Exploring SVA Insertion Polymorphisms in Shaping Differential Gene Expressions in the Central Nervous System
Abstract
1. Introduction
2. Materials and Methods
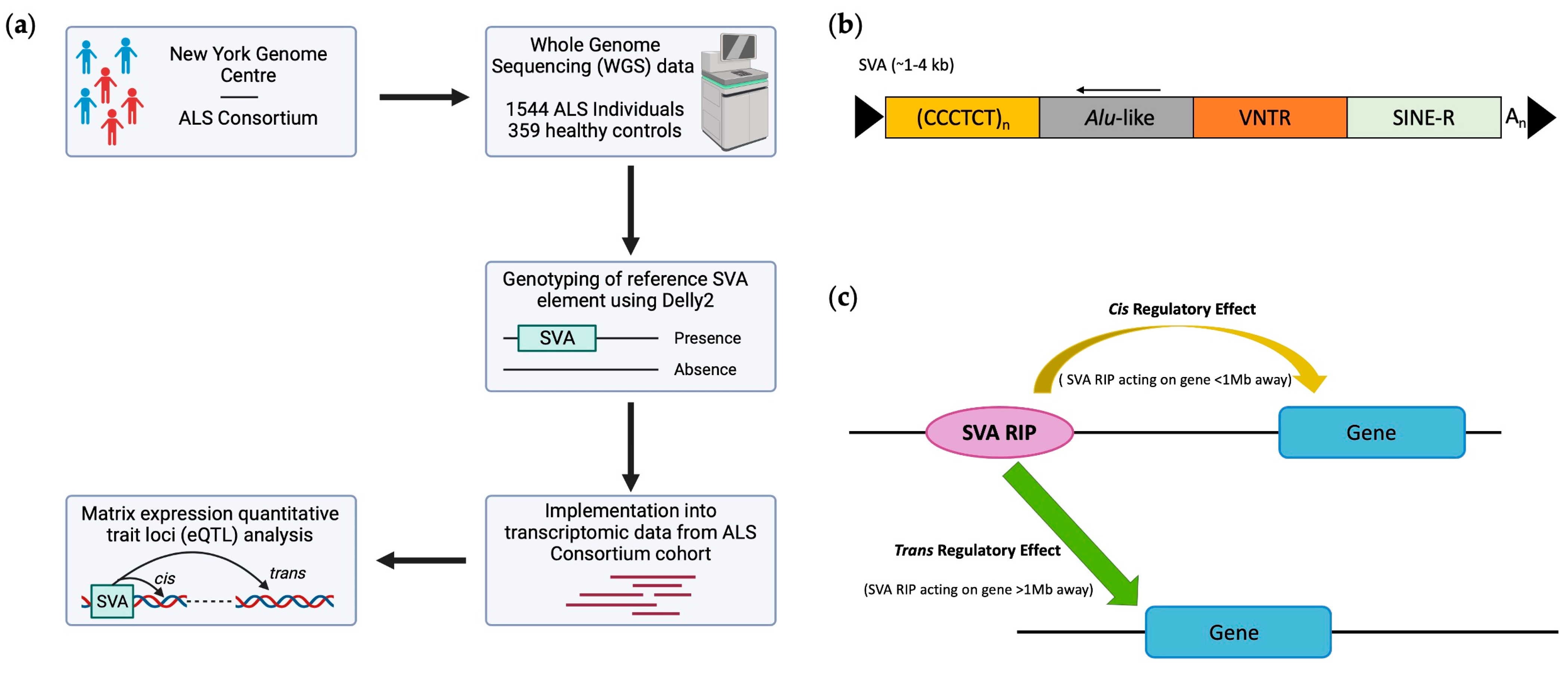
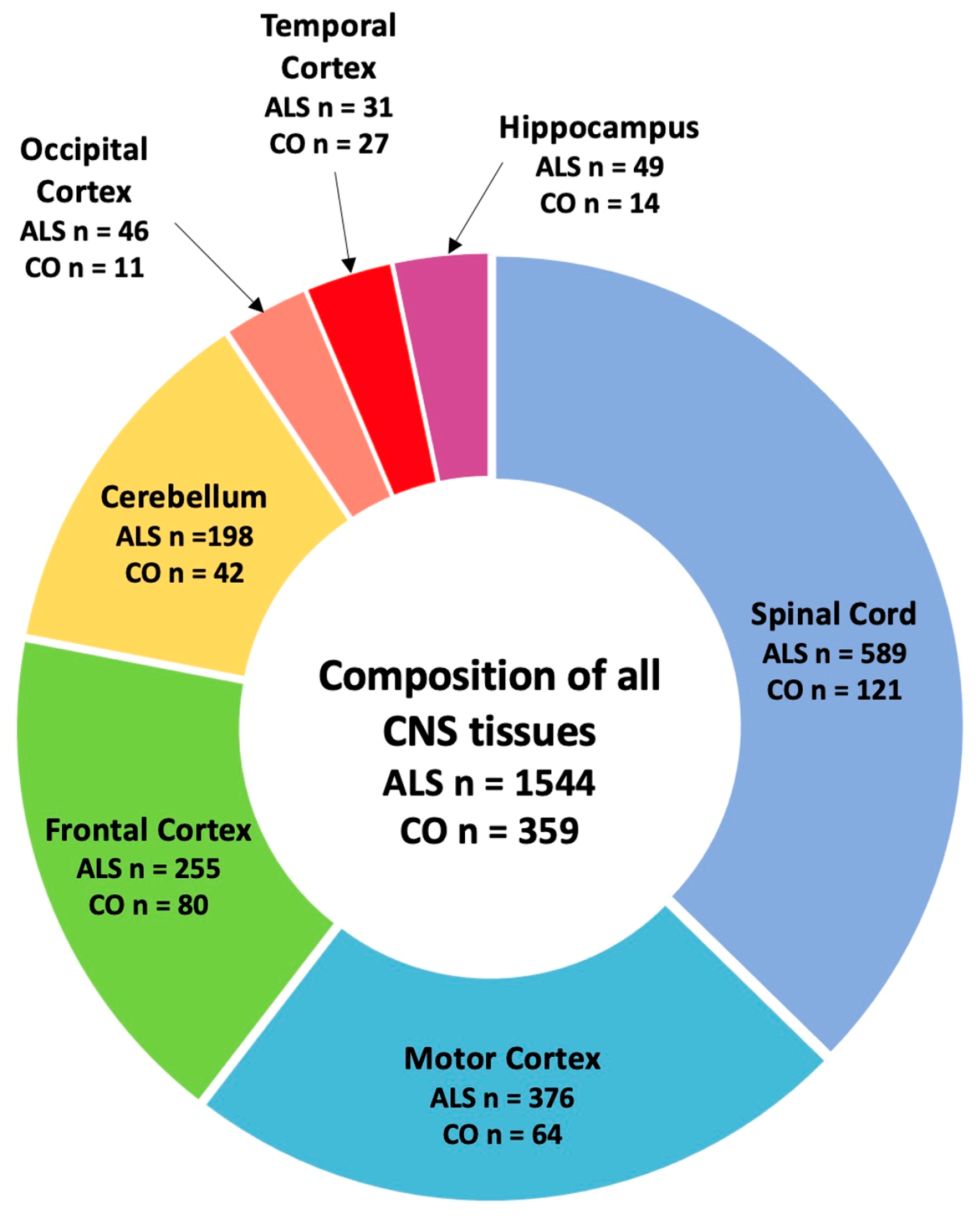
2.1. Genotyping Reference SVAs Polymorphic for Their Presence/Absence and Disease Associations from Whole-Genome Sequencing Data from ALS Consortium Dataset
2.2. RNA-Seq Differential Gene Expression Analysis
2.3. eQTL (Expression Quantitative Trait Loci) Analysis
3. Results
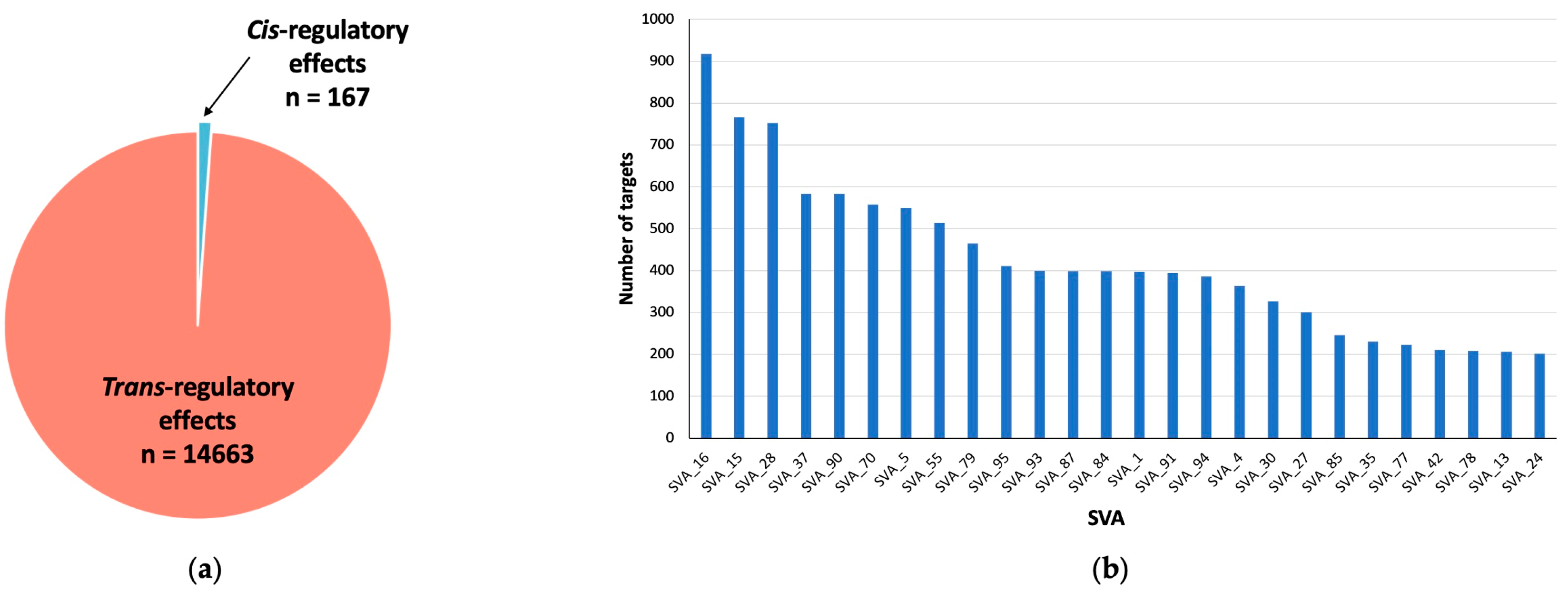
3.1. SVA RIPs Act as eQTL Genome-Wide in CNS Tissues
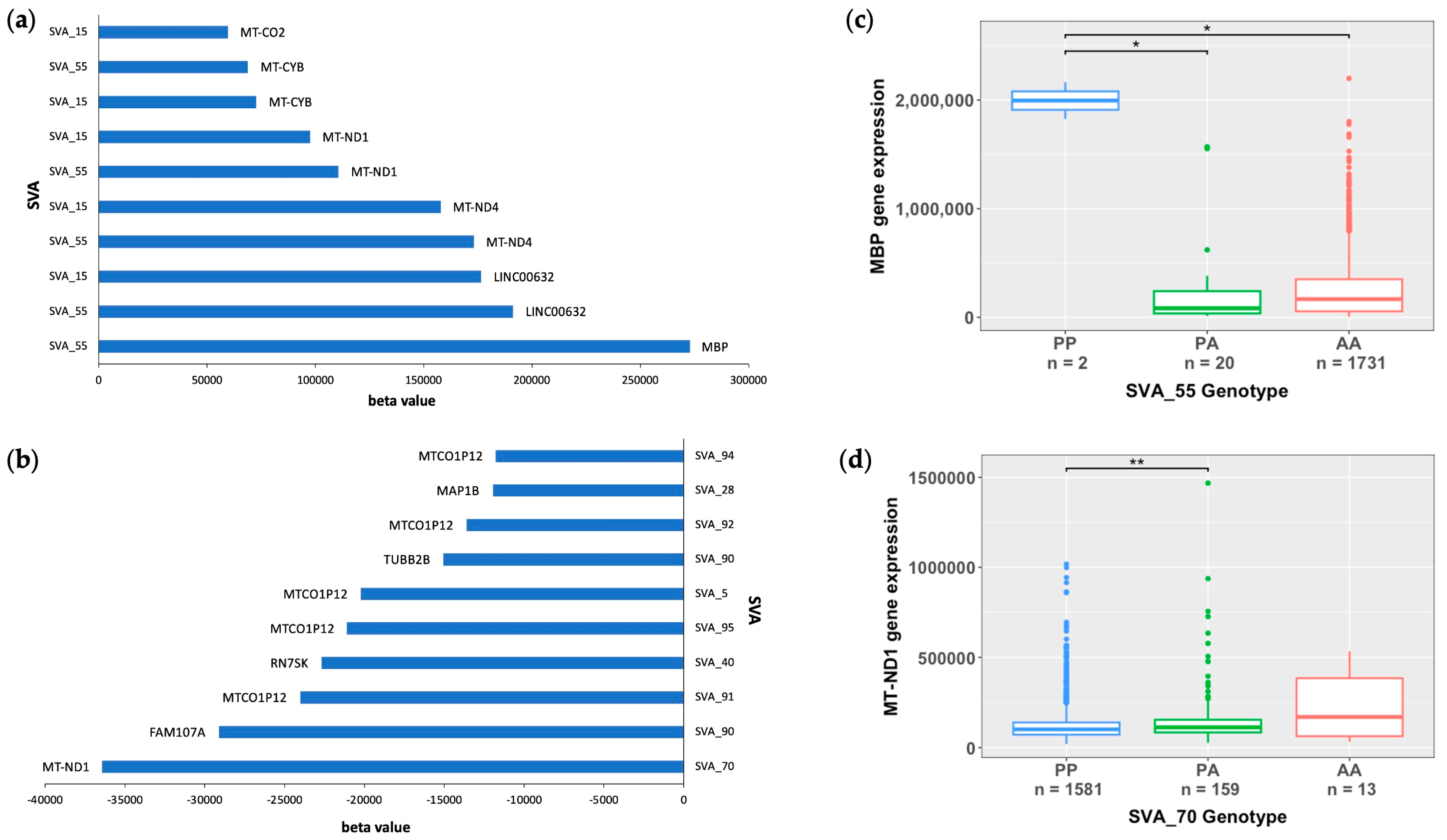
3.2. Mitochondrial Genes Are Significantly Modulated by SVA RIPs
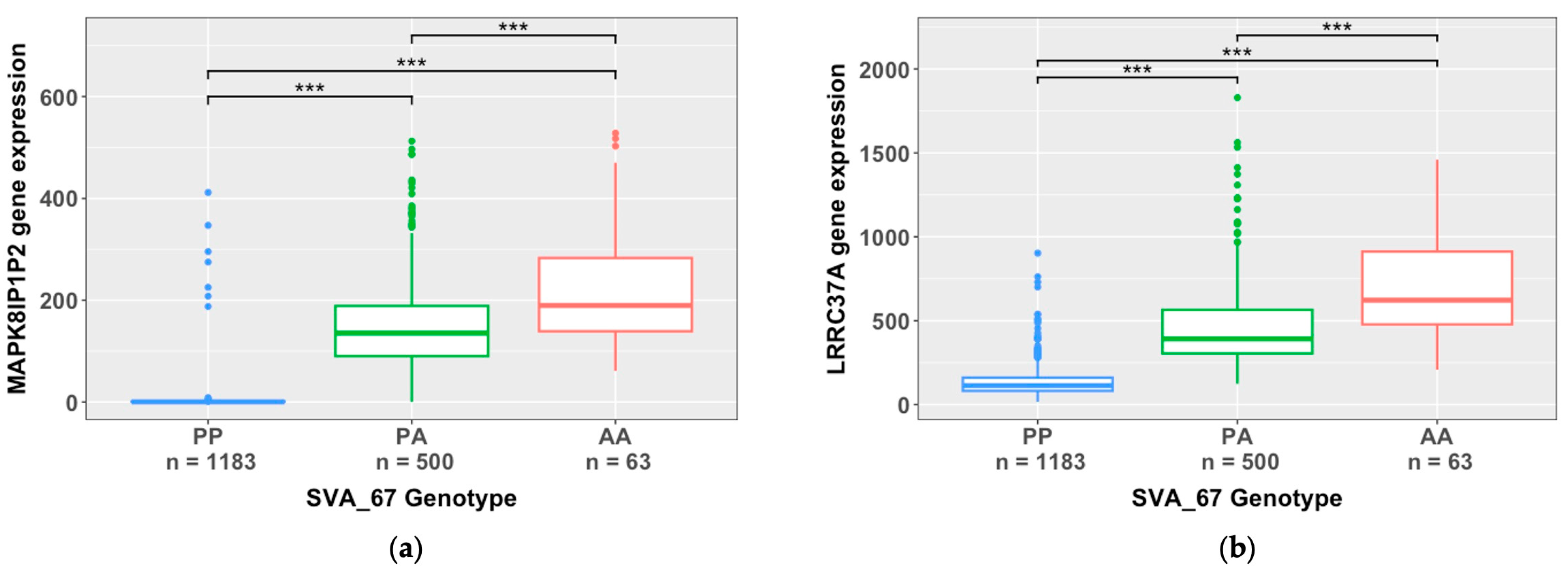
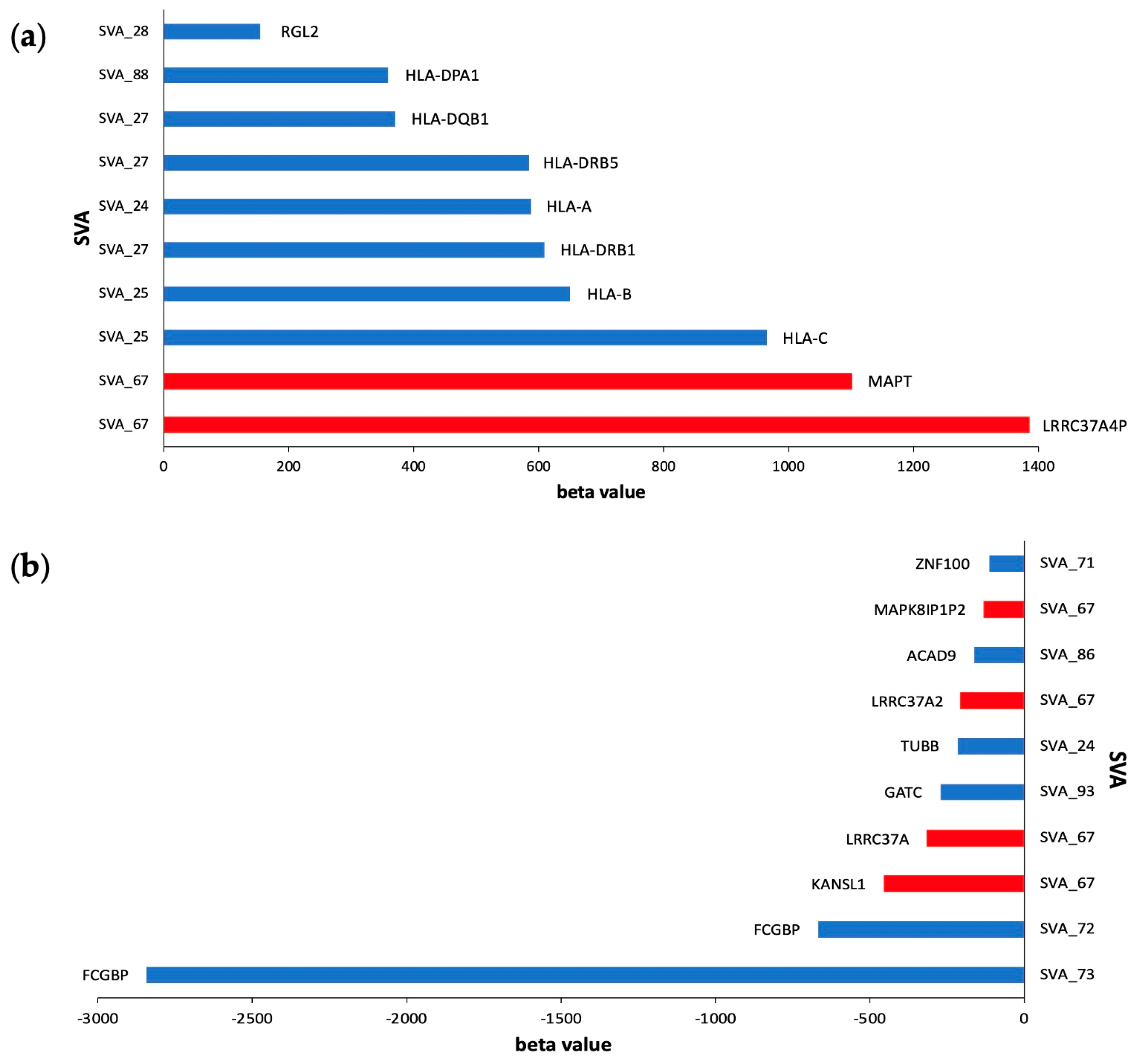
3.3. SVA RIPs Demonstrate Cis Effects on HLA and MAPT Loci
3.4. SVA RIPs Display Tissue-Specific Modulations of Gene Expressions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A Comprehensive Review of Amyotrophic Lateral Sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef]
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Euro J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Longinetti, E.; Fang, F. Epidemiology of Amyotrophic Lateral Sclerosis: An Update of Recent Literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Ajroud-Driss, S.; Siddique, T. Sporadic and Hereditary Amyotrophic Lateral Sclerosis (ALS). Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van Den Berg, L.H. Amyotrophic Lateral Sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Savage, A.L.; Schumann, G.G.; Breen, G.; Bubb, V.J.; Al-Chalabi, A.; Quinn, J.P. Retrotransposons in the Development and Progression of Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.M.; Elliott, E.; McDade, K.; Bak, T.; Pal, S.; Chandran, S.; Abrahams, S.; Smith, C. Neuronal Clusterin Expression Is Associated with Cognitive Protection in Amyotrophic Lateral Sclerosis. Neuropathol. Appl. Neurobio 2020, 46, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nishiyama, A.; Warita, H.; Aoki, M. Genetics of Amyotrophic Lateral Sclerosis: Seeking Therapeutic Targets in the Era of Gene Therapy. J. Hum. Genet. 2023, 68, 131–152. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Zhou, Z.-R.; Che, C.-H.; Liu, C.-Y.; He, R.-L.; Huang, H.-P. Genetic Epidemiology of Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 540–549. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Fang, F.; Hanby, M.F.; Leigh, P.N.; Shaw, C.E.; Ye, W.; Rijsdijk, F. An Estimate of Amyotrophic Lateral Sclerosis Heritability Using Twin Data. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1324–1326. [Google Scholar] [CrossRef]
- Theunissen, F.; Flynn, L.L.; Anderton, R.S.; Mastaglia, F.; Pytte, J.; Jiang, L.; Hodgetts, S.; Burns, D.K.; Saunders, A.; Fletcher, S.; et al. Structural Variants May Be a Source of Missing Heritability in sALS. Front. Neurosci. 2020, 14, 47. [Google Scholar] [CrossRef]
- Roses, A.D.; Akkari, P.A.; Chiba-Falek, O.; Lutz, M.W.; Gottschalk, W.K.; Saunders, A.M.; Saul, B.; Sundseth, S.; Burns, D. Structural Variants Can Be More Informative for Disease Diagnostics, Prognostics and Translation than Current SNP Mapping and Exon Sequencing. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 135–147. [Google Scholar] [CrossRef]
- GTEx Consortium; Chiang, C.; Scott, A.J.; Davis, J.R.; Tsang, E.K.; Li, X.; Kim, Y.; Hadzic, T.; Damani, F.N.; Ganel, L.; et al. The Impact of Structural Variation on Human Gene Expression. Nat. Genet. 2017, 49, 692–699. [Google Scholar] [CrossRef]
- Liscic, R.M. Als and Ftd: Insights into the Disease Mechanisms and Therapeutic Targets. Eur. J. Pharmacol. 2017, 817, 2–6. [Google Scholar] [CrossRef]
- Elbarbary, R.A.; Lucas, B.A.; Maquat, L.E. Retrotransposons as Regulators of Gene Expression. Science 2016, 351, aac7247. [Google Scholar] [CrossRef]
- Ayarpadikannan, S.; Kim, H.-S. The Impact of Transposable Elements in Genome Evolution and Genetic Instability and Their Implications in Various Diseases. Genom. Inf. 2014, 12, 98. [Google Scholar] [CrossRef]
- Cordaux, R.; Batzer, M.A. The Impact of Retrotransposons on Human Genome Evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef]
- Prudencio, M.; Gonzales, P.K.; Cook, C.N.; Gendron, T.F.; Daughrity, L.M.; Song, Y.; Ebbert, M.T.W.; Van Blitterswijk, M.; Zhang, Y.-J.; Jansen-West, K.; et al. Repetitive Element Transcripts Are Elevated in the Brain of C9orf72 ALS/FTLD Patients. Hum. Mol. Genet. 2017, 26, 3421–3431. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. Active Human Retrotransposons: Variation and Disease. Curr. Opin. Genet. Dev. 2012, 22, 191–203. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- De Koning, A.P.J.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive Elements May Comprise Over Two-Thirds of the Human Genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef]
- Fröhlich, A.; Pfaff, A.L.; Bubb, V.J.; Koks, S.; Quinn, J.P. Characterisation of the Function of a SINE-VNTR-Alu Retrotransposon to Modulate Isoform Expression at the MAPT Locus. Front. Mol. Neurosci. 2022, 15, 815695. [Google Scholar] [CrossRef]
- Wang, H.; Xing, J.; Grover, D.; Hedges, D.J.; Han, K.; Walker, J.A.; Batzer, M.A. SVA Elements: A Hominid-Specific Retroposon Family. J. Mol. Biol. 2005, 354, 994–1007. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. SVA Retrotransposons: Evolution and Genetic Instability. Semin. Cancer Biol. 2010, 20, 234–245. [Google Scholar] [CrossRef]
- Shen, L.; Wu, L.C.; Sanlioglu, S.; Chen, R.; Mendoza, A.R.; Dangel, A.W.; Carroll, M.C.; Zipf, W.B.; Yu, C.Y. Structure and Genetics of the Partially Duplicated Gene RP Located Immediately Upstream of the Complement C4A and the C4B Genes in the HLA Class III Region. Molecular Cloning, Exon-Intron Structure, Composite Retroposon, and Breakpoint of Gene Duplication. J. Biol. Chem. 1994, 269, 8466–8476. [Google Scholar] [CrossRef]
- Savage, A.L.; Bubb, V.J.; Breen, G.; Quinn, J.P. Characterisation of the Potential Function of SVA Retrotransposons to Modulate Gene Expression Patterns. BMC Evol. Biol. 2013, 13, 101. [Google Scholar] [CrossRef]
- Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Koks, S. Reference SVA Insertion Polymorphisms Are Associated with Parkinson’s Disease Progression and Differential Gene Expression. NPJ Park. Dis. 2021, 7, 44. [Google Scholar] [CrossRef]
- Quinn, J.P.; Bubb, V.J. SVA Retrotransposons as Modulators of Gene Expression. Mob. Genet. Elem. 2014, 4, e32102. [Google Scholar] [CrossRef]
- Makino, S.; Kaji, R.; Ando, S.; Tomizawa, M.; Yasuno, K.; Goto, S.; Matsumoto, S.; Tabuena, M.D.; Maranon, E.; Dantes, M.; et al. Reduced Neuron-Specific Expression of the TAF1 Gene Is Associated with X-Linked Dystonia-Parkinsonism. Am. J. Hum. Genet. 2007, 80, 393–406. [Google Scholar] [CrossRef]
- Aneichyk, T.; Hendriks, W.T.; Yadav, R.; Shin, D.; Gao, D.; Vaine, C.A.; Collins, R.L.; Domingo, A.; Currall, B.; Stortchevoi, A.; et al. Dissecting the Causal Mechanism of X-Linked Dystonia-Parkinsonism by Integrating Genome and Transcriptome Assembly. Cell 2018, 172, 897–909.e21. [Google Scholar] [CrossRef]
- Bragg, D.C.; Mangkalaphiban, K.; Vaine, C.A.; Kulkarni, N.J.; Shin, D.; Yadav, R.; Dhakal, J.; Ton, M.-L.; Cheng, A.; Russo, C.T.; et al. Disease Onset in X-Linked Dystonia-Parkinsonism Correlates with Expansion of a Hexameric Repeat within an SVA Retrotransposon in TAF1. Proc. Natl. Acad. Sci. USA 2017, 114, E11020–E11028. [Google Scholar] [CrossRef]
- Verpillat, P.; Camuzat, A.; Hannequin, D.; Thomas-Anterion, C.; Puel, M.; Belliard, S.; Dubois, B.; Didic, M.; Michel, B.-F.; Lacomblez, L.; et al. Association Between the Extended Tau Haplotype and Frontotemporal Dementia. Arch. Neurol. 2002, 59, 935. [Google Scholar] [CrossRef][Green Version]
- Wider, C.; Vilariño-Güell, C.; Jasinska-Myga, B.; Heckman, M.G.; Soto-Ortolaza, A.I.; Cobb, S.A.; Aasly, J.O.; Gibson, J.M.; Lynch, T.; Uitti, R.J.; et al. Association of the MAPT Locus with Parkinson’s Disease. Euro. J. Neurol. 2010, 17, 483–486. [Google Scholar] [CrossRef]
- Sánchez-Juan, P.; Moreno, S.; De Rojas, I.; Hernández, I.; Valero, S.; Alegret, M.; Montrreal, L.; García González, P.; Lage, C.; López-García, S.; et al. The MAPT H1 Haplotype Is a Risk Factor for Alzheimer’s Disease in APOE Ε4 Non-Carriers. Front. Aging Neurosci. 2019, 11, 327. [Google Scholar] [CrossRef]
- Fröhlich, A.; Hughes, L.S.; Middlehurst, B.; Pfaff, A.L.; Bubb, V.J.; Koks, S.; Quinn, J.P. CRISPR Deletion of a SINE-VNTR-Alu (SVA_67) Retrotransposon Demonstrates Its Ability to Differentially Modulate Gene Expression at the MAPT Locus. Front. Neurol. 2023, 14, 1273036. [Google Scholar] [CrossRef]
- Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Koks, S. A Genome-Wide Screen for the Exonisation of Reference SINE-VNTR-Alus and Their Expression in CNS Tissues of Individuals with Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 11548. [Google Scholar] [CrossRef]
- Koks, S.; Pfaff, A.L.; Bubb, V.J.; Quinn, J.P. Expression Quantitative Trait Loci (eQTLs) Associated with Retrotransposons Demonstrate Their Modulatory Effect on the Transcriptome. Int. J. Mol. Sci. 2021, 22, 6319. [Google Scholar] [CrossRef]
- Van Rheenen, W.; Van Der Spek, R.A.A.; Bakker, M.K.; Van Vugt, J.J.F.A.; Hop, P.J.; Zwamborn, R.A.J.; De Klein, N.; Westra, H.-J.; Bakker, O.B.; Deelen, P.; et al. Common and Rare Variant Association Analyses in Amyotrophic Lateral Sclerosis Identify 15 Risk Loci with Distinct Genetic Architectures and Neuron-Specific Biology. Nat. Genet. 2021, 53, 1636–1648. [Google Scholar] [CrossRef]
- Nona, R.J.; Greer, J.M.; Henderson, R.D.; McCombe, P.A. HLA and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 24–32. [Google Scholar] [CrossRef]
- Wang, L.; Rishishwar, L.; Mariño-Ramírez, L.; Jordan, I.K. Human Population-Specific Gene Expression and Transcriptional Network Modification with Polymorphic Transposable Elements. Nucleic Acids. Res. 2017, 45, 2318–2328. [Google Scholar] [CrossRef]
- Pugacheva, E.M.; Teplyakov, E.; Wu, Q.; Li, J.; Chen, C.; Meng, C.; Liu, J.; Robinson, S.; Loukinov, D.; Boukaba, A.; et al. The Cancer-Associated CTCFL/BORIS Protein Targets Multiple Classes of Genomic Repeats, with a Distinct Binding and Functional Preference for Humanoid-Specific SVA Transposable Elements. Epigenetics Chromatin 2016, 9, 35. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Cao, C. CTCF and Its Partners: Shaper of 3D Genome during Development. Genes 2022, 13, 1383. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Snaidero, N.; Pähler, G.; Frey, S.; Sánchez, P.; Zweckstetter, M.; Janshoff, A.; Schneider, A.; Weil, M.-T.; Schaap, I.A.T.; et al. Myelin Membrane Assembly Is Driven by a Phase Transition of Myelin Basic Proteins Into a Cohesive Protein Meshwork. PLoS Biol. 2013, 11, e1001577. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and Impaired Regeneration of Gray Matter Oligodendrocytes in Amyotrophic Lateral Sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Boccazzi, M.; Fumagalli, M. Oligodendrocyte Dysfunction in Amyotrophic Lateral Sclerosis: Mechanisms and Therapeutic Perspectives. Cells 2021, 10, 565. [Google Scholar] [CrossRef]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in Multiple Sclerosis: From Basic Science to Clinical Translation. Lancet Neurol. 2020, 19, 678–688. [Google Scholar] [CrossRef]
- Lorente Pons, A.; Higginbottom, A.; Cooper-Knock, J.; Alrafiah, A.; Alofi, E.; Kirby, J.; Shaw, P.J.; Wood, J.D.; Highley, J.R. Oligodendrocyte Pathology Exceeds Axonal Pathology in White Matter in Human Amyotrophic Lateral Sclerosis. J. Pathol. 2020, 251, 262–271. [Google Scholar] [CrossRef]
- Hübner, C.A.; Orth, U.; Senning, A.; Steglich, C.; Kohlschütter, A.; Korinthenberg, R.; Gal, A. Seventeen Novel PLP1 Mutations in Patients with Pelizaeus-Merzbacher Disease: MUTATIONS IN BRIEF. Hum. Mutat. 2005, 25, 321–322. [Google Scholar] [CrossRef]
- Cloake, N.; Yan, J.; Aminian, A.; Pender, M.; Greer, J. PLP1 Mutations in Patients with Multiple Sclerosis: Identification of a New Mutation and Potential Pathogenicity of the Mutations. J. Clin. Med. 2018, 7, 342. [Google Scholar] [CrossRef]
- Cruz-Rivera, Y.E.; Perez-Morales, J.; Santiago, Y.M.; Gonzalez, V.M.; Morales, L.; Cabrera-Rios, M.; Isaza, C.E. A Selection of Important Genes and Their Correlated Behavior in Alzheimer’s Disease. JAD 2018, 65, 193–205. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial Dysfunction in Neurodegenerative Diseases and the Potential Countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Misra, M.K.; Damotte, V.; Hollenbach, J.A. The Immunogenetics of Neurological Disease. Immunology 2018, 153, 399–414. [Google Scholar] [CrossRef]
- Antel, J.P.; Arnason, B.G.W.; Fuller, T.C.; Lehrich, J.R. Histocompatibility Typing in Amyotrophic Lateral Sclerosis. Arch. Neurol. 1976, 33, 423–425. [Google Scholar] [CrossRef]
- Behan, P.; Durward, W.; Dick, H. Histocompatibility antigens associated with motor-neurone disease. Lancet 1976, 308, 803. [Google Scholar] [CrossRef]
- Jokelainen, M.; Tiilikainen, A.; Lapinleimu, K. Polio Antibodies and HLA Antigens in Amyotrophic Lateral Sclerosis. Tissue Antigens 1977, 10, 259–266. [Google Scholar] [CrossRef]
- Kott, E.; Livni, E.; Zamir, R.; Kuritzky, A. Cell-Mediated Immunity to Polio and HLA Antigens in Amyotrophic Lateral Sclerosis. Neurology 1979, 29, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.K.; Pfaff, A.L.; Marney, L.D.; Fröhlich, A.; Bubb, V.J.; Quinn, J.P.; Koks, S. Regulation of Expression Quantitative Trait Loci by SVA Retrotransposons within the Major Histocompatibility Complex. Exp. Biol. Med. 2023, 248, 2304–2318. [Google Scholar] [CrossRef] [PubMed]
| SVA | Beta Value | False Discovery Rate (FDR) | Target Gene | Cis/trans Effect |
|---|---|---|---|---|
| SVA_67 | −131.2 | 1.93 × 10−303 | MAPK8IP1P2 | cis |
| SVA_67 | −5.2 | 1.93 × 10−303 | ENSG00000285668.1 | cis |
| SVA_67 | −315.1 | 5.12 × 10−299 | LRRC37A | cis |
| SVA_87 | −126.2 | 3.02 × 10−224 | MTND4P24 | trans |
| SVA_93 | −126.2 | 4.22 × 10−224 | MTND4P24 | trans |
| SVA_84 | −63.1 | 4.22 × 10−224 | MTND4P24 | trans |
| SVA_58 | 4.1 | 3.89 × 10−211 | LLPH-DT | cis |
| SVA_24 | 12.3 | 5.06 × 10−201 | HLA-K | cis |
| SVA_15 | 59.6 | 3.13 × 10−189 | MTND4P24 | trans |
| SVA_33 | 48.4 | 8.84 × 10−188 | ZFAND2A-DT | cis |
| SVA | Gene ID | FDR p Value | Beta Value | Gene | Chr | Cis/trans | Tissue |
|---|---|---|---|---|---|---|---|
| SVA_55 | ENSG00000197971.16 | 5.99 × 10−10 | 638,804.662 | MBP | 18 | trans | Spinal Cord |
| SVA_15 | ENSG00000197971.16 | 3.59 × 10−7 | 620,026.724 | MBP | 18 | trans | Spinal Cord |
| SVA_37 | ENSG00000197971.16 | 8.76 × 10−4 | 585,791.502 | MBP | 18 | trans | Spinal Cord |
| SVA_85 | ENSG00000197971.16 | 2.08 × 10−3 | 560,691.911 | MBP | 18 | trans | Spinal Cord |
| SVA_37 | ENSG00000123560.14 | 3.72 × 10−12 | 168,441.474 | PLP1 | X | trans | Spinal Cord |
| SVA_55 | ENSG00000198888.2 | 1.47 × 10−5 | 153,947.476 | MT-ND1 | MT | trans | Spinal Cord |
| SVA_55 | ENSG00000203930.12 | 5.97 × 10−4 | 148,616.177 | LINC00632 | X | trans | Motor Cortex |
| SVA_15 | ENSG00000198888.2 | 1.62 × 10−3 | 142,961.051 | MT-ND1 | MT | trans | Spinal Cord |
| SVA_55 | ENSG00000203930.12 | 1.25 × 10−16 | 115,977.491 | LINC00632 | X | trans | Spinal Cord |
| SVA_15 | ENSG00000203930.12 | 1.86 × 10−8 | 969,10.7666 | LINC00632 | X | trans | Spinal Cord |
| SVA_55 | ENSG00000180354.16 | 1.34× 10−22 | 90,266.4311 | MTURN | 7 | trans | Spinal Cord |
| SVA_15 | ENSG00000123560.14 | 3.43 × 10−3 | 80,380.5623 | PLP1 | X | trans | Spinal Cord |
| SVA_85 | ENSG00000203930.12 | 4.76 × 10−3 | 78,457.5282 | LINC00632 | X | trans | Spinal Cord |
| SVA_15 | ENSG00000180354.16 | 1.86 × 10−12 | 77,839.9088 | MTURN | 7 | trans | Spinal Cord |
| SVA_55 | ENSG00000198712.1 | 3.04 × 10−3 | 76,318.8326 | MT-CO2 | MT | trans | Spinal Cord |
| SVA_85 | ENSG00000180354.16 | 1.35 × 10−5 | 67,900.9382 | MTURN | 7 | trans | Spinal Cord |
| SVA_85 | ENSG00000237973.1 | 4.10 × 10−33 | 66,702.2584 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA_37 | ENSG00000168314.18 | 3.50 × 10−9 | 65,555.4973 | MOBP | 3 | trans | Spinal Cord |
| SVA_85 | ENSG00000237973.1 | 1.01 × 10−32 | 53,997.5748 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_15 | ENSG00000168314.18 | 5.02 × 10−9 | 53,362.8451 | MOBP | 3 | trans | Spinal Cord |
| SVA_55 | ENSG00000237973.1 | 3.61 × 10−22 | 52,048.0183 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA_55 | ENSG00000168314.18 | 1.38 × 10−9 | 49,216.1425 | MOBP | 3 | trans | Spinal Cord |
| SVA_15 | ENSG00000237973.1 | 2.83 × 10−26 | 48,449.3666 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA_85 | ENSG00000168314.18 | 3.76 × 10−4 | 47,350.2242 | MOBP | 3 | trans | Spinal Cord |
| SVA_85 | ENSG00000237973.1 | 8.82 × 10−42 | 39,521.0532 | MTCO1P12 | 1 | trans | Spinal Cord |
| SVA_55 | ENSG00000064787.13 | 1.62 × 10−30 | 36,504.4209 | BCAS1 | 20 | trans | Spinal Cord |
| SVA_37 | ENSG00000091513.16 | 1.65× 10−6 | 35,838.5557 | TF | 3 | trans | Spinal Cord |
| SVA_55 | ENSG00000237973.1 | 1.49 × 10−59 | 33,728.2747 | MTCO1P12 | 1 | trans | Spinal Cord |
| SVA_4 | ENSG00000237973.1 | 3.59 × 10−17 | 32,837.5825 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_37 | ENSG00000237973.1 | 1.52 × 10−23 | 32,783.3654 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA_37 | ENSG00000237973.1 | 1.72 × 10−31 | 32,728.512 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_15 | ENSG00000237973.1 | 8.40 × 10−41 | 32,193.3558 | MTCO1P12 | 1 | trans | Spinal Cord |
| SVA_37 | ENSG00000099194.6 | 2.82 × 10−4 | 31,333.7253 | SCD | 10 | trans | Spinal Cord |
| SVA_85 | ENSG00000064787.13 | 3.78 × 10−10 | 30,888.7058 | BCAS1 | 20 | trans | Spinal Cord |
| SVA_15 | ENSG00000237973.1 | 3.46 × 10−14 | 30,602.307 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_15 | ENSG00000064787.13 | 1.97 × 10−15 | 30,356.5865 | BCAS1 | 20 | trans | Spinal Cord |
| SVA_37 | ENSG00000237973.1 | 1.28 × 10−6 | 30,025.949 | MTCO1P12 | 1 | trans | Cerebellum |
| SVA_15 | ENSG00000237973.1 | 5.29 × 10−13 | 29,472.617 | MTCO1P12 | 1 | trans | Cerebellum |
| SVA_55 | ENSG00000237973.1 | 2.00 × 10−8 | 27,693.2393 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_4 | ENSG00000237973.1 | 2.56 × 10−9 | 27,128.1241 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA | Gene ID | FDR p-Value | Beta Value | Gene | Chr | Cis/trans | Tissue |
|---|---|---|---|---|---|---|---|
| SVA_90 | ENSG00000197971.16 | 3.28 × 10−3 | −1,337,611.1 | MBP | 18 | trans | Spinal Cord |
| SVA_5 | ENSG00000197971.16 | 3.06 × 10−3 | −351,064.11 | MBP | 18 | trans | Spinal Cord |
| SVA_87 | ENSG00000123560.14 | 1.98 × 10−7 | −193,423.77 | PLP1 | X | trans | Spinal Cord |
| SVA_93 | ENSG00000123560.14 | 2.05 × 10−7 | −193,266.83 | PLP1 | X | trans | Spinal Cord |
| SVA_30 | ENSG00000198888.2 | 8.47 × 10−3 | −143,159.48 | MT-ND1 | MT | trans | Cerebellum |
| SVA_70 | ENSG00000198886.2 | 1.029 × 10−2 | −133,464.74 | MT-ND4 | MT | trans | Cerebellum |
| SVA_30 | ENSG00000198763.3 | 7.85 × 10−4 | −131,182.45 | MT-ND2 | MT | trans | Motor Cortex |
| SVA_30 | ENSG00000198938.2 | 6.86 × 10−3 | −111,339.82 | MT-CO3 | MT | trans | Cerebellum |
| SVA_30 | ENSG00000198727.2 | 5.66 × 10−3 | −104,147.35 | MT-CYB | MT | trans | Cerebellum |
| SVA_84 | ENSG00000123560.14 | 2.11 × 10−7 | −96,556.036 | PLP1 | X | trans | Spinal Cord |
| SVA_30 | ENSG00000198786.2 | 7.25 × 10−3 | −94,294.133 | MT-ND5 | MT | trans | Motor Cortex |
| SVA_70 | ENSG00000198763.3 | 1.29 × 10−2 | −82,929.066 | MT-ND2 | MT | trans | Cerebellum |
| SVA_90 | ENSG00000259001.3 | 6.14 × 10−3 | −73,740.939 | ENSG00000259001 | 14 | trans | Spinal Cord |
| SVA_91 | ENSG00000203930.12 | 2.02 × 10−3 | −71,147.071 | LINC00632 | X | trans | Spinal Cord |
| SVA_87 | ENSG00000168314.18 | 3.60 × 10−4 | −67,162.942 | MOBP | 3 | trans | Spinal Cord |
| SVA_93 | ENSG00000168314.18 | 3.67 × 10−4 | −67,112.229 | MOBP | 3 | trans | Spinal Cord |
| SVA_91 | ENSG00000180354.16 | 2.99 × 10−5 | −57,438.97 | MTURN | 7 | trans | Spinal Cord |
| SVA_90 | ENSG00000168309.18 | 4.26 × 10−3 | −48,145.904 | FAM107A | 3 | trans | Spinal Cord |
| SVA_5 | ENSG00000180354.16 | 1.55 × 10−5 | −43,141.656 | MTURN | 7 | trans | Spinal Cord |
| SVA_70 | ENSG00000198712.1 | 6.03 × 10−3 | −43,049.326 | MT-CO2 | MT | trans | Cerebellum |
| SVA_90 | ENSG00000168309.18 | 2.45 × 10−3 | −40,637.548 | FAM107A | 3 | trans | Motor Cortex |
| SVA_91 | ENSG00000168314.18 | 5.48 × 10−4 | −40,457.332 | MOBP | 3 | trans | Spinal Cord |
| SVA_90 | ENSG00000177575.13 | 2.99 × 10−19 | −36,552.585 | CD163 | 12 | trans | Spinal Cord |
| SVA_84 | ENSG00000168314.18 | 3.83 × 10−4 | −33,494.891 | MOBP | 3 | trans | Spinal Cord |
| SVA_90 | ENSG00000087086.15 | 1.64 × 10−8 | −32,820.222 | FTL | 19 | trans | Motor Cortex |
| SVA_5 | ENSG00000168314.18 | 1.72 × 10−4 | −31,165.253 | MOBP | 3 | trans | Spinal Cord |
| SVA_16 | ENSG00000123560.14 | 8.97 × 10−7 | −31,144.963 | PLP1 | X | trans | Spinal Cord |
| SVA_91 | ENSG00000237973.1 | 6.16 × 10−30 | −29,834.037 | MTCO1P12 | 1 | trans | Spinal Cord |
| SVA_87 | ENSG00000173786.17 | 5.88 × 10−4 | −28,556.333 | CNP | 17 | trans | Spinal Cord |
| SVA_93 | ENSG00000173786.17 | 5.98 × 10−4 | −28,536.883 | CNP | 17 | trans | Spinal Cord |
| SVA_90 | ENSG00000137285.11 | 1.48 × 10−40 | −27,173.64 | TUBB2B | 6 | trans | Motor Cortex |
| SVA_5 | ENSG00000237973.1 | 3.92 × 10−10 | −24,995.474 | MTCO1P12 | 1 | trans | Motor Cortex |
| SVA_5 | ENSG00000237973.1 | 8.77 × 10−13 | −24,871.705 | MTCO1P12 | 1 | trans | Frontal Cortex |
| SVA_91 | ENSG00000064787.13 | 5.47 × 10−7 | −22,960.148 | BCAS1 | 20 | trans | Spinal Cord |
| SVA_93 | ENSG00000198840.2 | 3.12 × 10−3 | −22,049.981 | MT-ND3 | MT | trans | Spinal Cord |
| SVA_87 | ENSG00000198840.2 | 3.13 × 10−3 | −22,046.442 | MT-ND3 | MT | trans | Spinal Cord |
| SVA_90 | ENSG00000164733.22 | 1.39 × 10−4 | −21,828.547 | CTSB | 8 | trans | Spinal Cord |
| SVA_90 | ENSG00000079215.15 | 1.69 × 10−5 | −21,020.491 | SLC1A3 | 5 | trans | Motor Cortex |
| SVA_5 | ENSG00000237973.1 | 8.40 × 10−24 | −19,907.219 | MTCO1P12 | 1 | trans | Spinal Cord |
| SVA_87 | ENSG00000136541.15 | 1.45 × 10−10 | −19,551.323 | ERMN | 2 | trans | Spinal Cord |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, L.S.; Fröhlich, A.; Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Kõks, S. Exploring SVA Insertion Polymorphisms in Shaping Differential Gene Expressions in the Central Nervous System. Biomolecules 2024, 14, 358. https://doi.org/10.3390/biom14030358
Hughes LS, Fröhlich A, Pfaff AL, Bubb VJ, Quinn JP, Kõks S. Exploring SVA Insertion Polymorphisms in Shaping Differential Gene Expressions in the Central Nervous System. Biomolecules. 2024; 14(3):358. https://doi.org/10.3390/biom14030358
Chicago/Turabian StyleHughes, Lauren S., Alexander Fröhlich, Abigail L. Pfaff, Vivien J. Bubb, John P. Quinn, and Sulev Kõks. 2024. "Exploring SVA Insertion Polymorphisms in Shaping Differential Gene Expressions in the Central Nervous System" Biomolecules 14, no. 3: 358. https://doi.org/10.3390/biom14030358
APA StyleHughes, L. S., Fröhlich, A., Pfaff, A. L., Bubb, V. J., Quinn, J. P., & Kõks, S. (2024). Exploring SVA Insertion Polymorphisms in Shaping Differential Gene Expressions in the Central Nervous System. Biomolecules, 14(3), 358. https://doi.org/10.3390/biom14030358





