Post-Translational Modifications and Diabetes
Abstract
1. Introduction
2. PTMs in Type 1 Diabetes
2.1. Oxidation
2.2. Glycation and N-Glycosylation
2.3. Carbonylation
2.4. Citrullination
2.5. Deamidation
2.6. O-GlyNAcylation
2.7. SUMOylation
2.8. Methylation
3. PTMs in Type 2 Diabetes
3.1. O-GlcNAcylation
3.2. Phosphorylation
3.3. Acetylation
3.4. SUMOylation
3.5. Oxidation
3.6. AGEs
4. Clinical Trials of Diabetes by Targeting PTMs
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100, 153952. [Google Scholar] [CrossRef]
- Chakkalakal, R.J.; Galaviz, K.I.; Sathish, T.; Shah, M.K.; Narayan, K.M.V. Test and Treat for Prediabetes: A Review of the Health Effects of Prediabetes and the Role of Screening and Prevention. Annu. Rev. Public Health, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Diaz-Santana, M.V.; O’Brien, K.M.; Park, Y.M.; Sandler, D.P.; Weinberg, C.R. Persistence of Risk for Type 2 Diabetes after Gestational Diabetes Mellitus. Diabetes Care 2022, 45, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Uy, R.; Wold, F. Posttranslational covalent modification of proteins. Science 1977, 198, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zou, H.; Chen, B.; Zhong, J. Posttranslational modifications in diabetes: Mechanisms and functions. Rev. Endocr. Metab. Disord. 2022, 23, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.J.; Spindler, M.P.; van Lummel, M.; Roep, B.O. Where, How, and When: Positioning Posttranslational Modification Within Type 1 Diabetes Pathogenesis. Curr. Diabetes Rep. 2016, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, M.; Zhao, Y.; Yang, Z.; Song, M.; Mi, J.; Yang, X.; Tian, G. Regulation of insulin secretion by the post-translational modifications. Front. Cell Dev. Biol. 2023, 11, 1217189. [Google Scholar] [CrossRef]
- Sheppard, T.L. AMPing up click reactions. Nat. Chem. Biol. 2011, 7, 857. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Preissler, S.; Rato, C.; Perera, L.; Saudek, V.; Ron, D. FICD acts bifunctionally to AMPylate and de-AMPylate the endoplasmic reticulum chaperone BiP. Nat. Struct. Mol. Biol. 2017, 24, 23–29. [Google Scholar] [CrossRef]
- Perera, L.A.; Hattersley, A.T.; Harding, H.P.; Wakeling, M.N.; Flanagan, S.E.; Mohsina, I.; Raza, J.; Gardham, A.; Ron, D.; De Franco, E. Infancy-onset diabetes caused by de-regulated AMPylation of the human endoplasmic reticulum chaperone BiP. EMBO Mol. Med. 2023, 15, e16491. [Google Scholar] [CrossRef] [PubMed]
- Seeling, T.; Haucke, E.; Navarrete Santos, A.; Grybel, K.J.; Gurke, J.; Pendzialek, S.M.; Schindler, M.; Simm, A.; Navarrete Santos, A. Glyoxalase 1 expression is downregulated in preimplantation blastocysts of diabetic rabbits. Reprod. Domest. Anim. 2019, 54 (Suppl. S3), 4–11. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Thakur, S.S. Investigation of post-translational modifications in type 2 diabetes. Clin. Proteom. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Gnad, F.; Mann, M. The Case for Proteomics and Phospho-Proteomics in Personalized Cancer Medicine. Proteom. Clin. Appl. 2019, 13, e1800113. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, K.; Dowling, P.; Bazou, D.; O’Gorman, P. Current Methods of Post-Translational Modification Analysis and Their Applications in Blood Cancers. Cancers 2021, 13, 1930. [Google Scholar] [CrossRef]
- Sims, E.K.; Mirmira, R.G.; Evans-Molina, C. The role of beta-cell dysfunction in early type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 215–224. [Google Scholar] [CrossRef]
- Strollo, R.; Vinci, C.; Napoli, N.; Fioriti, E.; Maddaloni, E.; Akerman, L.; Casas, R.; Pozzilli, P.; Ludvigsson, J.; Nissim, A. Antibodies to oxidized insulin improve prediction of type 1 diabetes in children with positive standard islet autoantibodies. Diabetes Metab. Res. Rev. 2019, 35, e3132. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.; Neelofar, K.; Arfat, M.Y.; Zaman, A.; Tarannum, A.; Parveen, I.; Ahmad, S.; Khan, M.A.; Badar, A.; Islam, S.N. Hyperglycemia induced reactive species trigger structural changes in human serum albumin of type 1 diabetic subjects. Int. J. Biol. Macromol. 2018, 107 Pt B, 2141–2149. [Google Scholar] [CrossRef]
- Nuti, F.; Gallo, A.; Real-Fernandez, F.; Rentier, C.; Rossi, G.; Piarulli, F.; Traldi, P.; Carganico, S.; Rovero, P.; Lapolla, A.; et al. Study of Aberrant Modifications in Peptides as a Test Bench to Investigate the Immunological Response to Non-Enzymatic Glycation. Folia Biol. 2019, 65, 195–202. [Google Scholar] [CrossRef]
- Rudman, N.; Kifer, D.; Kaur, S.; Simunovic, V.; Cvetko, A.; Pociot, F.; Morahan, G.; Gornik, O. Children at onset of type 1 diabetes show altered N-glycosylation of plasma proteins and IgG. Diabetologia 2022, 65, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Soic, D.; Keser, T.; Stambuk, J.; Kifer, D.; Pociot, F.; Lauc, G.; Morahan, G.; Novokmet, M.; Gornik, O. High-Throughput Human Complement C3 N-Glycoprofiling Identifies Markers of Early Onset Type 1 Diabetes Mellitus in Children. Mol. Cell. Proteom. 2022, 21, 100407. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Connolly, S.E.; Gee, R.J.; Lam, T.T.; Kanyo, J.; Peng, J.; Guyer, P.; Syed, F.; Tse, H.M.; Clarke, S.G.; et al. Carbonyl Posttranslational Modification Associated With Early-Onset Type 1 Diabetes Autoimmunity. Diabetes 2022, 71, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Horstman, S.; Gee, R.; Guyer, P.; Lam, T.T.; Kanyo, J.; Perdigoto, A.L.; Speake, C.; Greenbaum, C.J.; Callebaut, A.; et al. Citrullination of glucokinase is linked to autoimmune diabetes. Nat. Commun. 2022, 13, 1870. [Google Scholar] [CrossRef]
- Callebaut, A.; Bruggeman, Y.; Zamit, C.; Sodre, F.M.C.; Irla, M.; Mathieu, C.; Buitinga, M.; Overbergh, L. Aberrant expression of transglutaminase 2 in pancreas and thymus of NOD mice underscores the importance of deamidation in neoantigen generation. Front. Endocrinol. 2022, 13, 908248. [Google Scholar] [CrossRef]
- De Jesus, T.J.; Tomalka, J.A.; Centore, J.T.; Staback Rodriguez, F.D.; Agarwal, R.A.; Liu, A.R.; Kern, T.S.; Ramakrishnan, P. Negative regulation of FOXP3 expression by c-Rel O-GlcNAcylation. Glycobiology 2021, 31, 812–826. [Google Scholar] [CrossRef]
- Kronlage, M.; Dewenter, M.; Grosso, J.; Fleming, T.; Oehl, U.; Lehmann, L.H.; Falcao-Pires, I.; Leite-Moreira, A.F.; Volk, N.; Grone, H.J.; et al. O-GlcNAcylation of Histone Deacetylase 4 Protects the Diabetic Heart From Failure. Circulation 2019, 140, 580–594. [Google Scholar] [CrossRef]
- Wang, F.; Sun, F.; Luo, J.; Yue, T.; Chen, L.; Zhou, H.; Zhang, J.; Yang, C.; Luo, X.; Zhou, Q.; et al. Loss of ubiquitin-conjugating enzyme E2 (Ubc9) in macrophages exacerbates multiple low-dose streptozotocin-induced diabetes by attenuating M2 macrophage polarization. Cell Death Dis. 2019, 10, 892. [Google Scholar] [CrossRef]
- Yonamine, C.Y.; Alves-Wagner, A.B.; Esteves, J.V.; Okamoto, M.M.; Correa-Giannella, M.L.; Giannella-Neto, D.; Machado, U.F. Diabetes induces tri-methylation at lysine 9 of histone 3 at Slc2a4 gene in skeletal muscle: A new target to improve glycemic control. Mol. Cell. Endocrinol. 2019, 481, 26–34. [Google Scholar] [CrossRef]
- Von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Jin, Z.; Fu, Z.; Yang, J.; Troncosco, J.; Everett, A.D.; Van Eyk, J.E. Identification and characterization of citrulline-modified brain proteins by combining HCD and CID fragmentation. Proteomics 2013, 13, 2682–2691. [Google Scholar] [CrossRef]
- Matschinsky, F.M.; Wilson, D.F. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Gerondakis, S. The c-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, S.; Xiong, F.; Eizirik, D.L.; Wang, C.Y. SUMOylation, a multifaceted regulatory mechanism in the pancreatic beta cells. Semin. Cell Dev. Biol. 2020, 103, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, M.; Geng, M.; Chen, S.; Little, P.J.; Xu, S.; Weng, J. Targeting protein modifications in metabolic diseases: Molecular mechanisms and targeted therapies. Signal Transduct. Target Ther. 2023, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Type 2 diabetes: Etiology and reversibility. Diabetes Care 2013, 36, 1047–1055. [Google Scholar] [CrossRef]
- Gonzalez-Rellan, M.J.; Fondevila, M.F.; Fernandez, U.; Rodriguez, A.; Varela-Rey, M.; Veyrat-Durebex, C.; Seoane, S.; Bernardo, G.; Lopitz-Otsoa, F.; Fernandez-Ramos, D.; et al. O-GlcNAcylated p53 in the liver modulates hepatic glucose production. Nat. Commun. 2021, 12, 5068. [Google Scholar] [CrossRef]
- Oliveri, L.M.; Buzaleh, A.M.; Gerez, E.N. An increase in O-GlcNAcylation of Sp1 down-regulates the gene expression of pi class glutathione S-transferase in diabetic mice. Biochem. Biophys. Rep. 2021, 27, 101049. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Maniyadath, B.; Bagul, H.P.; Chakraborty, A.; Shukla, N.; Budnar, S.; Rajendran, A.; Shukla, A.; Kamat, S.S.; Kolthur-Seetharam, U. Spatiotemporal gating of SIRT1 functions by O-GlcNAcylation is essential for liver metabolic switching and prevents hyperglycemia. Proc. Natl. Acad. Sci. USA 2020, 117, 6890–6900. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lockridge, A.; Alejandro, E.U. eIF4G1 and carboxypeptidase E axis dysregulation in O-GlcNAc transferase-deficient pancreatic beta-cells contributes to hyperproinsulinemia in mice. J. Biol. Chem. 2019, 294, 13040–13050. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, A.; Jo, S.; Gustafson, E.; Damberg, N.; Mohan, R.; Olson, M.; Abrahante, J.E.; Alejandro, E.U. Islet O-GlcNAcylation Is Required for Lipid Potentiation of Insulin Secretion through SERCA2. Cell Rep. 2020, 31, 107609. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, M.; Li, M.D.; Zhang, K.; Zhang, B.; Wang, S.; Liu, Y.; Ni, W.; Ong, Q.; Mi, J.; et al. O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat. Commun. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Sacco, F.; Seelig, A.; Humphrey, S.J.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets. Cell Metab. 2019, 29, 1422–1432.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, W.K.; Oh, K.J.; Lee, E.W.; Han, B.S.; Lee, S.C.; Bae, K.H. Protein Tyrosine Phosphatase, Receptor Type B (PTPRB) Inhibits Brown Adipocyte Differentiation through Regulation of VEGFR2 Phosphorylation. J. Microbiol. Biotechnol. 2019, 29, 645–650. [Google Scholar] [CrossRef]
- Cochrane, V.A.; Wu, Y.; Yang, Z.; ElSheikh, A.; Dunford, J.; Kievit, P.; Fortin, D.A.; Shyng, S.L. Leptin modulates pancreatic beta-cell membrane potential through Src kinase-mediated phosphorylation of NMDA receptors. J. Biol. Chem. 2020, 295, 17281–17297. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Xiao, H.; Xu, F.; Yang, Y.; Lin, Z.; Chen, Z.; Quan, S.; Huang, H. PAQR3 regulates phosphorylation of FoxO1 in insulin-resistant HepG2 cells via NF-kappaB signaling pathway. Exp. Cell Res. 2019, 381, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, J.; Chen, J.; Yao, C.; Yang, Y.; Wang, J.; Zhuang, H.; Hua, Z.C. FADD Phosphorylation Modulates Blood Glucose Levels by Decreasing the Expression of Insulin-Degrading Enzyme. Mol. Cells 2020, 43, 373–383. [Google Scholar] [PubMed]
- Harding, H.P.; Zeng, H.; Zhang, Y.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Matsumoto, M.; Kaneko, S.; Kasuga, M.; Inoue, H. Sirt2 facilitates hepatic glucose uptake by deacetylating glucokinase regulatory protein. Nat. Commun. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, T.; Li, Y.; Li, X.; Yuan, X.; Liu, L.; Wu, W.; Wu, J.; Wu, Y.; Liang, R.; et al. The hepatic AMPK-TET1-SIRT1 axis regulates glucose homeostasis. Elife 2021, 10, e70672. [Google Scholar] [CrossRef]
- Yuan, W.; Ma, C.; Zhou, Y.; Wang, M.; Zeng, G.; Huang, Q. Negative regulation of eNOS-NO signaling by over-SUMOylation of PPARgamma contributes to insulin resistance and dysfunction of vascular endothelium in rats. Vasc. Pharmacol. 2019, 122–123, 106597. [Google Scholar] [CrossRef]
- Katafuchi, T.; Holland, W.L.; Kollipara, R.K.; Kittler, R.; Mangelsdorf, D.J.; Kliewer, S.A. PPARgamma-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12102–12111. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Shen, X.; Yuan, W.; Zhou, Y.; Huang, Q. Sumoylation of PPARgamma contributes to vascular endothelium insulin resistance through stabilizing the PPARgamma-NcoR complex. J. Cell. Physiol. 2019, 234, 19663–19674. [Google Scholar] [CrossRef]
- Hoard, T.M.; Yang, X.P.; Jetten, A.M.; ZeRuth, G.T. PIAS-family proteins negatively regulate Glis3 transactivation function through SUMO modification in pancreatic beta cells. Heliyon 2018, 4, e00709. [Google Scholar] [CrossRef]
- Arunagiri, A.; Haataja, L.; Pottekat, A.; Pamenan, F.; Kim, S.; Zeltser, L.M.; Paton, A.W.; Paton, J.C.; Tsai, B.; Itkin-Ansari, P.; et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. Elife 2019, 8, e44532. [Google Scholar] [CrossRef]
- Pinto-Junior, D.C.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci. Rep. 2018, 8, 8109. [Google Scholar] [CrossRef]
- McClain, D.A.; Lubas, W.A.; Cooksey, R.C.; Hazel, M.; Parker, G.J.; Love, D.C.; Hanover, J.A. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc. Natl. Acad. Sci. USA 2002, 99, 10695–10699. [Google Scholar] [CrossRef] [PubMed]
- Vosseller, K.; Wells, L.; Lane, M.D.; Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 5313–5318. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.A.; Dias, W.B.; Thiruneelakantapillai, L.; Lane, M.D.; Hart, G.W. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-Linked beta-N-acetylglucosamine in 3T3-L1 adipocytes. J. Biol. Chem. 2010, 285, 5204–5211. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Yi, W. O-GlcNAcylation, a sweet link to the pathology of diseases. J. Zhejiang Univ. Sci. B 2019, 20, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ongusaha, P.P.; Miles, P.D.; Havstad, J.C.; Zhang, F.; So, W.V.; Kudlow, J.E.; Michell, R.H.; Olefsky, J.M.; Field, S.J.; et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 2008, 451, 964–969. [Google Scholar] [CrossRef]
- Parker, G.J.; Lund, K.C.; Taylor, R.P.; McClain, D.A. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J. Biol. Chem. 2003, 278, 10022–10027. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Yang, T.; Fu, M.; Pestell, R.; Sauve, A.A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006, 17, 186–191. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Wu, M.; Wang, H. Emerging Roles of SIRT1 in Alcoholic Liver Disease. Int. J. Biol. Sci. 2020, 16, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Pfutzner, A.; Kunt, T.; Hohberg, C.; Mondok, A.; Pahler, S.; Konrad, T.; Lubben, G.; Forst, T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care 2004, 27, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Mykkanen, L.; Zaccaro, D.J.; Hales, C.N.; Festa, A.; Haffner, S.M. The relation of proinsulin and insulin to insulin sensitivity and acute insulin response in subjects with newly diagnosed type II diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia 1999, 42, 1060–1066. [Google Scholar] [CrossRef]
- Klein, S. The case of visceral fat: Argument for the defense. J. Clin. Investig. 2004, 113, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.H.; Jokubka, R.; Tojjar, D.; Granhall, C.; Hansson, O.; Li, D.Q.; Nagaraj, V.; Reinbothe, T.M.; Tuncel, J.; Eliasson, L.; et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 2010, 327, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.J.; Selander, L.; Carlsson, L.; Edlund, H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J. Biol. Chem. 2006, 281, 6395–6403. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Annamalai, P.; Enerback, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Hanssen, M.J.; Hoeks, J.; Brans, B.; van der Lans, A.A.; Schaart, G.; van den Driessche, J.J.; Jorgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diabetes Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Barsh, G.S.; Schwartz, M.W. Genetic approaches to studying energy balance: Perception and integration. Nat. Rev. Genet. 2002, 3, 589–600. [Google Scholar] [CrossRef]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980225. [Google Scholar] [CrossRef]
- Rada, P.; Mosquera, A.; Muntane, J.; Ferrandiz, F.; Rodriguez-Manas, L.; de Pablo, F.; Gonzalez-Canudas, J.; Valverde, A.M. Differential effects of metformin glycinate and hydrochloride in glucose production, AMPK phosphorylation and insulin sensitivity in hepatocytes from non-diabetic and diabetic mice. Food Chem. Toxicol. 2019, 123, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Teaney, N.A.; Cyr, N.E. FoxO1 as a tissue-specific therapeutic target for type 2 diabetes. Front. Endocrinol. 2023, 14, 1286838. [Google Scholar] [CrossRef]
- Nakae, J.; Park, B.C.; Accili, D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J. Biol. Chem. 1999, 274, 15982–15985. [Google Scholar] [CrossRef]
- Brownawell, A.M.; Kops, G.J.; Macara, I.G.; Burgering, B.M. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 2001, 21, 3534–3546. [Google Scholar] [CrossRef]
- Yao, C.; Zhuang, H.; Cheng, W.; Lin, Y.; Du, P.; Yang, B.; Huang, X.; Chen, S.; Hu, Q.; Hua, Z.C. FADD phosphorylation impaired islet morphology and function. J. Cell. Physiol. 2015, 230, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Colon-Negron, K.; Papa, F.R. Endoplasmic reticulum stress, degeneration of pancreatic islet beta-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol. Metab. 2019, 27, S60–S68. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic beta-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Gao, Y.; Sartori, D.J.; Li, C.; Yu, Q.C.; Kushner, J.A.; Simon, M.C.; Diehl, J.A. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol. Cell. Biol. 2012, 32, 5129–5139. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Chakraborty, P.; Gangopadhyay, M.; Sahu, R.; Medala, V.; John, A.; Reddy, P.H.; De Feo, V.; et al. The Emerging Role of HDACs: Pathology and Therapeutic Targets in Diabetes Mellitus. Cells 2021, 10, 1340. [Google Scholar] [CrossRef]
- Kumar, S.; Chinnusamy, V.; Mohapatra, T. Epigenetics of Modified DNA Bases: 5-Methylcytosine and Beyond. Front. Genet. 2018, 9, 640. [Google Scholar] [CrossRef]
- Rajan, S.; Torres, J.; Thompson, M.S.; Philipson, L.H. SUMO downregulates GLP-1-stimulated cAMP generation and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E714–E723. [Google Scholar] [CrossRef][Green Version]
- Dai, X.Q.; Plummer, G.; Casimir, M.; Kang, Y.; Hajmrle, C.; Gaisano, H.Y.; Manning Fox, J.E.; MacDonald, P.E. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 2011, 60, 838–847. [Google Scholar] [CrossRef]
- Ohshima, T.; Koga, H.; Shimotohno, K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J. Biol. Chem. 2004, 279, 29551–29557. [Google Scholar] [CrossRef] [PubMed]
- ZeRuth, G.T.; Takeda, Y.; Jetten, A.M. The Kruppel-like protein Gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol. Endocrinol. 2013, 27, 1692–1705. [Google Scholar] [CrossRef]
- Scoville, D.W.; Jetten, A.M. GLIS3: A Critical Transcription Factor in Islet beta-Cell Generation. Cells 2021, 10, 3471. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yang, Y. Emerging roles of GLIS3 in neonatal diabetes, type 1 and type 2 diabetes. J. Mol. Endocrinol. 2017, 58, R73–R85. [Google Scholar] [CrossRef] [PubMed]
- Haataja, L.; Manickam, N.; Soliman, A.; Tsai, B.; Liu, M.; Arvan, P. Disulfide Mispairing During Proinsulin Folding in the Endoplasmic Reticulum. Diabetes 2016, 65, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, Y.; Zhang, M.; Deng, K.Y.; Singh, R.; Tian, Q.; Gong, Y.; Pan, Z.; Liu, Q.; Boisclair, Y.R.; et al. Endoplasmic Reticulum-Associated Degradation (ERAD) Has a Critical Role in Supporting Glucose-Stimulated Insulin Secretion in Pancreatic beta-Cells. Diabetes 2019, 68, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Pottekat, A.; Poothong, J.; Yong, J.; Lagunas-Acosta, J.; Charbono, A.; Chen, Z.; Scheuner, D.L.; Liu, M.; Itkin-Ansari, P.; et al. PDIA1/P4HB is required for efficient proinsulin maturation and ss cell health in response to diet induced obesity. eLife 2019, 8, e44528. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Chekol Abebe, E.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Agidew, M.M.; Teshome Azezew, M.; Zewde, E.A.; Agegnehu Teshome, A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Mori, R.C.; Hirabara, S.M.; Hirata, A.E.; Okamoto, M.M.; Machado, U.F. Glimepiride as insulin sensitizer: Increased liver and muscle responses to insulin. Diabetes Obes. Metab. 2008, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Baum, S.J.; Brinton, E.A.; Plutzky, J.; Hanselman, J.C.; Teng, R.; Ballantyne, C.M. Effect of bempedoic acid plus ezetimibe fixed-dose combination vs ezetimibe or placebo on low-density lipoprotein cholesterol in patients with type 2 diabetes and hypercholesterolemia not treated with statins. Am. J. Prev. Cardiol. 2021, 8, 100278. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, A.; Hayashi, N.; Komori, H.; Leonsson-Zachrisson, M.; Johnsson, E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2013, 15, 923–930. [Google Scholar] [CrossRef]
- Wilding, J.P.; Leonsson-Zachrisson, M.; Wessman, C.; Johnsson, E. Dose-ranging study with the glucokinase activator AZD1656 in patients with type 2 diabetes mellitus on metformin. Diabetes Obes. Metab. 2013, 15, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Gan, S.; Liu, Y.; Ma, J.; Dong, X.; Song, W.; Zeng, J.; Wang, G.; Zhao, W.; Zhang, Q.; et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: A dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018, 6, 627–636. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, D.; Gan, S.; Dong, X.; Su, J.; Li, W.; Jiang, H.; Zhao, W.; Yao, M.; Song, W.; et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 974–981. [Google Scholar] [CrossRef]
- Vanweert, F.; Neinast, M.; Tapia, E.E.; van de Weijer, T.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Blair, M.C.; Bornstein, M.R.; Hesselink, M.K.C.; Schrauwen, P.; et al. A randomized placebo-controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nat. Commun. 2022, 13, 3508. [Google Scholar] [CrossRef]
- Klein, K.R.; Freeman, J.L.R.; Dunn, I.; Dvergsten, C.; Kirkman, M.S.; Buse, J.B.; Valcarce, C.; Simplici, T.R.G. The SimpliciT1 Study: A Randomized, Double-Blind, Placebo-Controlled Phase 1b/2 Adaptive Study of TTP399, a Hepatoselective Glucokinase Activator, for Adjunctive Treatment of Type 1 Diabetes. Diabetes Care 2021, 44, 960–968. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Filippov, S.; Srivastava, R.A.; Hanselman, J.C.; Bradshaw, C.D.; Hurley, T.R.; Cramer, C.T.; Spahr, M.A.; Brant, A.F.; Houghton, J.L.; et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 2013, 54, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.S.; Kwon, O.S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Zhu, X.X.; Zhu, D.L.; Li, X.Y.; Li, Y.L.; Jin, X.W.; Hu, T.X.; Zhao, Y.; Li, Y.G.; Zhao, G.Y.; Ren, S.; et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic beta-cell function in patients with type 2 diabetes: A 28-day treatment study using biomarker-guided patient selection. Diabetes Obes. Metab. 2018, 20, 2113–2120. [Google Scholar] [CrossRef]
- Burrage, L.C.; Jain, M.; Gandolfo, L.; Lee, B.H.; Members of the Urea Cycle Disorders Consortium; Nagamani, S.C. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol. Genet. Metab. 2014, 113, 131–135. [Google Scholar] [CrossRef]
- Vella, A.; Freeman, J.L.R.; Dunn, I.; Keller, K.; Buse, J.B.; Valcarce, C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci. Transl. Med. 2019, 11, eaau3441. [Google Scholar] [CrossRef]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, J.; Inuzuka, H.; Wei, W. Targeted protein posttranslational modifications by chemically induced proximity for cancer therapy. J. Biol. Chem. 2023, 299, 104572. [Google Scholar] [CrossRef] [PubMed]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Henning, N.J.; Boike, L.; Spradlin, J.N.; Ward, C.C.; Liu, G.; Zhang, E.; Belcher, B.P.; Brittain, S.M.; Hesse, M.J.; Dovala, D.; et al. Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 2022, 18, 412–421. [Google Scholar] [CrossRef]
- Chen, P.H.; Hu, Z.; An, E.; Okeke, I.; Zheng, S.; Luo, X.; Gong, A.; Jaime-Figueroa, S.; Crews, C.M. Modulation of Phosphoprotein Activity by Phosphorylation Targeting Chimeras (PhosTACs). ACS Chem. Biol. 2021, 16, 2808–2815. [Google Scholar] [CrossRef]
- Kabir, M.; Sun, N.; Hu, X.; Martin, T.C.; Yi, J.; Zhong, Y.; Xiong, Y.; Kaniskan, H.U.; Gu, W.; Parsons, R.; et al. Acetylation Targeting Chimera Enables Acetylation of the Tumor Suppressor p53. J. Am. Chem. Soc. 2023, 145, 14932–14944. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.D.; Kim, K.K.; Lee, K.R. Does Weight Gain Associated with Thiazolidinedione Use Negatively Affect Cardiometabolic Health? J. Obes. Metab. Syndr. 2017, 26, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.G.; Augsornworawat, P.; Velazco-Cruz, L.; Kim, M.H.; Asada, R.; Hogrebe, N.J.; Morikawa, S.; Urano, F.; Millman, J.R. Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020, 12, eaax9106. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Zidek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Yamashita, D.; Yamaguchi, T.; Shimizu, M.; Nakata, N.; Hirose, F.; Osumi, T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells 2004, 9, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
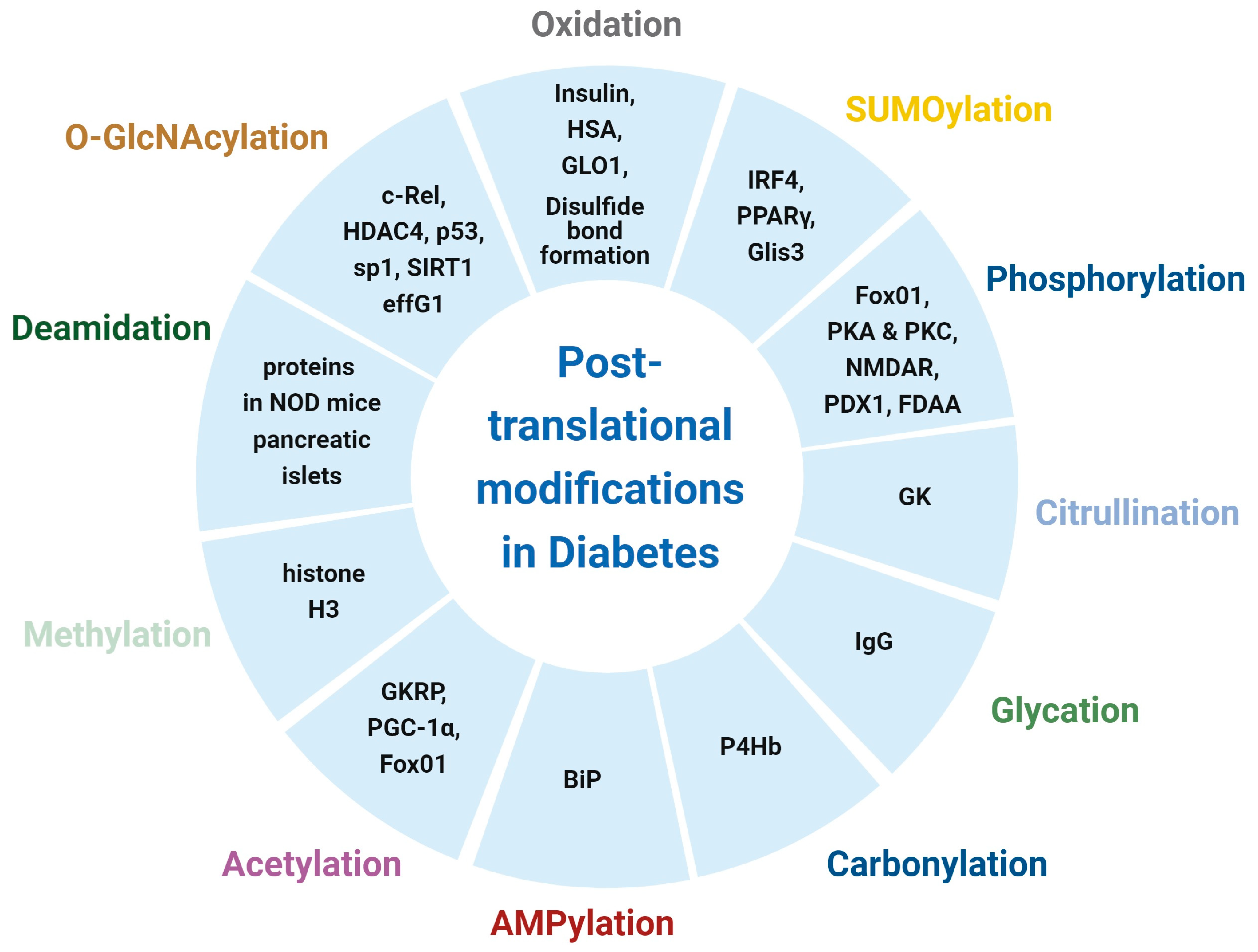
| PTM | Proteins | Function | Reference |
|---|---|---|---|
| Oxidation | Insulin | Antibodies to oxidized insulin (oxPTM-INS-Ab) improve T1D risk assessment | [21] |
| HSA | Initiation and progression of T1D | [22] | |
| Glycation | lgG | Decreased IgG function | [23] |
| N-Glycosylation | Plasma proteins, IgG, and C3 | Risk factor for early-onset T1D | [24,25] |
| Carbonylation | P4Hb | Decreases glucose-stimulated insulin secretion and alters proinsulin-to-insulin ratios | [26] |
| Citrullination | GK | Impairs islet response to glucose and overall glucose homeostasis | [27] |
| Deamidation | Proteins in NOD mice and pancreatic islets | Generation of deamidated autoantigens in T1D | [28] |
| O-GlcNAcylation | c-Rel | Reduces immunosuppressive FOXP3 expression | [29] |
| HDAC4 | Enhances the production of the cardio-protective N terminal of HDAC4 | [30] | |
| SUMOylation | IRF4 | Promotes macrophage M2 polarization and energy homeostasis | [31] |
| Methylation | Histone H3 | Suppresses GLUT4 expression and worsens glycemic impairment | [32] |
| PTM | Proteins | Function | Reference |
|---|---|---|---|
| O-GlcNAcylation | p53 | Activates gluconeogenic PCK1 transcription | [42] |
| Sp1 | Downregulation of GTSP expression for the development of oxidative stress | [43] | |
| SIRT1 | Controls liver metabolic switching and hyperglycemia prevention | [44] | |
| eIF4G1 | reverses hyperproinsulinemia | [45] | |
| SERCA2 | Restores insulin secretion | [46] | |
| PLIN1 | Retains fat mass in adipose tissue, triggers diet-induced obesity, and leads to whole-body insulin resistance | [47] | |
| Phosphorylation | PKA and PKC | Hyperglycemia | [48] |
| PDX1 | Impairment of β-cell functions | [48] | |
| VEGFR2 | Regulates brown adipocyte differentiation | [49] | |
| NMDAR | Results in β-cell hyperpolarization and a reduction in glucose-mediated insulin secretion | [50] | |
| FoxO1 | Insulin resistance | [51] | |
| FDAA | Insulin degradation | [52] | |
| eIF2α | Reduced protein synthesis | [53] | |
| Acetylation | GKRP | Impairment of hepatic glucose uptake | [54] |
| PGC-1α and FoxO1 | Regulates hepatic glucose homeostasis | [55] | |
| SUMOylation | PPARγ | Endothelial insulin resistance | [56,57,58] |
| Glis3 | Reduction in insulin transcription | [59] | |
| Oxidation | Proinsulin | Proinsulin misfolding, ER stress, and β-cell failure | [60] |
| AGEs | Albumin | Insulin resistance and decreased GLUT4 expression | [61] |
| Drug | Disease | Clinical Trial ID | PTM | Outcome | Reference |
|---|---|---|---|---|---|
| Bempedoic acid/Ezetimibe | T2D | NCT03531905 | Phosphorylation | Lowered low-density lipoprotein cholesterol and improved high-sensitivity C-reactive protein | [105] |
| Curcumin | T2D | NCT01052025 | Phosphorylation Ubiquitination O-GlcNAcylation | Delayed onset of T2D from prediabetes | [106] |
| AZD1656 | T2D | NCT01152385 NCT01020123 | S-nitrosylation SUMOylation | Reduction in HbA1C after short-term treatment; Improvement of glycemic control in combination with metformin up to 4 months | [107,108] |
| Dorzagliatin | T2D | NCT02561338 NCT03141073 | S-nitrosylation SUMOylation | Beneficial effect on glycemic control; Effective glycemic control in combination with metformin | [109,110] |
| Sodium phenylbutyrate | T2D | NTR7426 | Acetylation | Increased peripheral insulin sensitivity and reduced plasma branched-chain amino acids and glucose levels | [111] |
| TTP399 | T1D | NCT03335371 | S-nitrosylation SUMOylation | Lowered HbA1C and reduced hypoglycemia | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, C.; Hamza, A.; Boyle, E.; Donu, D.; Cen, Y. Post-Translational Modifications and Diabetes. Biomolecules 2024, 14, 310. https://doi.org/10.3390/biom14030310
Sharma C, Hamza A, Boyle E, Donu D, Cen Y. Post-Translational Modifications and Diabetes. Biomolecules. 2024; 14(3):310. https://doi.org/10.3390/biom14030310
Chicago/Turabian StyleSharma, Chiranjeev, Abu Hamza, Emily Boyle, Dickson Donu, and Yana Cen. 2024. "Post-Translational Modifications and Diabetes" Biomolecules 14, no. 3: 310. https://doi.org/10.3390/biom14030310
APA StyleSharma, C., Hamza, A., Boyle, E., Donu, D., & Cen, Y. (2024). Post-Translational Modifications and Diabetes. Biomolecules, 14(3), 310. https://doi.org/10.3390/biom14030310






