Rational Design of Key Enzymes to Efficiently Synthesize Phycocyanobilin in Escherichia coli
Abstract
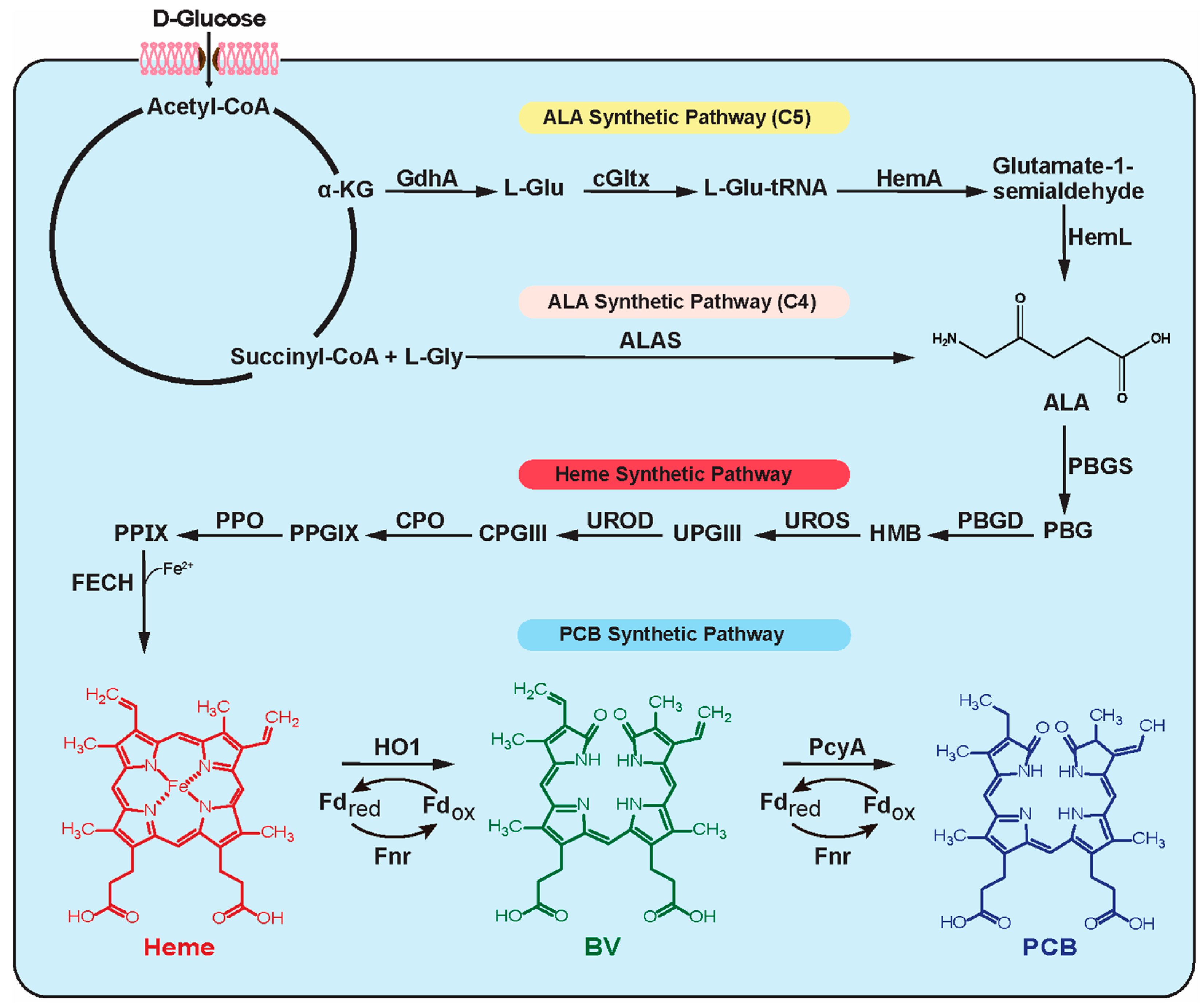
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Plasmids and DNA Manipulations
2.3. Culture Conditions
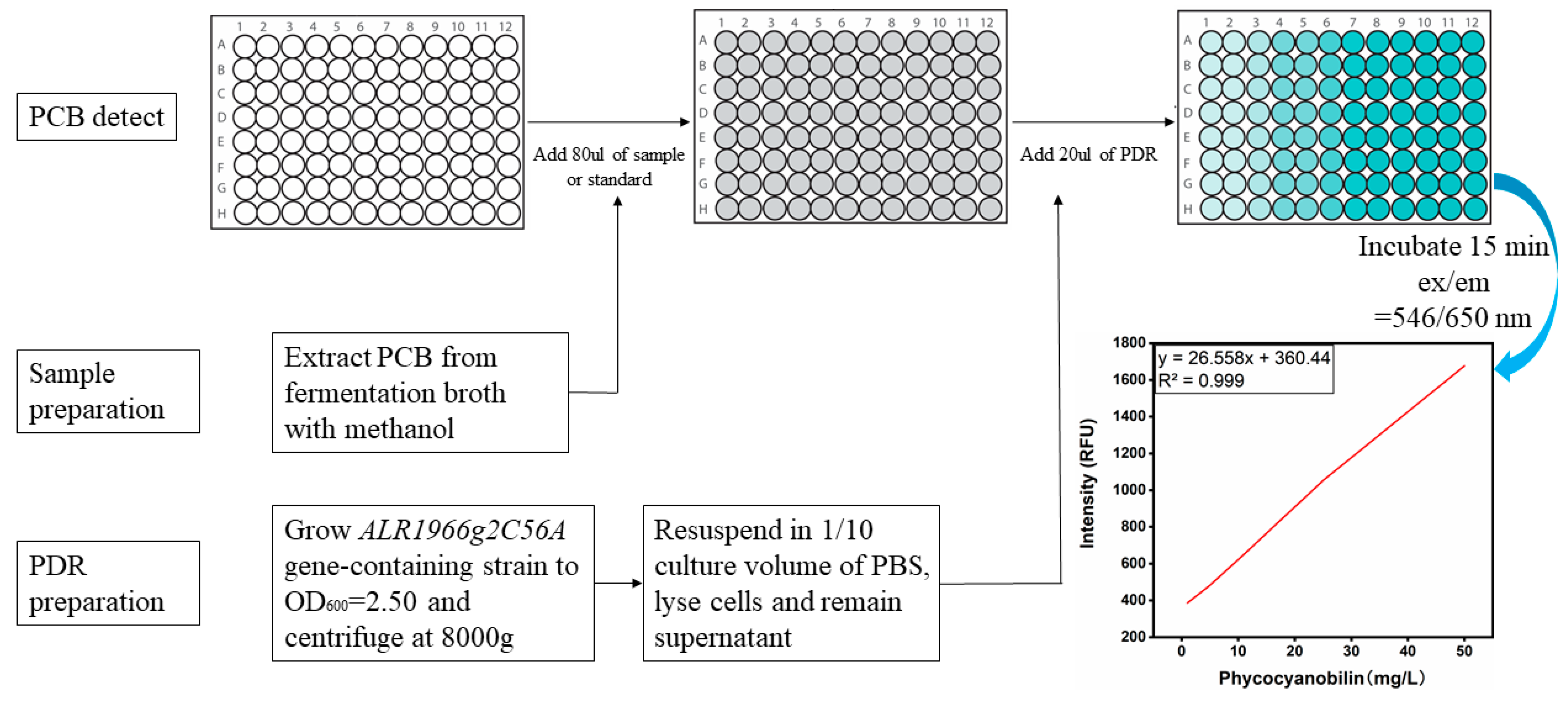
2.4. Preparation of Detection Reagents (BDR/PDR) and Standard Curves of BV and PCB for High-Throughput Screening Assays
2.5. Analytical Procedures
2.6. Homology Modeling and Ligand Docking
3. Results and Discussion
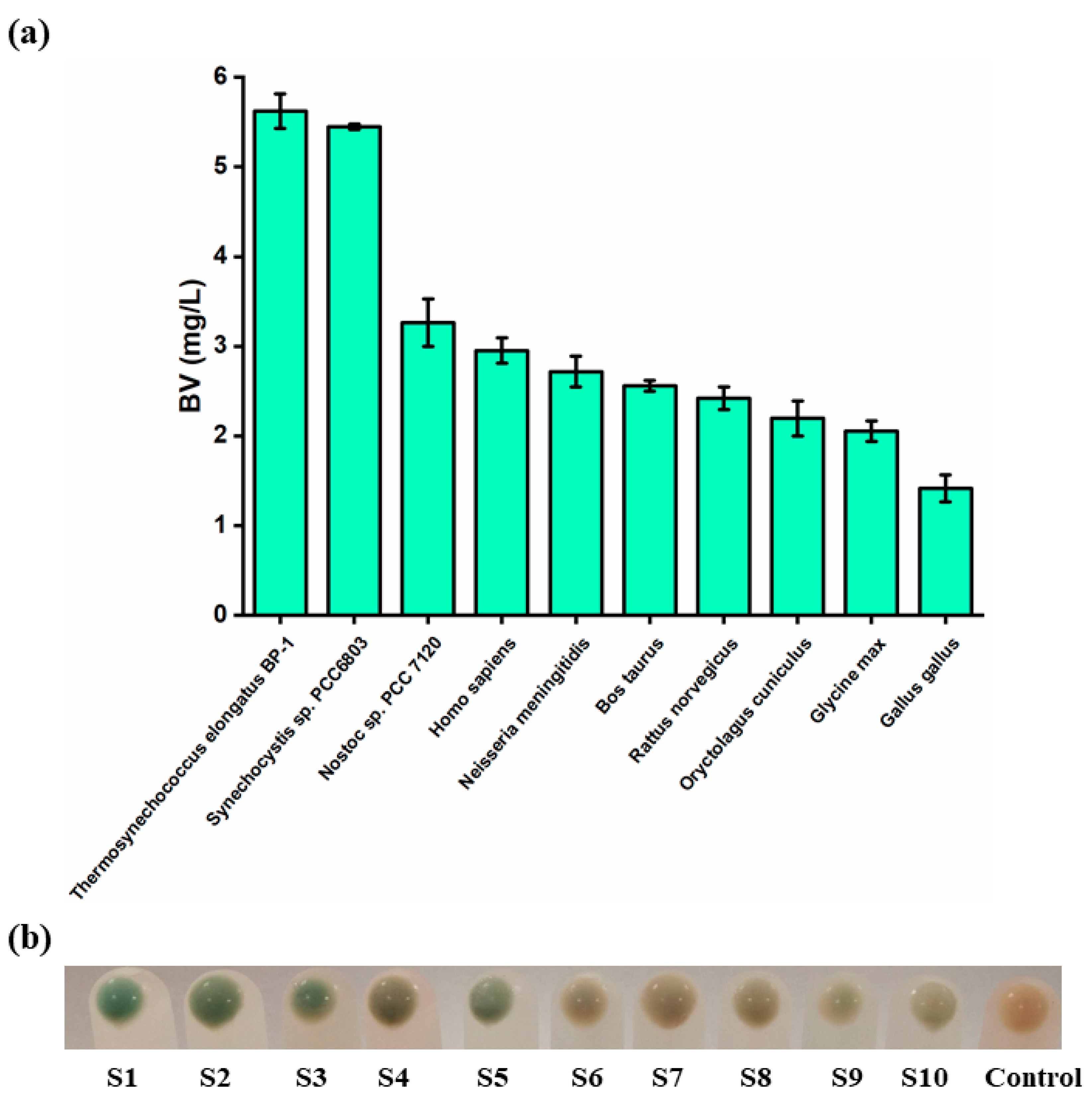
3.1. Screening of the Optimal HO Source for the Synthesis of BV
3.2. Development of a High-Throughput Assay for the Detection of BV
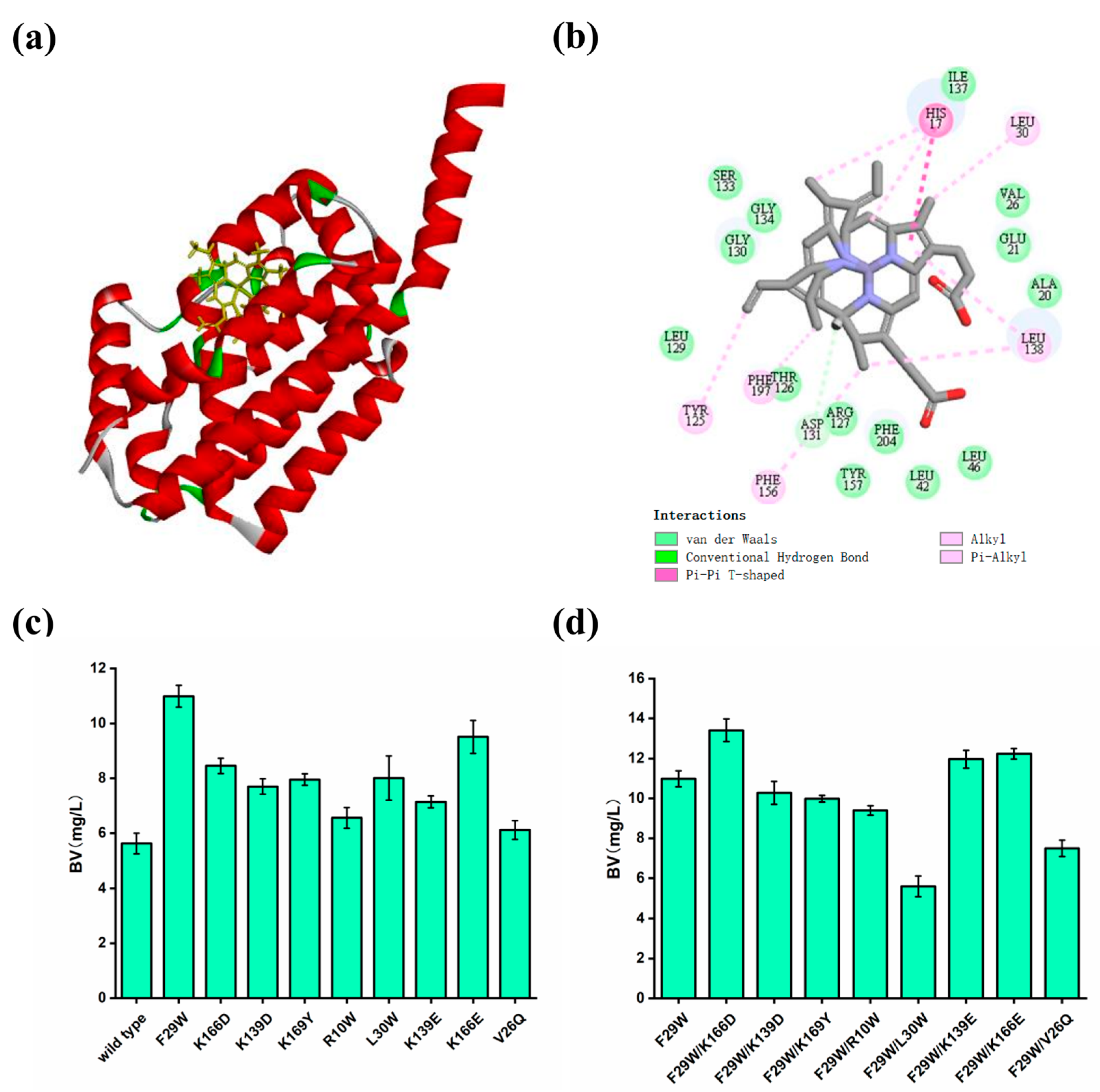
3.3. Improving the Catalytic Activity of HO by Rational Design
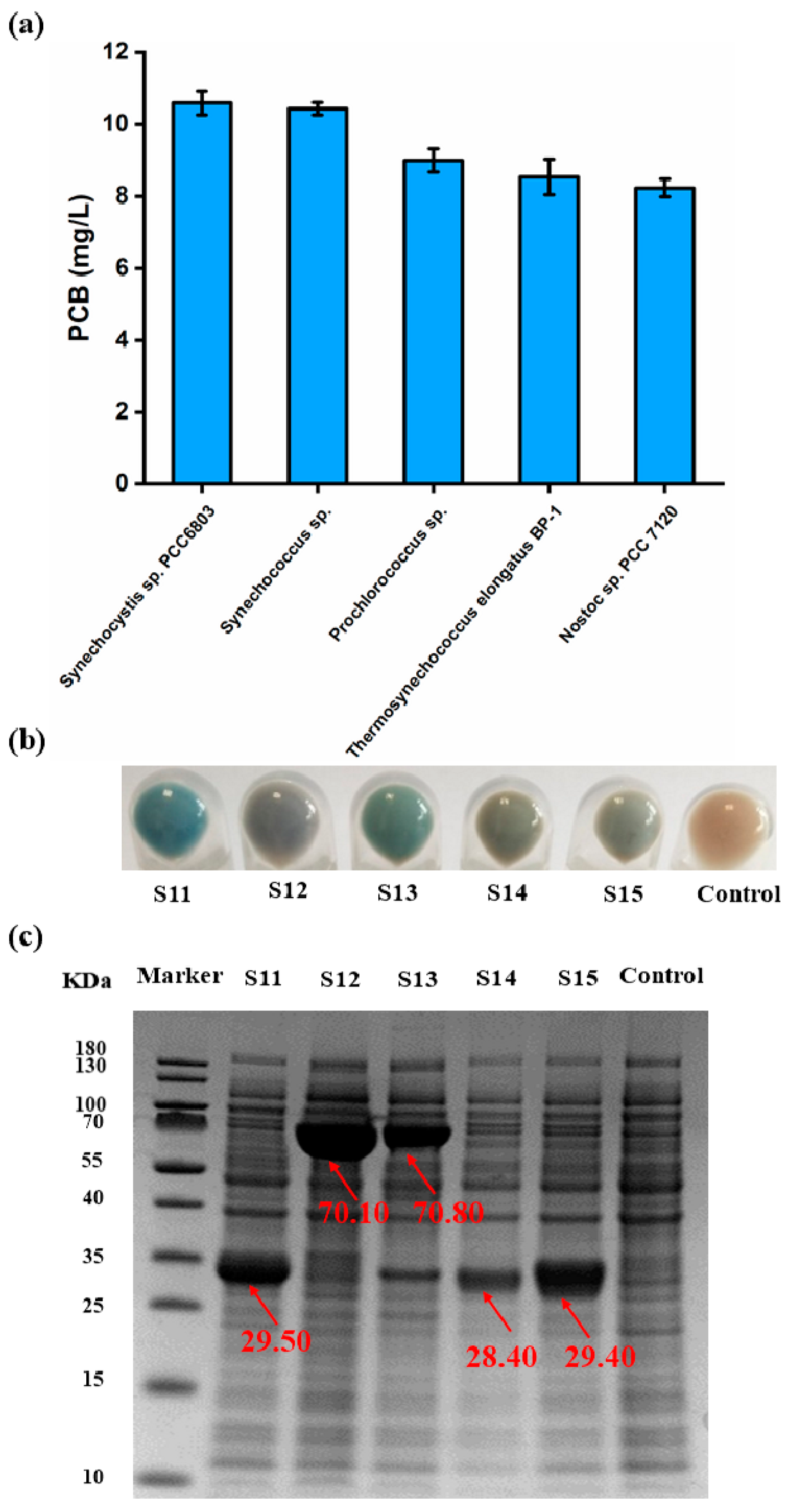
3.4. Screening of the Ideal PcyA Source for the Synthesis of PCB
3.5. Establishment of a High-Throughput Assay for the Detection of PCB
3.6. Enhancing the Catalytic Activity of PcyA by Rational Design
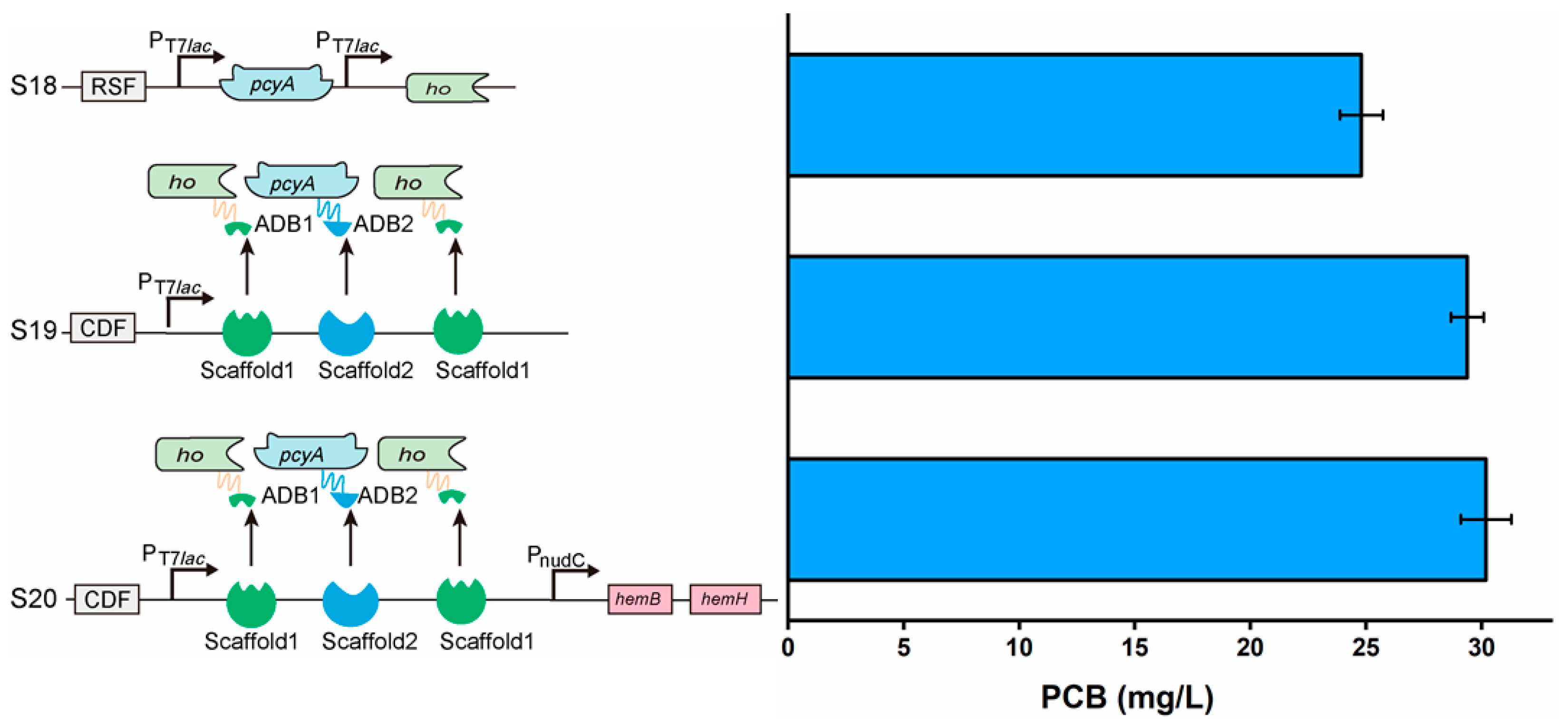
3.7. Assembling of Key Enzymes and Enhancement in Heme Supply to Improve PCB Synthesis
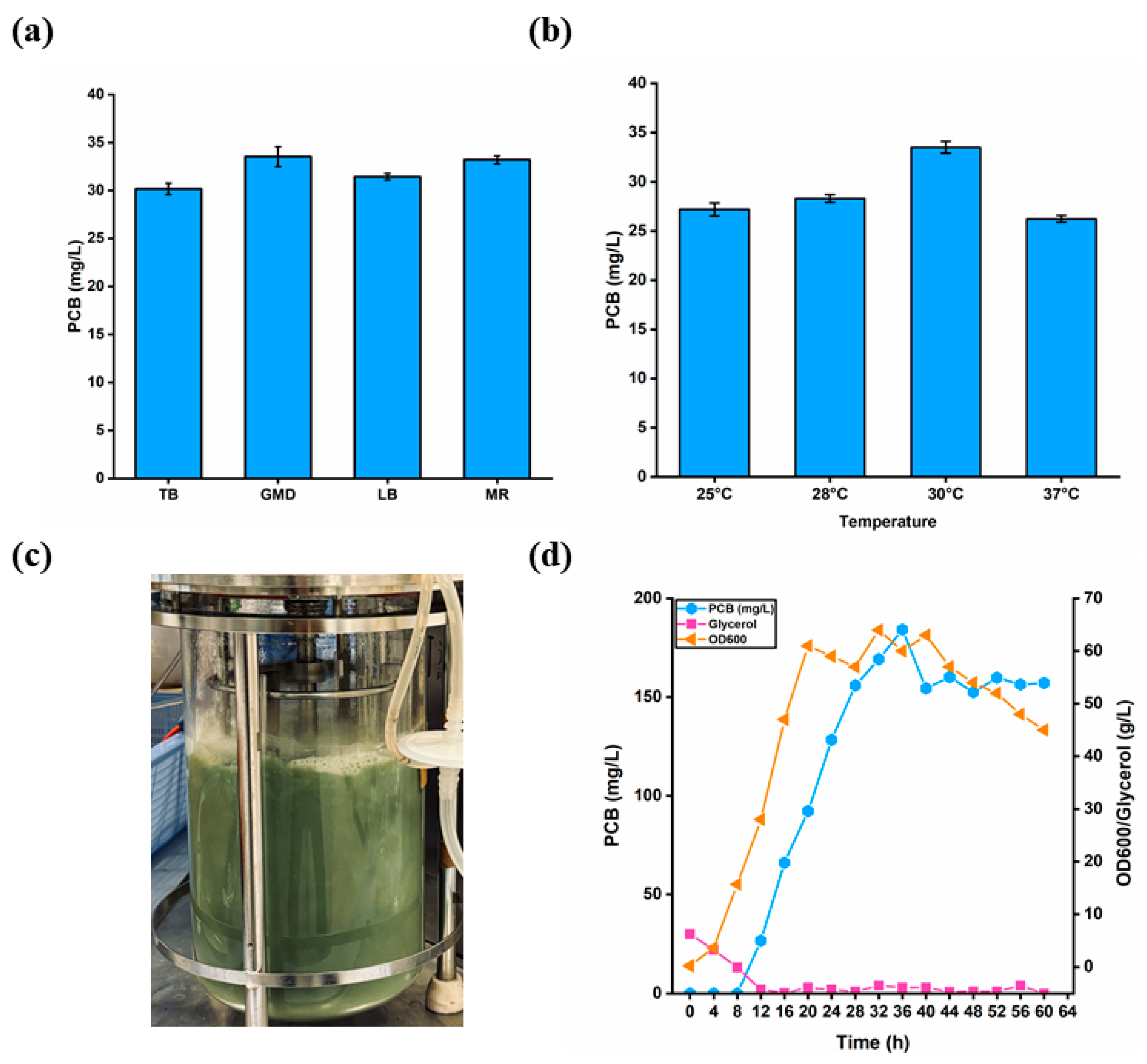
3.8. Scale-Up of Fed-Batch Fermentation in 5 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, D.; Ding, W.L.; Zhao, B.Q.; Lu, L.; Xu, Q.Z.; Scheer, H.; Zhao, K.H. Adapting Photosynthesis to the Near-Infrared: Non-Covalent Binding of Phycocyanobilin Provides an Extreme Spectral Red-Shift to Phycobilisome Core-Membrane Linker from Synechococcus sp. PCC7335. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 688–694. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health Applications of Bioactive Compounds from Marine Microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant Activities of Phycocyanobilin Prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant Activity and Associated Structural Attributes of Halomicronema Phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and Phycocyanobilin from Spirulina platensis Protect against Diabetic Nephropathy by Inhibiting Oxidative Stress. Am. J. Physiol. Integr. Comp. Physiol. 2012, 304, R110–R120. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Piniella-Matamoros, B.; Marín-Prida, J.; Pentón-Rol, G. Nutraceutical and Therapeutic Potential of Phycocyanobilin for Treating Alzheimer’s Disease. J. Biosci. 2021, 46, 42. [Google Scholar] [CrossRef]
- Ge, B.; Chen, Y.; Yu, Q.; Lin, X.; Li, J.; Qin, S. Regulation of the Heme Biosynthetic Pathway for Combinational Biosynthesis of Phycocyanobilin in Escherichia coli. Process Biochem. 2018, 71, 23–30. [Google Scholar] [CrossRef]
- O’Carra, P.; Murphy, R.F.; Killilea, S.D. The Native Forms of the Phycobilin Chromophores of Algal Biliproteins. A Clarification. Biochem. J. 1980, 187, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Kamiya, A.; Hammam, M.A.S.; Kinoshita, H.; Fujinami, S.; Ukaji, Y.; Inomata, K. Total Syntheses of Sterically Locked Phycocyanobilin Derivatives Bearing a 15Z-Antior a 15E-Anti CD-Ring Component. Bull. Chem. Soc. Jpn. 2010, 83, 1309–1322. [Google Scholar] [CrossRef]
- Kang, Z.; Ding, W.; Gong, X.; Liu, Q.; Du, G.; Chen, J. Recent Advances in Production of 5-Aminolevulinic Acid Using Biological Strategies. World J. Microbiol. Biotechnol. 2017, 33, 200. [Google Scholar] [CrossRef] [PubMed]
- Layer, G.; Reichelt, J.; Jahn, D.; Heinz, D.W. Structure and Function of Enzymes in Heme Biosynthesis. Protein Sci. 2010, 19, 1137–1161. [Google Scholar] [CrossRef] [PubMed]
- Okada, K. HO1 and PcyA Proteins Involved in Phycobilin Biosynthesis Form a 1:2 Complex with Ferredoxin-1 Required for Photosynthesis. FEBS Lett. 2009, 583, 1251–1256. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Sugishima, M.; Khawn, H.; Kinoshita, H.; Inomata, K.; Shang, L.; Lagarias, J.C.; Takahashi, Y.; Fukuyama, K. Structural Insights into Vinyl Reduction Regiospecificity of Phycocyanobilin:Ferredoxin Oxidoreductase (PcyA). J. Biol. Chem. 2010, 285, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic Engineering of Escherichia coli for Natural Product Biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Lagarias, J.C. Genetic Engineering of Phytochrome Biosynthesis in Bacteria. Proc. Natl. Acad. Sci. USA 2001, 98, 10566–10571. [Google Scholar] [CrossRef]
- Castillo-Hair, S.M.; Baerman, E.A.; Fujita, M.; Igoshin, O.A.; Tabor, J.J. Optogenetic Control of Bacillus subtilis Gene Expression. Nat. Commun. 2019, 10, 3099. [Google Scholar] [CrossRef]
- Ma, C.; Li, W.; Ge, B.; Lin, J.; Qin, S. Biosynthesis of Phycocyanobilin in Recombinant Escherichia coli. J. Oceanol. Limnol. 2020, 38, 529–538. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, H.; Wang, Y.; Wang, Z.; Zhou, J. Efficient Synthesis of Phycocyanobilin by Combinatorial Metabolic Engineering in Escherichia coli. ACS Synth. Biol. 2022, 11, 2089–2097. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Shan, X.; Zhao, X.; Sun, Y.; Zhou, J. Enhancement of Phycocyanobilin Biosynthesis in Escherichia coli by Strengthening the Supply of Precursor and Artificially Self-Assembly Complex. Synth. Syst. Biotechnol. 2023, 8, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.A.; Tran, G.N.; Gross, L.A.; Crisp, J.L.; Shu, X.; Lin, J.Y.; Tsien, R.Y. A Far-Red Fluorescent Protein Evolved from a Cyanobacterial Phycobiliprotein. Nat. Methods 2016, 13, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lan, D.; Shao, A.; Li, Y.; Zhang, X. Red Fluorescent Protein from Cyanobacteriochrome Chromophorylated with Phycocyanobilin and Biliverdin. Anal. Biochem. 2022, 642, 114557. [Google Scholar] [CrossRef] [PubMed]
- Porebski, B.T.; Buckle, A.M. Consensus Protein Design. Protein Eng. Des. Sel. 2016, 29, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sugishima, M.; Migita, C.T.; Zhang, X.; Yoshida, T.; Fukuyama, K. Crystal Structure of Heme Oxygenase-1 from Cyanobacterium Synechocystis sp. PCC 6803 in Complex with Heme. Eur. J. Biochem. 2004, 271, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Brooks III, C.L. Covalent Docking in CDOCKER. J. Comput. Aided. Mol. Des. 2022, 36, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhao, X.; Zhou, J.; Li, J.; Chen, J.; Du, G. Efficient Hydroxylation of Flavonoids by Using Whole-Cell P450 Sca-2 Biocatalyst in Escherichia coli. Front. Bioeng. Biotechnol. 2023, 11, 1138376. [Google Scholar] [CrossRef]
- Wu, G.; Robertson, D.H.; Brooks III, C.L.; Vieth, M. Detailed Analysis of Grid-Based Molecular Docking: A Case Study of CDOCKER—A CHARMm-Based MD Docking Algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, P. Metabolic Engineering of Escherichia coli for Efficient Biosynthesis of Fluorescent Phycobiliprotein. Microb. Cell Fact. 2019, 18, 58. [Google Scholar] [CrossRef]
- Filonov, G.S.; Piatkevich, K.D.; Ting, L.-M.; Zhang, J.; Kim, K.; Verkhusha, V. V Bright and Stable Near-Infrared Fluorescent Protein for in Vivo Imaging. Nat. Biotechnol. 2011, 29, 757–761. [Google Scholar] [CrossRef]
- Tu, S.L.; Sughrue, W.; Britt, R.D.; Lagarias, J.C. A Conserved Histidine-Aspartate Pair Is Required for Exovinyl Reduction of Biliverdin by a Cyanobacterial Phycocyanobilin: Ferredoxin Oxidoreductase. J. Biol. Chem. 2006, 281, 3127–3136. [Google Scholar] [CrossRef]
- Ma, Q.; Hua, H.H.; Chen, Y.; Liu, B.B.; Krämer, A.L.; Scheer, H.; Zhao, K.H.; Zhou, M. A Rising Tide of Blue-Absorbing Biliprotein Photoreceptors- Characterization of Seven Such Bilin-Binding GAF Domains in Nostoc sp. PCC7120. FEBS J. 2012, 279, 4095–4108. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Sugishima, M.; Takahashi, Y.; Fukuyama, K. Crystal Structure of Phycocyanobilin:Ferredoxin Oxidoreductase in Complex with Biliverdin IXα, a Key Enzyme in the Biosynthesis of Phycocyanobilin. Proc. Natl. Acad. Sci. USA 2006, 103, 27–32. [Google Scholar] [CrossRef]
- Xu, X.; Tian, L.; Tang, S.; Xie, C.; Xu, J.; Jiang, L. Design and Tailoring of an Artificial DNA Scaffolding System for Efficient Lycopene Synthesis Using Zinc-Finger-Guided Assembly. J. Ind. Microbiol. Biotechnol. 2020, 47, 209–222. [Google Scholar] [CrossRef]
- Conrado, R.J.; Wu, G.C.; Boock, J.T.; Xu, H.; Chen, S.Y.; Lebar, T.; Turnšek, J.; Tomšič, N.; Avbelj, M.; Gaber, R.; et al. DNA-Guided Assembly of Biosynthetic Pathways Promotes Improved Catalytic Efficiency. Nucleic Acids Res. 2012, 40, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Ma, W.; Shin, H.; Li, J.; Liu, L.; Du, G.; Chen, J. Spatial Modulation of Key Pathway Enzymes by DNA-Guided Scaffold System and Respiration Chain Engineering for Improved N-Acetylglucosamine Production by Bacillus subtilis. Metab. Eng. 2014, 24, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Choi, K.R.; Lee, S.Y. Metabolic Engineering of Escherichia coli for Secretory Production of Free Haem. Nat. Catal. 2018, 1, 720–728. [Google Scholar] [CrossRef]
- Wilks, A.; Burkhard, K.A. Heme and Virulence: How Bacterial Pathogens Regulate, Transport and Utilize Heme. Nat. Prod. Rep. 2007, 24, 511–522. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, J.; Li, J.; Du, G.; Chen, J.; Zhao, X. Rational Design of Key Enzymes to Efficiently Synthesize Phycocyanobilin in Escherichia coli. Biomolecules 2024, 14, 301. https://doi.org/10.3390/biom14030301
Wang Z, Zhou J, Li J, Du G, Chen J, Zhao X. Rational Design of Key Enzymes to Efficiently Synthesize Phycocyanobilin in Escherichia coli. Biomolecules. 2024; 14(3):301. https://doi.org/10.3390/biom14030301
Chicago/Turabian StyleWang, Ziwei, Jingwen Zhou, Jianghua Li, Guocheng Du, Jian Chen, and Xinrui Zhao. 2024. "Rational Design of Key Enzymes to Efficiently Synthesize Phycocyanobilin in Escherichia coli" Biomolecules 14, no. 3: 301. https://doi.org/10.3390/biom14030301
APA StyleWang, Z., Zhou, J., Li, J., Du, G., Chen, J., & Zhao, X. (2024). Rational Design of Key Enzymes to Efficiently Synthesize Phycocyanobilin in Escherichia coli. Biomolecules, 14(3), 301. https://doi.org/10.3390/biom14030301





