DPP Inhibition Enhances the Efficacy of PD-1 Blockade by Remodeling the Tumor Microenvironment in Lewis Lung Carcinoma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Cell Lines and Tumor Models
2.4. Cell Viability Assays
2.5. In Vivo Treatment
2.6. Flow Cytometry
2.7. RNA Extraction and Real-Time PCR Analysis
2.8. Immunohistochemical (IHC) Staining
2.9. ELISA
2.10. Statistical Analysis
3. Results
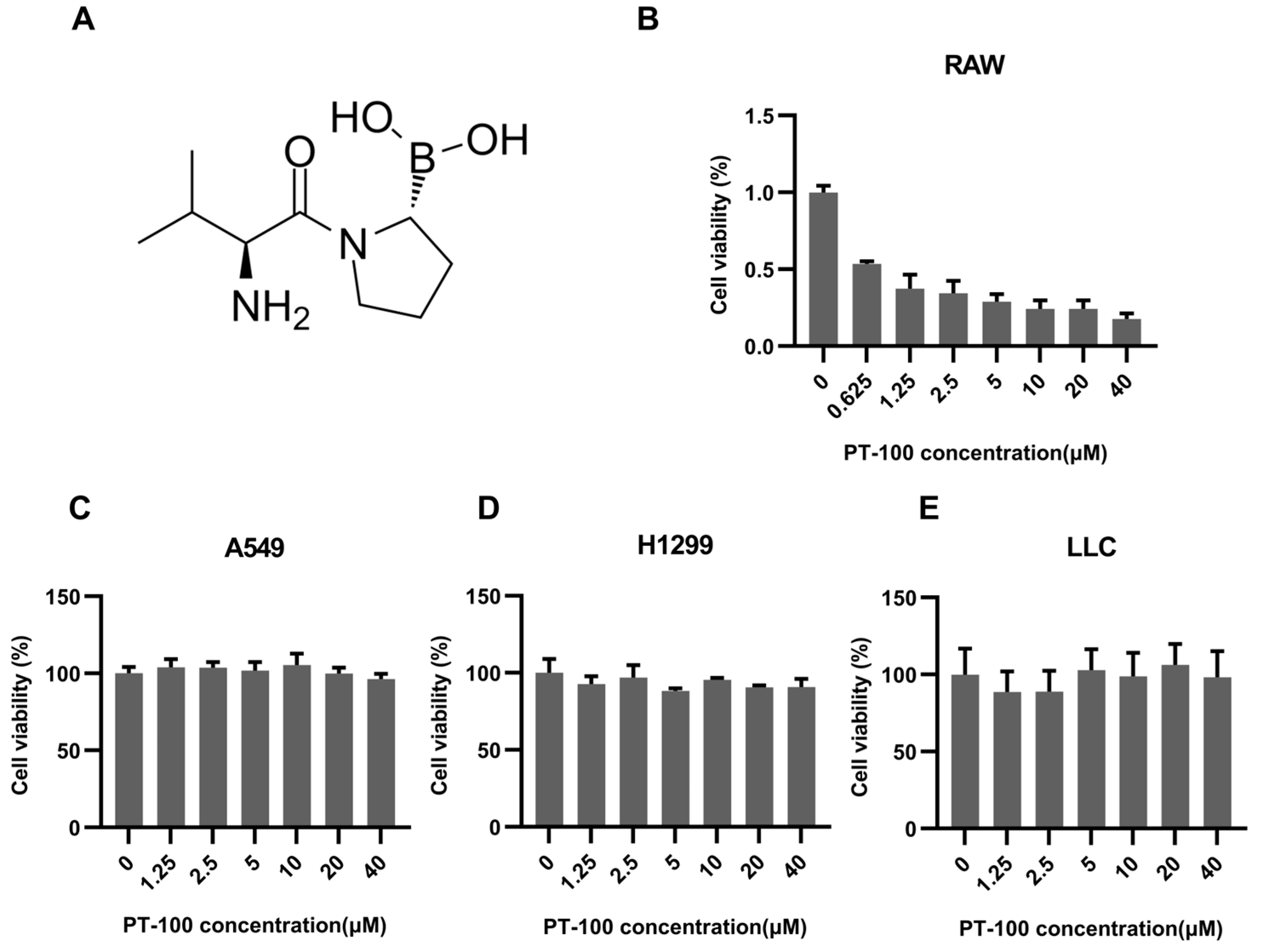
3.1. PT-100 Inhibited Macrophage Viability In Vitro
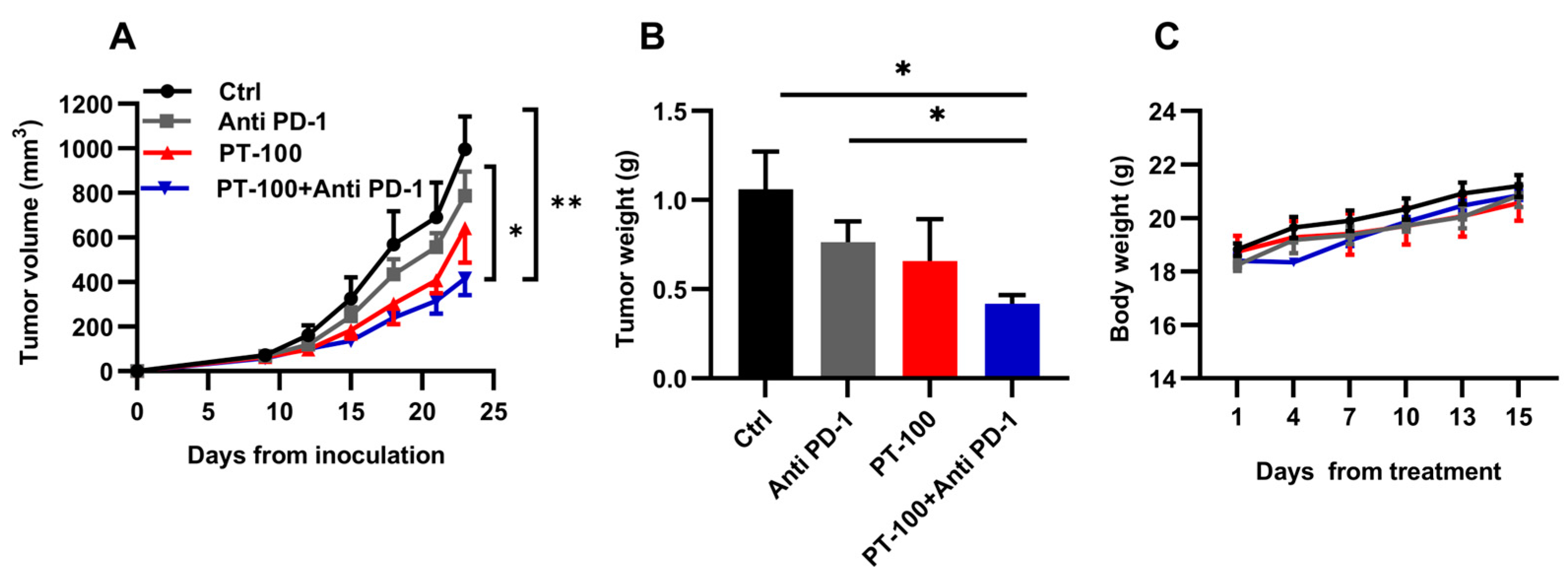
3.2. Combination Therapy of PT-100 with Anti-PD-1 Antibody Potently Inhibited Tumor Growth
3.3. Combination of PT-100 with Anti-PD-1 Antibody Increased the Infiltration of CD4+ and CD8+ T Cells in the TME
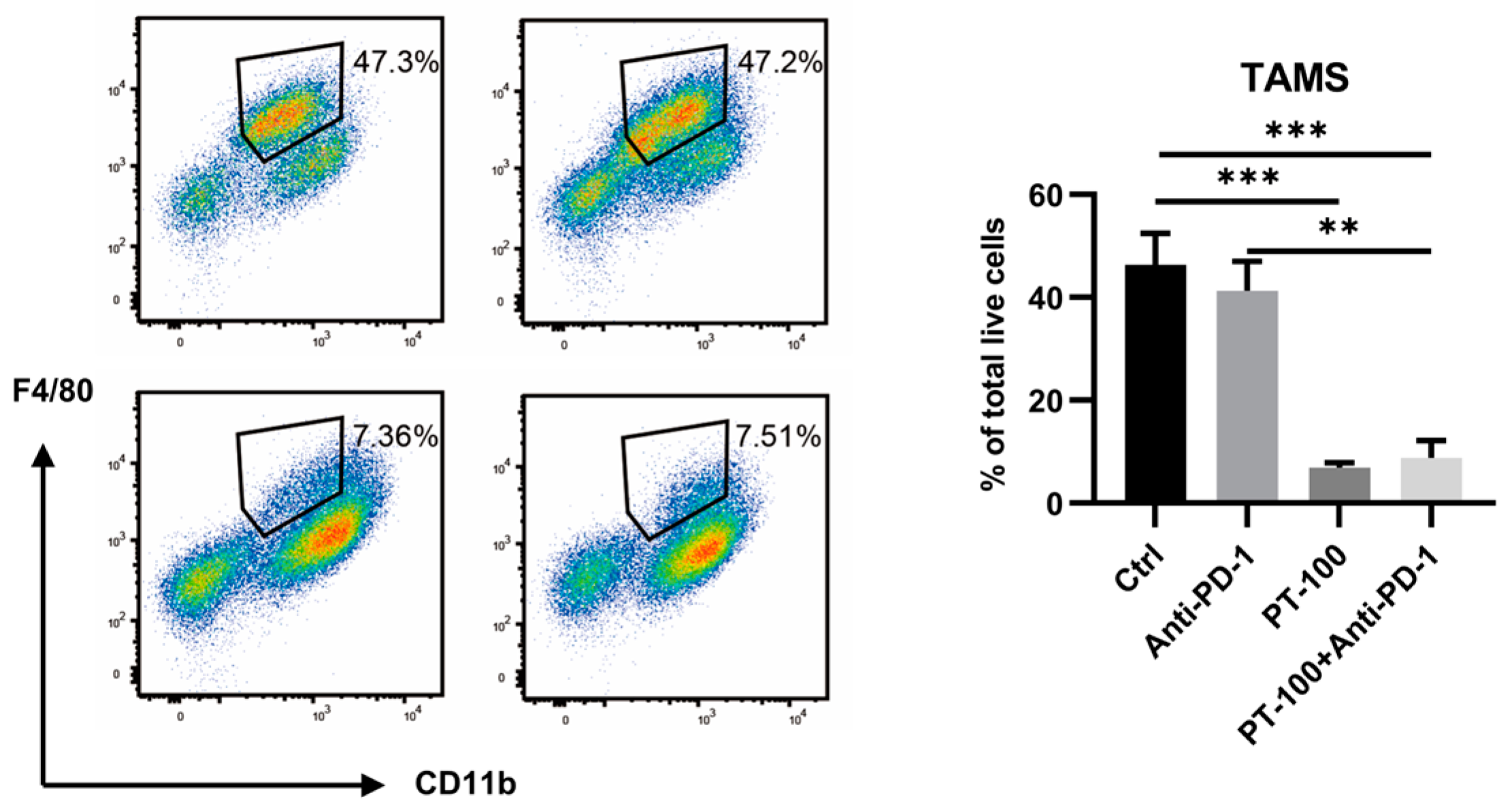
3.4. Combination of PT-100 with Anti-PD-1 Antibody Reduced the Infiltration of Tumor-Associated Macrophages (TAMs)
3.5. Characterization of Chemokines and Cytokines in Tumors Treated with Combination Therapy
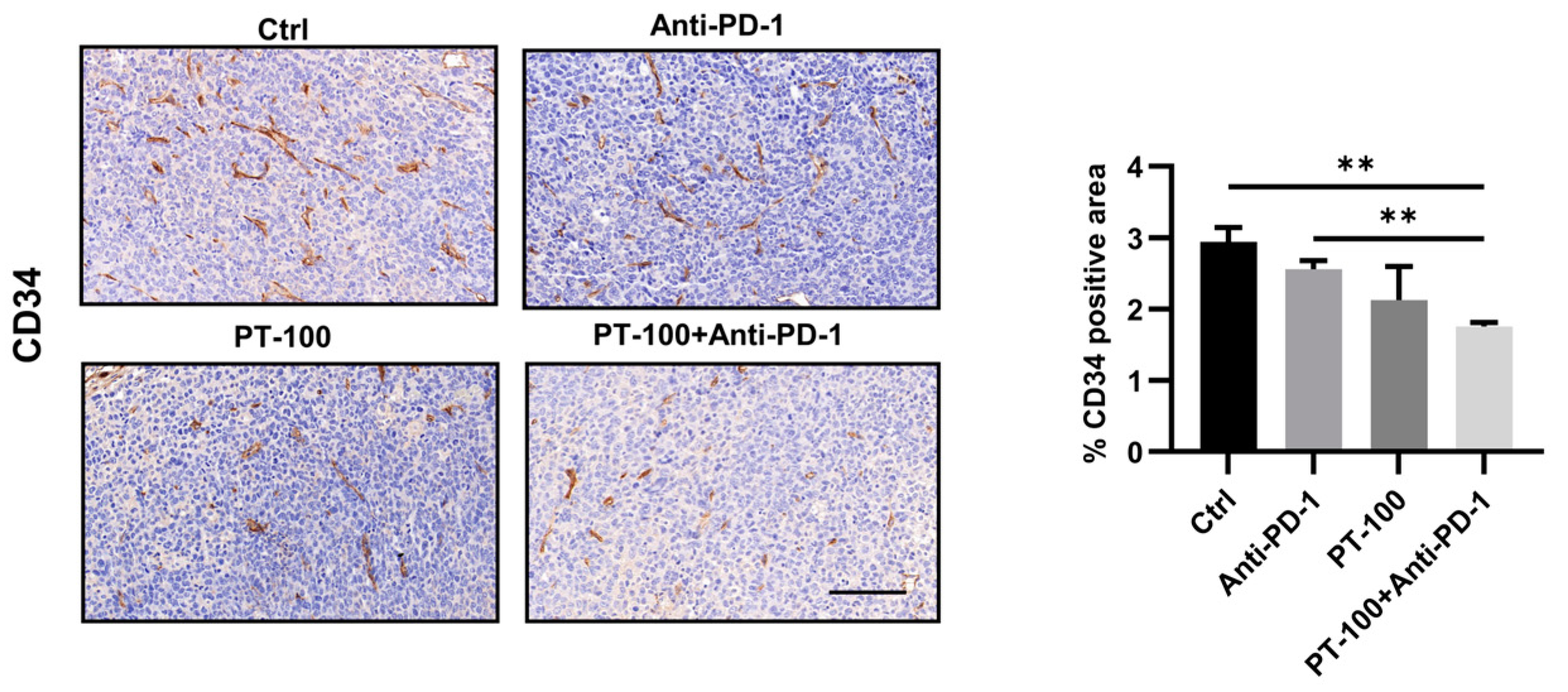
3.6. Influence of Combination Therapy on Angiogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Morgensztern, D. Treatment Advances in Small Cell Lung Cancer (SCLC). Pharmacol. Ther. 2017, 180, 16–23. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Neftelino, S.T.; Hodge, J.P.; Oliva, C.; Campbell, J.R.; Yu, J.X. Combinations Take Centre Stage in PD1/PDL1 Inhibitor Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 168–170. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune Checkpoint Therapy for Solid Tumours: Clinical Dilemmas and Future Trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef]
- Connolly, B.A.; Sanford, D.G.; Chiluwal, A.K.; Healey, S.E.; Peters, D.E.; Dimare, M.T.; Wu, W.; Liu, Y.; Maw, H.; Zhou, Y. Dipeptide Boronic Acid Inhibitors of Dipeptidyl Peptidase IV: Determinants of Potency and in Vivo Efficacy and Safety. J. Med. Chem. 2008, 51, 6005–6013. [Google Scholar] [CrossRef]
- Adams, S.; Miller, G.T.; Jesson, M.I.; Watanabe, T.; Jones, B.; Wallner, B.P. PT-100, a Small Molecule Dipeptidyl Peptidase Inhibitor, Has Potent Antitumor Effects and Augments Antibody-Mediated Cytotoxicity via a Novel Immune Mechanism. Cancer Res. 2004, 64, 5471–5480. [Google Scholar] [CrossRef]
- Walsh, M.P.; Duncan, B.; Larabee, S.; Krauss, A.; Davis, J.P.; Cui, Y.; Kim, S.Y.; Guimond, M.; Bachovchin, W.; Fry, T.J. Val-boroPro Accelerates T Cell Priming via Modulation of Dendritic Cell Trafficking Resulting in Complete Regression of Established Murine Tumors. PLoS ONE 2013, 8, e58860. [Google Scholar] [CrossRef]
- Uprichard, M.J.; Jones, B. Phase 1 Rising Multiple-Dose Study of Talabostat (PT-100) in Healthy Subjects. Blood 2004, 104, 4215. [Google Scholar] [CrossRef]
- Redman, B.G.; Ernstoff, M.S.; Gajewski, T.F.; Cunningham, C.; Lawson, D.H.; Gregoire, L.; Haltom, E.; Uprichard, M.J. Phase 2 Trial of Talabostat in Stage IV Melanoma. J. Clin. Oncol. 2005, 23, 7570. [Google Scholar] [CrossRef]
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non–Small-Cell Lung Cancer. Eur. J. Cancer 2019, 106, 144–159. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D. Tumor-Associated Macrophages and Survival in Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cui, J. Anti-Angiogenesis: Opening a New Window for Immunotherapy. Life Sci. 2020, 258, 118163. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Man, S.; Sun, R.; Li, Z.; Wu, Y.; Zuo, D. Recent Advances and Challenges of Immune Checkpoint Inhibitors in Immunotherapy of Non-Small Cell Lung Cancer. Int. Immunopharmacol. 2020, 85, 106613. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-Infiltrating Lymphocytes in the Immunotherapy Era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; O’Leary, H.A.; Broxmeyer, H.E. Implications of DPP4 Modification of Proteins That Regulate Stem/Progenitor and More Mature Cell Types. Blood J. Am. Soc. Hematol. 2013, 122, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 Inhibition Enhances Lymphocyte Trafficking, Improving Both Naturally Occurring Tumor Immunity and Immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 2016, 30, 365. [Google Scholar] [CrossRef] [PubMed]
- Okondo, M.C.; Johnson, D.C.; Sridharan, R.; Go, E.B.; Chui, A.J.; Wang, M.S.; Poplawski, S.E.; Wu, W.; Liu, Y.; Lai, J.H.; et al. DPP8 and DPP9 Inhibition Induces Pro-Caspase-1-Dependent Monocyte and Macrophage Pyroptosis. Nat. Chem. Biol. 2017, 13, 46–53. [Google Scholar] [CrossRef]
- Wang, L.; Qin, X.; Liang, J.; Ge, P. Induction of Pyroptosis: A Promising Strategy for Cancer Treatment. Front. Oncol. 2021, 11, 635774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Ding, J.; Wang, C.; Zhou, X.; Gao, W.; Huang, H.; Shao, F.; Liu, Z. A Bioorthogonal System Reveals Antitumour Immune Function of Pyroptosis. Nature 2020, 579, 421–426. [Google Scholar] [CrossRef]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular Normalization as an Emerging Strategy to Enhance Cancer ImmunotherapyVascular Normalization to Boost Immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Huang, Y.; Simms, A.E.; Mazur, A.; Wang, S.; León, N.R.; Jones, B.; Aziz, N.; Kelly, T. Fibroblast Activation Protein-α Promotes Tumor Growth and Invasion of Breast Cancer Cells through Non-Enzymatic Functions. Clin. Exp. Metastasis 2011, 28, 567–579. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Kelly, T. Seprase Promotes Rapid Tumor Growth and Increased Microvessel Density in a Mouse Model of Human Breast Cancer. Cancer Res. 2004, 64, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Eager, R.M.; Cunningham, C.C.; Senzer, N.N.; Stephenson, J.; Anthony, S.P.; O’Day, S.J.; Frenette, G.; Pavlick, A.C.; Jones, B.; Uprichard, M.; et al. Phase II Assessment of Talabostat and Cisplatin in Second-Line Stage IV Melanoma. BMC Cancer 2009, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Eager, R.M.; Cunningham, C.C.; Senzer, N.; Richards, D.A.; Raju, R.N.; Jones, B.; Uprichard, M.; Nemunaitis, J. Phase II Trial of Talabostat and Docetaxel in Advanced Non-Small Cell Lung Cancer. Clin. Oncol. 2009, 21, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, L.; Gupta, S.; Dahiya, A.; Jagga, Z.; Nandabalan, K.; Upmanyu, S. The Synergy between BXCL701, a DPP Inhibitor, and Immune Checkpoint Inhibitors Discovered Using AI and Big Data Analytics. Cancer Res. 2017, 77, 2629. [Google Scholar] [CrossRef]
- Wang, S.; Fitzgerald, A.; Ajina, R.; Jablonsk, S.A.; Weiner, L.M.; MacDougall, J.; Agarwal, V.; O’Neill, V.J. Therapy with BXCL701 (B), a DPP8, DPP9, DPPIV and FAP Inhibitor, in Combination with Anti-PD1 Antibody (PD1) in a Syngeneic Murine Pancreatic Ductal Adenocarcinoma (PDAC) Model Improves Treatment Outcomes and Induces Intratumoral NK Cell Infiltrates and a Marked Reduction in Tumor Stromal Fibrosis. Cancer Res. 2020, 80, 6636. [Google Scholar]
- Rastelli, L.; Gupta, S.; Jagga, Z.; Charych, D.H.; Zalevsky, J. Efficacy and Immune Modulation by BXCL701 a Dipeptidyl Peptidase Inhibitor, NKTR-214 a CD122-Biased Immune Agonist with PD1 Blockade in Murine Pancreatic Tumors. J. Clin. Oncol. 2018, 36, 3085. [Google Scholar] [CrossRef]


| Gene | Primer Pair | Primer Sequence (5′-3′) |
|---|---|---|
| Ccl3 | forward | TTCTCTGTACCATGACACTCTGC |
| reverse | CGTGGAATCTTCCGGCTGTAG | |
| Cxcl10 | forward | CCAAGTGCTGCCGTCATTTTC |
| reverse | GGCTCGCAGGGATGATTTCAA | |
| Tnf-α | forward | CAGGCGGTGCCTATGTCTC |
| reverse | CGATCACCCCGAAGTTCAGTAG | |
| Ifn-γ | forward | ATGAACGCTACACACTGCATC |
| reverse | CCATCCTTTTGCCAGTTCCTC | |
| Granzyme B | forward | TCATGCTGCTAAAGCTGAAGAG |
| reverse | CCCGCACATATCTGATTGGTTT | |
| Perforin | forward | CAAGGTAGCCAATTTTGCAGC |
| reverse | GTACATGCGACACTCTACTGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.; Liu, J.; Gao, Y.; Dai, W.; Huang, H.; Jiang, Q.; Liu, Z. DPP Inhibition Enhances the Efficacy of PD-1 Blockade by Remodeling the Tumor Microenvironment in Lewis Lung Carcinoma Model. Biomolecules 2024, 14, 391. https://doi.org/10.3390/biom14040391
Lei M, Liu J, Gao Y, Dai W, Huang H, Jiang Q, Liu Z. DPP Inhibition Enhances the Efficacy of PD-1 Blockade by Remodeling the Tumor Microenvironment in Lewis Lung Carcinoma Model. Biomolecules. 2024; 14(4):391. https://doi.org/10.3390/biom14040391
Chicago/Turabian StyleLei, Mengrong, Junyan Liu, Ying Gao, Wenting Dai, Hanxue Huang, Qingqing Jiang, and Zhaoqian Liu. 2024. "DPP Inhibition Enhances the Efficacy of PD-1 Blockade by Remodeling the Tumor Microenvironment in Lewis Lung Carcinoma Model" Biomolecules 14, no. 4: 391. https://doi.org/10.3390/biom14040391
APA StyleLei, M., Liu, J., Gao, Y., Dai, W., Huang, H., Jiang, Q., & Liu, Z. (2024). DPP Inhibition Enhances the Efficacy of PD-1 Blockade by Remodeling the Tumor Microenvironment in Lewis Lung Carcinoma Model. Biomolecules, 14(4), 391. https://doi.org/10.3390/biom14040391


_Kwok.png)



