFF | 412.2231 | 412.2643 |
| β12 | KGAIIGL | 671.4450 | 671.4257 |
| β13 | FRH | 459.2463 | 459.2905 |
| β14 | KGAIIGLM | 802.4855 | 802.4546 |
| β15 | FFAEDVG | 784.3512 | 784.5042 |
| β16 | LMVGGVVI | 787.4746 | 787.4581 |
| β17 | EVHHQKLVFFAED | 1598.7961 | 1598.9801 |
| NKGAIIGLMVGGV | 1228.7082 | 1228.7654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatakawa, Y.; Nakamura, R.; Akizawa, T.; Konishi, M.; Matsuda, A.; Oe, T.; Saito, M.; Ito, F. SKGQA, a Peptide Derived from the ANA/BTG3 Protein, Cleaves Amyloid-β with Proteolytic Activity. Biomolecules 2024, 14, 586. https://doi.org/10.3390/biom14050586
Hatakawa Y, Nakamura R, Akizawa T, Konishi M, Matsuda A, Oe T, Saito M, Ito F. SKGQA, a Peptide Derived from the ANA/BTG3 Protein, Cleaves Amyloid-β with Proteolytic Activity. Biomolecules. 2024; 14(5):586. https://doi.org/10.3390/biom14050586
Chicago/Turabian StyleHatakawa, Yusuke, Rina Nakamura, Toshifumi Akizawa, Motomi Konishi, Akira Matsuda, Tomoyuki Oe, Motoaki Saito, and Fumiaki Ito. 2024. "SKGQA, a Peptide Derived from the ANA/BTG3 Protein, Cleaves Amyloid-β with Proteolytic Activity" Biomolecules 14, no. 5: 586. https://doi.org/10.3390/biom14050586
APA StyleHatakawa, Y., Nakamura, R., Akizawa, T., Konishi, M., Matsuda, A., Oe, T., Saito, M., & Ito, F. (2024). SKGQA, a Peptide Derived from the ANA/BTG3 Protein, Cleaves Amyloid-β with Proteolytic Activity. Biomolecules, 14(5), 586. https://doi.org/10.3390/biom14050586








