Steroid Metabolomic Signature in Term and Preterm Infants
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Urine Collection Procedure
2.3. Laboratory Analyses
2.4. Statistical Analysis of Metabolomic Data
3. Results
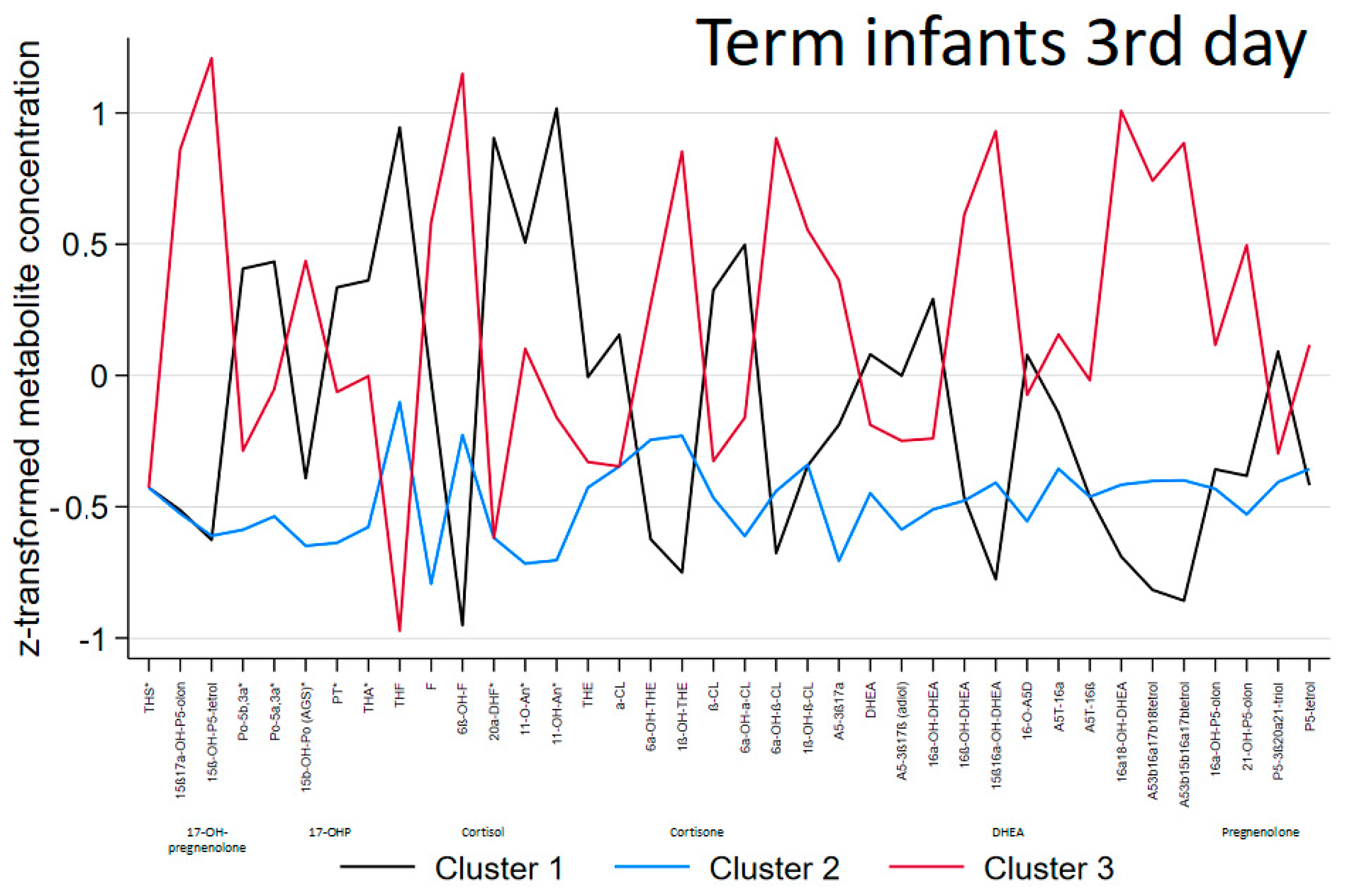
3.1. Term Infants
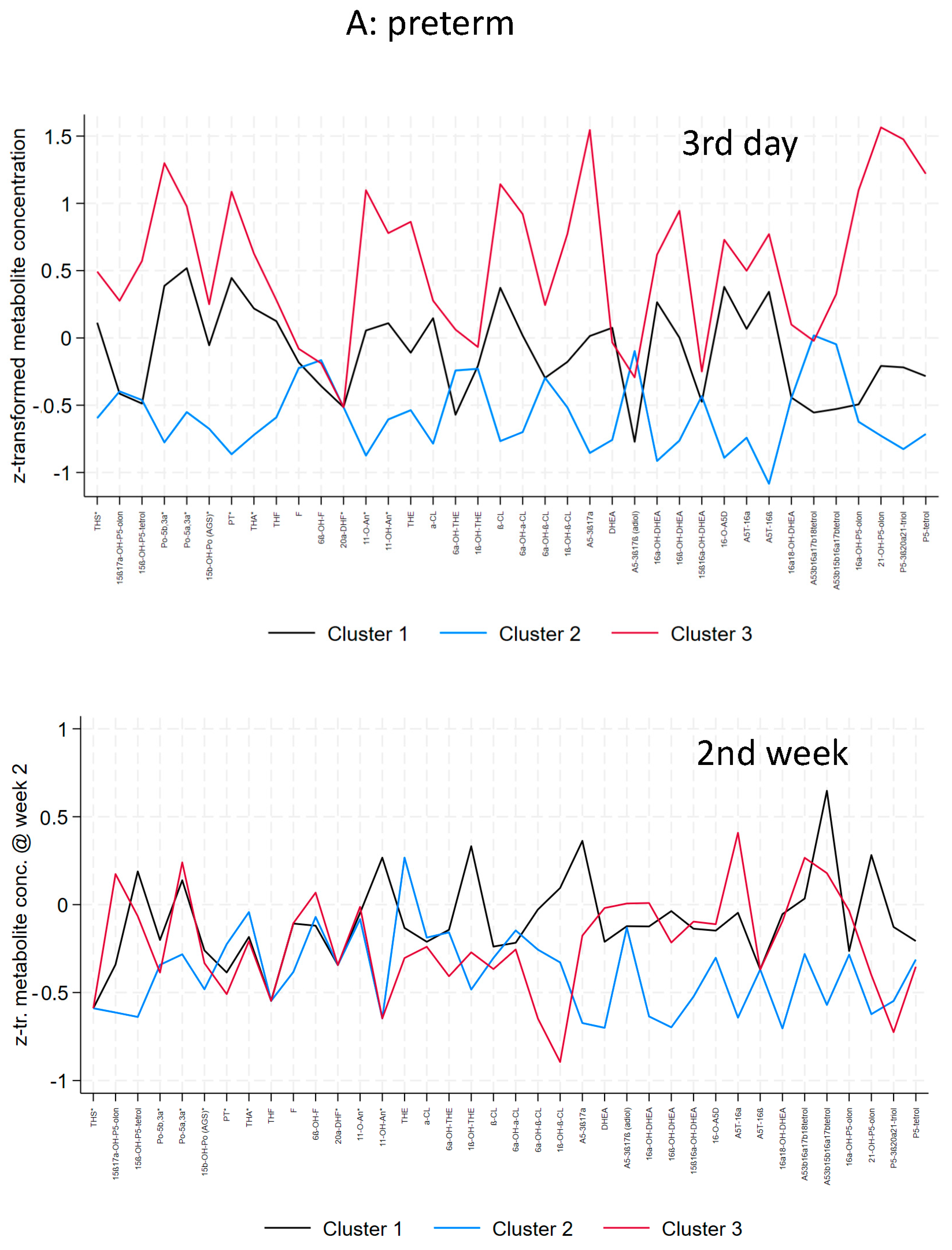
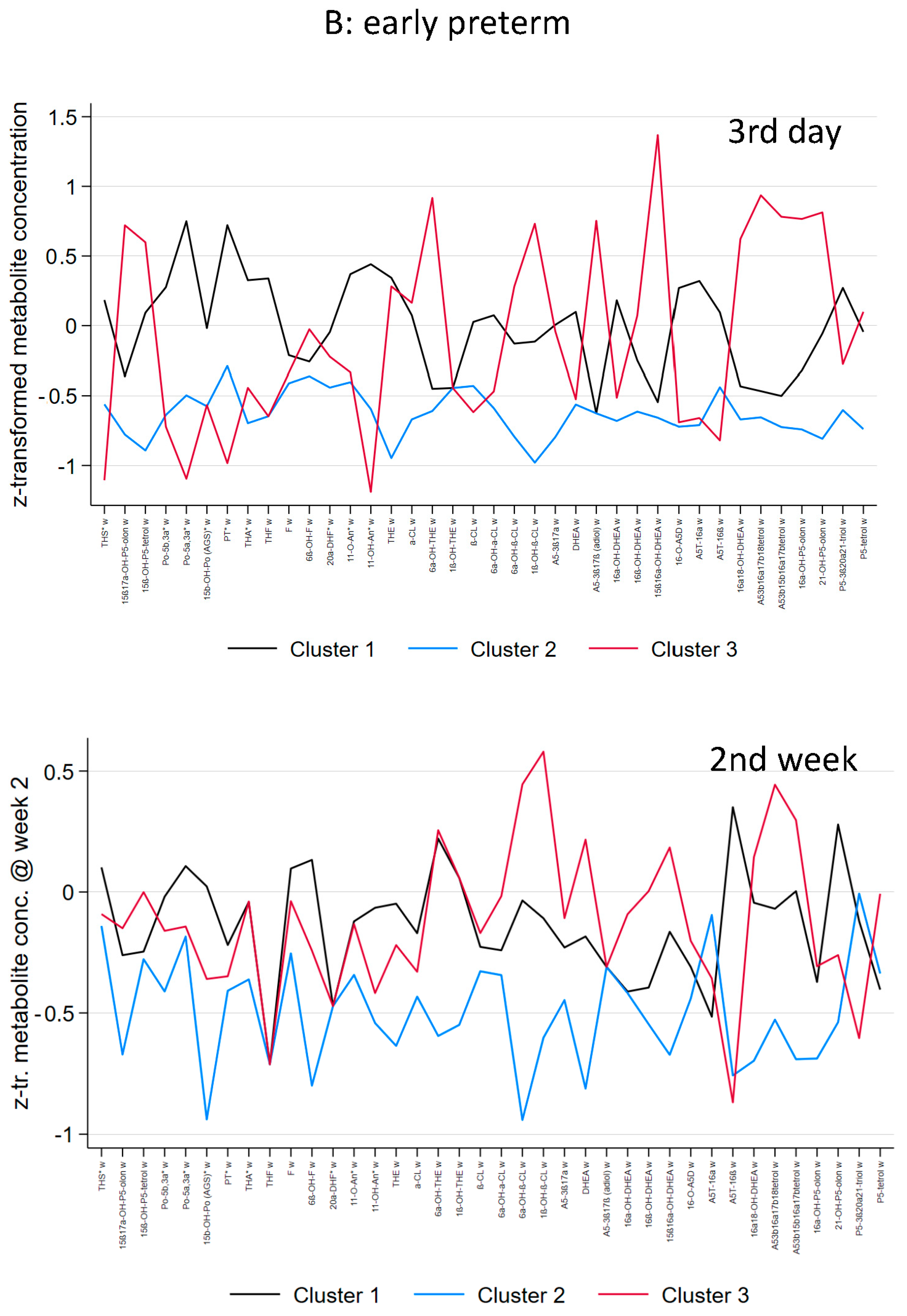
3.2. Preterm and Early Preterm Infants
3.3. Biomarker Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, E.; Biermann, A.; Gunster, C.; Bohler, T.; Heller, G.; Hummler, H.D.; Buhrer, C.; Routine Data-Based Quality Improvement Panel. Mortality and Major. Morbidity of Very-Low-Birth-Weight. Infants in Germany 2008–2012: A Report Based on Administrative Data. Front. Pediatr. 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Myrhaug, H.T.; Brurberg, K.G.; Hov, L.; Markestad, T. Survival and Impairment of Extremely Premature Infants: A Meta-analysis. Pediatrics 2019, 143, e20180933. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, H.; Jaffe, R.B. Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocr. Rev. 2011, 32, 317–355. [Google Scholar] [CrossRef] [PubMed]
- Fluck, C.E.; Kuiri-Hanninen, T.; Silvennoinen, S.; Sankilampi, U.; Groessl, M. The Androgen Metabolome of Preterm Infants Reflects Fetal Adrenal Gland Involution. J. Clin. Endocrinol. Metab. 2022, 107, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Hartmann, M.; Kampschulte, B.; Gack, H.; Boedeker, R.H.; Gortner, L.; Wudy, S.A. Persistent high activity of the fetal adrenal cortex in preterm infants: Is there a clinical significance? J. Pediatr. Endocrinol. Metab. 2006, 19, 1303–1312. [Google Scholar] [CrossRef]
- Midgley, P.C.; Russell, K.; Oates, N.; Holownia, P.; Shaw, J.C.; Honour, J.W. Adrenal function in preterm infants: ACTH may not be the sole regulator of the fetal zone. Pediatr. Res. 1998, 44, 887–893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Banna, N.; Pavlovic, D.; Sharawi, N.; Bac, V.H.; Jaskulski, M.; Balzer, C.; Weber, S.; Nedeljkov, V.; Lehmann, C. Combination of dehydroepiandrosterone and orthovanadate administration reduces intestinal leukocyte recruitment in models of experimental sepsis. Microvasc. Res. 2014, 95, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Dumas de la Roque, E.; Quignard, J.F.; Ducret, T.; Dahan, D.; Courtois, A.; Begueret, H.; Marthan, R.; Savineau, J.P. Beneficial effect of dehydroepiandrosterone on pulmonary hypertension in a rodent model of pulmonary hypertension in infants. Pediatr. Res. 2013, 74, 163–169. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Cui, S.; Zhang, Z.; Zhou, R.; Ge, Y.; Sokabe, M.; Chen, L. DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2009, 29, 287–296. [Google Scholar] [CrossRef]
- Suzuki, M.; Wright, L.S.; Marwah, P.; Lardy, H.A.; Svendsen, C.N. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 3202–3207. [Google Scholar] [CrossRef]
- Hubner, S.; Reich, B.; Heckmann, M. Role of sex steroids and their receptors in human preterm infants: Impacts on future treatment strategies for cerebral development. Biochem. Pharmacol. 2015, 98, 556–563. [Google Scholar] [CrossRef]
- Sunny, D.E.; Hammer, E.; Ittermann, T.; Kruger, E.L.; Hubner, S.; Hartmann, M.F.; Wudy, S.A.; Volker, U.; Heckmann, M. Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage. Int J Mol Sci 2021, 12, 6586. [Google Scholar] [CrossRef]
- Fernandez, E.F.; Watterberg, K.L. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 2009, 29 (Suppl. 2), S44–S49. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Teblick, A.; Langouche, L.; Gunst, J. The hypothalamus-pituitary-adrenal axis in sepsis- and hyperinflammation-induced critical illness: Gaps in current knowledge and future translational research directions. EBioMedicine 2022, 84, 104284. [Google Scholar] [CrossRef]
- Boonen, E.; Vervenne, H.; Meersseman, P.; Andrew, R.; Mortier, L.; Declercq, P.E.; Vanwijngaerden, Y.M.; Spriet, I.; Wouters, P.J.; Vander Perre, S.; et al. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 2013, 368, 1477–1488. [Google Scholar] [CrossRef]
- Chan, W.L.; Carrell, R.W.; Zhou, A.; Read, R.J. How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J. Clin. Endocrinol. Metab. 2013, 98, 3315–3322. [Google Scholar] [CrossRef]
- Poomthavorn, P.; Lertbunrian, R.; Preutthipan, A.; Sriphrapradang, A.; Khlairit, P.; Mahachoklertwattana, P. Serum free cortisol index, free cortisol, and total cortisol in critically ill children. Intensive Care Med. 2009, 35, 1281–1285. [Google Scholar] [CrossRef]
- Teblick, A.; Gunst, J.; Van den Berghe, G. Critical Illness-induced Corticosteroid Insufficiency: What It Is Not and What It Could Be. J. Clin. Endocrinol. Metab. 2022, 107, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Hartmann, M.; Kampschulte, B.; Gack, H.; Bödeker, R.-H.; Gortner, L.; Wudy, S.A. Cortisol production rates in preterm infants in relation to growth and illness: A non invasive prospective study using GC-MS. J. Clin. Endocrinol. Metab. 2005, 90, 5737–5742. [Google Scholar] [CrossRef] [PubMed]
- Grofer, B.; Bodeker, R.H.; Gortner, L.; Heckmann, M. Maturation of adrenal function determined by urinary glucocorticoid steroid excretion rates in preterm infants of more than 30 weeks of gestational age. Neonatology 2010, 98, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ruhnau, J.; Hubner, S.; Sunny, D.; Ittermann, T.; Hartmann, M.F.; De Lafollie, J.; Wudy, S.A.; Heckmann, M. Impact of Gestational and Postmenstrual Age on Excretion of Fetal Zone Steroids in Preterm Infants Determined by Gas Chromatography-Mass Spectrometry. J. Clin. Endocrinol. Metab. 2021, 106, e3725–e3738. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Richardson, D.K.; Gray, J.E.; McCormick, M.C.; Workman, K.; Goldmann, D.A. Score for Neonatal Acute Physiology: A physiologic severity index for neonatal intensive care. Pediatrics 1993, 91, 617–623. [Google Scholar] [CrossRef]
- Gawlik, A.; Shmoish, M.; Hartmann, M.F.; Malecka-Tendera, E.; Wudy, S.A.; Hochberg, Z. Steroid Metabolomic Disease Signature of Nonsyndromic Childhood Obesity. J. Clin. Endocrinol. Metab. 2016, 101, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Aucott, S.W.; Watterberg, K.L.; Shaffer, M.L.; Donohue, P.K.; PROPHET Study Group. Early cortisol values and long-term outcomes in extremely low birth weight infants. J. Perinatol. 2010, 30, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Wong, S.P.; Chan, I.H.; Lam, H.S.; Lee, C.H.; Lam, C.W. A prospective longitudinal study to estimate the “adjusted cortisol percentile” in preterm infants. Pediatr. Res. 2011, 69, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Renolleau, C.; Toumazi, A.; Bourmaud, A.; Benoist, J.F.; Chevenne, D.; Mohamed, D.; Alberti, C.; Biran, V.; Baud, O.; PREMILOC Trial Study Group. Association between Baseline Cortisol Serum Concentrations and the Effect of Prophylactic Hydrocortisone in Extremely Preterm Infants. J. Pediatr. 2021, 234, 65–70.e63. [Google Scholar] [CrossRef] [PubMed]
- Diczfalusy, E. Endocrine Functions of the Human Fetoplacental Unit. Fed. Proc. 1964, 23, 791–798. [Google Scholar] [CrossRef]
- Siiteri, P.K.; MacDonald, P.C. Placental estrogen biosynthesis during human pregnancy. J. Clin. Endocrinol. Metab. 1966, 26, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; Thijs, L.G.; Vermes, I. Decreased levels of dehydroepiandrosterone sulphate in severe critical illness: A sign of exhausted adrenal reserve? Crit. Care 2002, 6, 434–438. [Google Scholar] [CrossRef]
- Arlt, W.; Hammer, F.; Sanning, P.; Butcher, S.K.; Lord, J.M.; Allolio, B.; Annane, D.; Stewart, P.M. Dissociation of serum dehydroepiandrosterone and dehydroepiandrosterone sulfate in septic shock. J. Clin. Endocrinol. Metab. 2006, 91, 2548–2554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foster, M.A.; Taylor, A.E.; Hill, N.E.; Bentley, C.; Bishop, J.; Gilligan, L.C.; Shaheen, F.; Bion, J.F.; Fallowfield, J.L.; Woods, D.R.; et al. Mapping the Steroid Response to Major Trauma From Injury to Recovery: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2020, 105, 925–937. [Google Scholar] [CrossRef] [PubMed]
- van den Berghe, G.; Weekers, F.; Baxter, R.C.; Wouters, P.; Iranmanesh, A.; Bouillon, R.; Veldhuis, J.D. Five-day pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic-pituitary-gonadal defects underlying profound hypoandrogenism in men with prolonged critical illness. J. Clin. Endocrinol. Metab. 2001, 86, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.; Hazeldine, J.; Greig, C.; Lord, J.; Foster, M. Dehydroepiandrosterone: A potential therapeutic agent in the treatment and rehabilitation of the traumatically injured patient. Burns Trauma. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Arlt, W.; Lord, J.M. Dehydroepiandrosterone as a regulator of immune cell function. J. Steroid Biochem. Mol. Biol. 2010, 120, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.; Potter, C.; Yakoub, K.M.; Brock, K.; Homer, V.; Toman, E.; Taylor, A.E.; Shaheen, F.; Gilligan, L.C.; Athwal, A.; et al. A prospective, phase II, single-centre, cross-sectional, randomised study investigating Dehydroepiandrosterone supplementation and its Profile in Trauma: ADaPT. BMJ Open 2021, 11, e040823. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Yang, Z.; Roy, M.; Guo, Q. The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis. Burns J. Int. Soc. Burn. Inj. 2016, 42, 717–727. [Google Scholar] [CrossRef]
- Mesiano, S.; Jaffe, R.B. Role of growth factors in the developmental regulation of the human fetal adrenal cortex. Steroids 1997, 62, 62–72. [Google Scholar] [CrossRef]
- Aucott, S.W.; Watterberg, K.L.; Shaffer, M.L.; Donohue, P.K. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 2008, 122, 775–781. [Google Scholar] [CrossRef]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchee, A.; Bolot, P.; et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E.; et al. Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2019, 321, 354–363. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Walsh, M.C.; Li, L.; Chawla, S.; D’Angio, C.T.; Goldberg, R.N.; Hintz, S.R.; Laughon, M.M.; Yoder, B.A.; Kennedy, K.A.; et al. Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia. N. Engl. J. Med. 2022, 386, 1121–1131. [Google Scholar] [CrossRef]
- Prete, A.; Taylor, A.E.; Bancos, I.; Smith, D.J.; Foster, M.A.; Kohler, S.; Fazal-Sanderson, V.; Komninos, J.; O’Neil, D.M.; Vassiliadi, D.A.; et al. Prevention of Adrenal Crisis: Cortisol Responses to Major Stress Compared to Stress Dose Hydrocortisone Delivery. J. Clin. Endocrinol. Metab. 2020, 105, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Rezai, M.; Fullwood, C.; Hird, B.; Chawla, M.; Tetlow, L.; Banerjee, I.; Patel, L. Cortisol Levels During Acute Illnesses in Children and Adolescents: A Systematic Review. JAMA Netw. Open 2022, 5, e2217812. [Google Scholar] [CrossRef] [PubMed]
- Midgley, P.C.; Russell, K.; Oates, N.; Shaw, J.C.; Honour, J.W. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr Res 1996, 22, 729–733. [Google Scholar] [CrossRef] [PubMed]
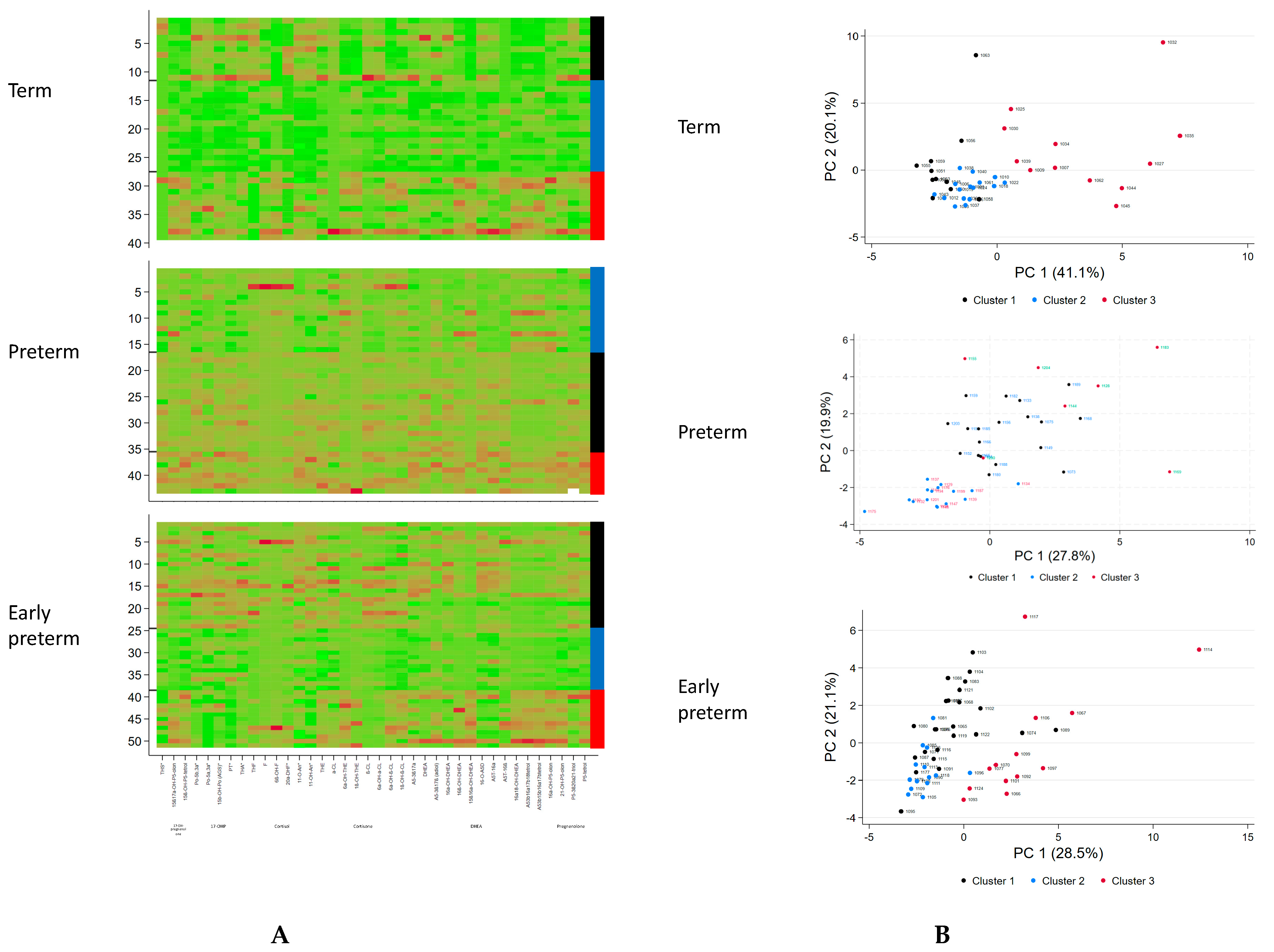
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| n | |||
| Distinctive metabolic characteristics | Mild elevation of C21-steroids (glucocorticoid precursors, cortisol metabolites and less cortisone metabolites) and C19-steroids (DHEA-metabolites) | No specific abnormality, lowest excretion in general | Highest elevation of C21-steroids (glucocorticoids: both cortisol and cortisone metabolites), 17-OH-pregnenolone metabolites and C19-steroids (DHEA-metabolites) |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Group (n) | Well (0) | Ill (11) | Well (13) | Ill (3) | Well (9) | Ill (3) | |
| GA | 39 (38–41) | 40 (37–42) | 41 (40–41) | 39 (37–41) | 39 (38–39) | 0.08 | |
| BW | 3050 (1860–3750) | 3260 (2710–4280) | 3620 (3120–3700) | 3410 (2910–3930) | 3310 (2710–3650) | 0.25 | |
| Sex (female) | 4 (11) | 8/13 | 2/3 | 6/9 | 1/3 | ||
| SGA | 5/11 | 2/13 | 0 | 0 | 1/2 | 0.05 | |
| Apgar 5 min | 10 (8–10) | 10 (9–10) | 10 (8–10) | 10 (7–10) | 8 (8–10) | 0.76 | |
| C-section | 2/11 | 7/13 | 0 | 6/9 | 1/3 | 0.14 | |
| SNAP | 2 (0–19) | 0 | 2 (0–7) | 0 | 5 (1–6) | 0.002C1>2 | |
| Infection | 8/11 | 0 | 1/3 | 0 | 2/3 | 0.0005C1>2 | |
| RDS | 7/11 | 0 | 0 | 0 | 2/3 | 0.0005C1>2 | |
| CHD | 1/11 | 0 | 1/3 | 0 | 1/3 | 0.96 | |
| Hypo-glycemia | 1/11 | 0 | 1/3 | 0 | 1/3 | 0.96 | |
| Cluster 1 | Cluster 2 | Cluster 3 | p | |
|---|---|---|---|---|
| n | 11 (28.2%) | 16 (41.0%) | 12 (30.8%) | |
| THS | 0.397 (1.213) | −0.206 (0.612) | −0.088 (1.174) | 0.293 |
| 15ß,17OH-P5o | −0.440 (0.362) | −0.502 (0.284) | 1.073 (1.186) | <0.001 |
| P5-tetrol-15ß | −0.414 (0.655) | −0.557 (0.338) | 1.122 (0.955) | <0.001 |
| 17OHPo | 0.889 (1.356) | −0.561 (0.191) | −0.067 (0.693) | <0.001 |
| 17OHPo-5α | 0.538 (0.580) | −0.565 (0.432) | 0.259 (1.438) | 0.007 |
| 15ß,17OHPo | 0.220 (1.370) | −0.604 (0.331) | 0.603 (0.790) | 0.003 |
| PT | 0.866 (1.403) | −0.630 (0.155) | 0.046 (0.551) | <0.001 |
| THA | 0.800 (1.335) | −0.634 (0.285) | 0.112 (0.697) | <0.001 |
| THF | 1.100 (0.835) | −0.276 (0.535) | −0.641 (0.814) | <0.001 |
| F | 0.153 (0.946) | −0.588 (0.520) | 0.644 (1.136) | 0.002 |
| 6ß-OH-F | −0.847 (0.246) | −0.191 (0.481) | 1.031 (1.074) | <0.001 |
| 20α-DHF | 0.624 (1.257) | −0.442 (0.486) | 0.018 (1.011) | 0.020 |
| 11-O-An | 0.658 (0.873) | −0.674 (0.394) | 0.295 (1.167) | <0.001 |
| 11-OH-An | 0.938 (1.003) | −0.677 (0.267) | 0.043 (0.946) | <0.001 |
| THE | 0.508 (1.352) | −0.472 (0.187) | 0.164 (1.065) | 0.030 |
| α-Cl | 0.315 (0.431) | −0.332 (0.039) | 0.154 (1.732) | 0.212 |
| 6α-OH-THE | −0.495 (0.470) | −0.266 (0.281) | 0.809 (1.443) | 0.001 |
| 1ß-OH-THE | −0.740 (0.504) | −0.190 (0.442) | 0.932 (1.187) | <0.001 |
| ß-Cl | 0.828 (1.546) | −0.458 (0.130) | −0.149 (0.495) | 0.002 |
| 6α-OH-α-Cl | 0.600 (1.130) | −0.547 (0.502) | 0.179 (1.058) | 0.007 |
| 6α-OH-ß-Cl | −0.551 (0.579) | −0.368 (0.557) | 0.996 (1.069) | <0.001 |
| 1ß-OH-ß-Cl | −0.140 (0.812) | −0.383 (0.525) | 0.639 (1.342) | 0.020 |
| A5-3ß,17α | 0.336 (1.349) | −0.575 (0.345) | 0.459 (0.902) | 0.007 |
| DHEA | 0.534 (1.597) | −0.390 (0.273) | 0.030 (0.725) | 0.057 |
| Adiol | 0.415 (1.217) | −0.266 (0.836) | −0.026 (0.938) | 0.223 |
| 16α-OH-DHEA | 0.431 (1.173) | −0.429 (0.264) | 0.176 (1.273) | 0.065 |
| 16ß-OH-DHEA | −0.377 (0.303) | −0.453 (0.463) | 0.950 (1.286) | <0.001 |
| 15ß,16α -OH-DHEA | −0.781 (0.118) | −0.322 (0.209) | 1.146 (1.090) | <0.001 |
| 16-O-A5D | 0.314 (0.857) | −0.502 (0.373) | 0.382 (1.404) | 0.028 |
| A5T-16α | −0.012 (0.510) | −0.359 (0.415) | 0.490 (1.591) | 0.081 |
| A5T-16ß | −0.462 (0.000) | −0.189 (0.637) | 0.676 (1.462) | 0.011 |
| 16α,18-OH-DHEA | −0.639 (0.205) | −0.397 (0.208) | 1.114 (1.168) | <0.001 |
| A5-3ß16α17ß18-tetrol | −0.672 (0.345) | −0.274 (0.399) | 0.981 (1.232) | <0.001 |
| A5-3ß15ß16α17ß-tetrol | −0.729 (0.368) | −0.269 (0.464) | 1.026 (1.119) | <0.001 |
| 16OHP5o | −0.336 (0.262) | −0.234 (0.439) | 0.620 (1.587) | 0.030 |
| 21OHP5o | 0.001 (0.874) | −0.521 (0.270) | 0.694 (1.320) | 0.004 |
| P5-3ß20α21-triol | 0.506 (1.282) | −0.438 (0.287) | 0.121 (1.138) | 0.044 |
| P5-tetrol | −0.057 (1.025) | −0.375 (0.474) | 0.551 (1.286) | 0.047 |
| 11ß-HSD * | −0.136 (0.752) | −0.017 (1.232) | 0.147 (0.910) | 0.800 |
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| n | |||
| Distinctive metabolic characteristics (day 3) | General mild elevation of C21-and C19-steroids (no specific pattern) | No specific abnormality, lowest excretion in general | Highest elevation of C21-steroids (17-OH-progesterone, cortisone metabolites), 17-OH-pregnenolone metabolites and C19-steroids (DHEA-metabolites) |
| Distinctive metabolic characteristics (week 2) | General mild elevation of C21-and C19-steroids (no specific pattern) | No specific abnormality, lowest excretion in general | Mild elevation of 17-OH-progesterone metabolites, low cortisone metabolites, and mild elevation of C19-steroids but considerably lower than cluster 3 at day 3 |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Preterm | Well (n = 14) | Ill (n = 5) | Well (n = 10) | Ill (n = 6) | Well (n = 5) | Ill (n = 2) | |
| GA | 32 (30–35) | 32 (31–33) | 33 (32–36) | 34 (31–35) | 34 (32–35) | 32.4, 33.3 | 0.66 |
| BW | 1790 (1120–2600) | 1730 (1150–2250) | 2130 (1700–2690) | 1630 (1470–2630) | 1800 (1220–2455) | 2160, 2320 | 0.52 |
| Sex (female) | 3/14 | 2/5 | 3/10 | 1/6 | 3/5 | 1/2 | |
| SGA | 0/14 | 1/5 | 2/10 | 2/6 | 0/5 | 0/2 | 0.11 |
| Apgar 5 min | 8 (7–10) | 9 (2–10) | 9 (7–10) | 8 (4–9) | 9 (9–10) | 9,10 | 0.05 |
| C-section | 13/14 | 4/5 | 9/10 | 5/6 | 0/5 | 1/2 | 1.00 |
| pH | 7.35 (7.21–7.39) | 7.3 (7.27–7.37) | 7.3 (7.23–7.41) | 7.32 (7.23–7.62) | 7.3 (7.3–7.36) | 7.33, 7.36 | 0.88 |
| Prenatal Steroids | 5/14 | 2/5 | 6/10 | 1/6 | 1/5 | 0.94 | |
| SNAP D3 | 2 (0–7) | 9 (7–12) | 5 (2–7) | 6 (3–15) | 2 (0–5) | 6 | 0.92 |
| SNAP W2 | 2 (0–6) | 4 (1–6) | 1 (0–6) | 2 (1–7) | 1 (0–2) | 1,2 | 0.10 |
| RDS | 1/5 | 4/10 | 1/6 | 2/5 | 0.63 | ||
| Infection | 0/14 | 2/5 | 1/10 | 3/6 | 0/5 | 0.24 | |
| Cluster 1 | Cluster 2 | Cluster 3 | p | |
|---|---|---|---|---|
| n | 19 (45.2%) | 16 (38.1%) | 7 (16.7%) | |
| THS | 0.184 (0.710) | −0.576 (0.916) | 0.760 (1.302) | 0.005 |
| 15ß,17OH-P5o | −0.308 (0.397) | −0.030 (1.259) | 0.809 (1.192) | 0.038 |
| P5-tetrol-15ß | −0.225 (0.579) | −0.280 (0.865) | 1.268 (1.370) | <0.001 |
| 17OHPo | 0.444 (0.669) | −0.903 (0.381) | 1.023 (1.028) | <0.001 |
| 17OHPo-5α | 0.043 (0.928) | −0.396 (0.788) | 0.942 (1.100) | 0.009 |
| 15ß,17OHPo | 0.126 (0.656) | −0.579 (0.353) | 1.043 (1.776) | <0.001 |
| PT | 0.405 (0.546) | −0.865 (0.453) | 1.068 (1.206) | <0.001 |
| THA | 0.282 (0.912) | −0.732 (0.438) | 1.011 (1.031) | <0.001 |
| THF | −0.069 (0.446) | 0.043 (1.512) | 0.172 (0.756) | 0.861 |
| F | −0.177 (0.100) | 0.193 (1.629) | 0.067 (0.358) | 0.562 |
| 6ß-OH-F | −0.270 (0.143) | 0.360 (1.584) | −0.103 (0.271) | 0.180 |
| 20α DHF | −0.126 (0.548) | 0.146 (1.451) | 0.084 (0.823) | 0.724 |
| 11-O-An | 0.261 (0.616) | −0.864 (0.616) | 1.349 (0.728) | <0.001 |
| 11-OH-An | 0.168 (0.597) | −0.675 (0.493) | 1.184 (1.518) | <0.001 |
| THE | 0.033 (0.733) | −0.513 (0.673) | 1.079 (1.481) | 0.001 |
| α-Cl | 0.201 (0.654) | −0.478 (1.077) | 0.262 (1.156) | 0.071 |
| 6α-OH-THE | −0.437 (0.357) | 0.246 (1.242) | 0.344 (1.101) | 0.053 |
| 1ß-OH-THE | −0.209 (0.088) | −0.093 (0.352) | −0.111 (0.191) | 0.332 |
| ß-Cl | 0.358 (0.878) | −0.644 (0.585) | 0.641 (1.298) | 0.001 |
| 6α-OH-α-Cl | 0.107 (0.707) | −0.464 (0.978) | 0.884 (1.216) | 0.008 |
| 6α-OH-ß-Cl | −0.262 (0.312) | 0.135 (1.472) | 0.357 (0.926) | 0.303 |
| 1ß-OH-ß-Cl | −0.164 (0.582) | 0.008 (1.330) | 0.359 (1.134) | 0.512 |
| A5-3ß,17α | 0.084 (0.744) | −0.730 (0.556) | 1.441 (0.835) | <0.001 |
| DHEA | 0.431 (0.978) | −0.720 (0.132) | 0.561 (1.300) | <0.001 |
| Adiol | −0.044 (0.978) | −0.127 (0.631) | 0.365 (1.708) | 0.561 |
| 16α-OH-DHEA | 0.424 (0.800) | −0.847 (0.264) | 0.840 (1.255) | <0.001 |
| 16ß-OH-DHEA | −0.090 (0.540) | −0.391 (1.210) | 1.065 (0.816) | 0.003 |
| 15ß,16αOH-DHEA | −0.390 (0.276) | 0.290 (1.316) | 0.122 (1.052) | 0.100 |
| 16-O-A5D | 0.461 (0.717) | −0.901 (0.192) | 0.909 (1.216) | <0.001 |
| A5T-16α | 0.230 (0.647) | −0.750 (0.282) | 1.097 (1.573) | <0.001 |
| A5T-16ß | 0.315 (0.619) | −0.798 (0.408) | 0.598 (1.368) | <0.001 |
| 16α,18OH-DHEA | −0.375 (0.431) | 0.183 (1.338) | 0.405 (0.962) | 0.108 |
| A5-3ß16α17ß18-tetrol | −0.412 (0.512) | 0.284 (1.290) | 0.343 (1.014) | 0.067 |
| A5-3ß15ß16α17ß-tetrol | −0.329 (0.777) | 0.245 (1.276) | 0.284 (0.705) | 0.174 |
| 16OHP5o | −0.372 (0.381) | 0.028 (1.332) | 0.888 (0.861) | 0.015 |
| 21OHP5o | −0.166 (0.453) | −0.530 (0.816) | 1.562 (0.978) | <0.001 |
| P5-3ß20α21-triol | 0.235 (1.002) | −0.723 (0.298) | 1.014 (0.914) | <0.001 |
| P5-tetrol | −0.039 (0.570) | −0.601 (0.784) | 1.446 (1.034) | <0.001 |
| 11ß-HSD * | −0.366 (0.490) | 0.479 (1.349) | −0.056 (0.821) | 0.043 |
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| n | |||
| Distinctive metabolic characteristics (day 3) | Mild Elevation of C21-steroids (particular 17-OH-progesterone, and cortisone metabolites), and C19-steroids (DHEA-metabolites) | No specific abnormality, lowest excretion in general | Highest elevation of C21-steroids (17-OH-pregnenolone metabolites, and cortisone metabolites) and C19-steroids (DHEA-metabolites) |
| Distinctive metabolic characteristics (week 2) | Mild Elevation of C21-steroids (particular 17-OH-progesterone, cortisol and cortisone metabolites), pregnenolone-metabolites and C19-steroids (DHEA-metabolites) compared to cluster 2 but slightly lower than cluster 1 at day 3 | No specific abnormality, lowest excretion in general but higher compared to day 3 | Elevation of C21-steroids (particular cortisone metabolites) and C19-steroids (DHEA-metabolites) compared to cluster 1 and 2 but lower compared cluster 3 at day 3 |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Early preterm | Well (n = 6) | Ill (n = 18) | Well (n = 3) | Ill (n = 11) | Well (n = 6) | Ill (n = 7) | |
| GA | 29 (25.6–30) | 27.6 (24.3–29.9) | 28.7 (28.6–28.7) | 27.9 (25.1–30) | 27.9 (24–29.6) | 27.0 (26.3–27.9) | 0.50 |
| BW | 1155 (700–1460) | 1046 (460–1650) | 1150 (660–1240) | 850 (580–1180) | 910 (640–1590) | 1050 (880–1340) | 0.16 |
| Sex (female) | 3/6 | 11/18 | 6/11 | 2/6 | 2/7 | ||
| SGA | 0/6 | 2/18 | 3/11 | 1/6 | 0/7 | 0.17 | |
| Apgar 5 min | 9 (8–10) | 9 (5–10) | 9 | 8 (7–9) | 9.5 (8–10) | 8 (7–9) | 0.31 |
| C-section | 15/18 | 11/11 | 2/6 | 7/7 | 0.07 | ||
| pH | 7.35 (7.30–7.39) | 7.31 (7.15–7.46) | 7.26 (7.24–7.4) | 7.29 (7.12–7.39) | 7.31 (7.14–7.42) | 7.35 (7.22–7.37) | 0.59 |
| Prenatal Steroids | 1/6 | 10/18 | 5/11 | 5/6 | 4/7 | 0.32 | |
| SNAP D3 | 8.5 (3–17) | 17 (4–33) | 6 (4–7) | 19 (6–33) | 9.5 (2–16) | 25.5 (11–31) | 0.81 |
| SNAP W2 | 5 (2–7) | 5.5 (2–23) | 5 (4–5) | 5 (2–28) | 5 (3–14) | 8 (5–11) | 0.69 |
| RDS >II° | 0 | 4/18 | 0 | 7/11 | 0 | 4/7 | 0.49 |
| Infection | 0 | 15/18 | 0 | 11/11 | 0 | 5/7 | 0.10 |
| IVH > 2 | 0/6 | 6/18 | 0 | 1/11 | 0 | 0 | 0.08 |
| NEC | 0 | 2/18 | 0 | 1/11 | 0 | 1/7 | 0.80 |
| ROP | 1/6 | 1/18 | 1/11 | 1/6 | 0.23 | ||
| PVL | 0 | 1/18 | 0 | 1/7 | 0.57 | ||
| Cluster 1 | Cluster 2 | Cluster 3 | p | |
|---|---|---|---|---|
| n | 24 (47.1%) | 14 (27.5%) | 13 (25.5%) | |
| THS | 0.533 (0.991) | −0.638 (0.322) | −0.296 (1.018) | <0.001 |
| 15ß,17OHP5o | −0.059 (0.957) | −0.662 (0.377) | 0.821 (1.004) | <0.001 |
| P5-tetrol-15ß | 0.061 (0.826) | −0.860 (0.575) | 0.814 (0.956) | <0.001 |
| 17OHPo | 0.610 (1.090) | −0.508 (0.570) | −0.578 (0.360) | <0.001 |
| 17OHPo-5α | 0.645 (0.883) | −0.417 (0.730) | −0.742 (0.680) | <0.001 |
| 15ß,17OHPo | 0.454 (1.081) | −0.513 (0.534) | −0.285 (0.901) | 0.006 |
| PT | 0.645 (0.904) | −0.217 (0.803) | −0.957 (0.156) | <0.001 |
| THA | 0.499 (1.189) | −0.537 (0.583) | −0.343 (0.358) | 0.002 |
| THF | 0.371 (1.172) | −0.541 (0.234) | −0.102 (0.920) | 0.020 |
| F | 0.262 (1.316) | −0.396 (0.070) | −0.058 (0.719) | 0.143 |
| 6ß-OH-F | −0.052 (0.907) | −0.324 (0.069) | 0.445 (1.497) | 0.128 |
| 20α DHF | 0.276 (1.248) | −0.418 (0.293) | −0.060 (0.853) | 0.114 |
| 11-O-An | 0.490 (0.913) | −0.414 (0.679) | −0.460 (1.076) | 0.003 |
| 11-OH-An | 0.678 (0.879) | −0.485 (0.516) | −0.730 (0.790) | <0.001 |
| THE | 0.335 (0.896) | −0.997 (0.685) | 0.455 (0.715) | <0.001 |
| α-Cl | 0.336 (1.245) | −0.566 (0.526) | −0.012 (0.544) | 0.024 |
| 6α-OH-THE | −0.283 (0.581) | −0.578 (0.157) | 1.145 (1.235) | <0.001 |
| 1ß-OH-THE | −0.095 (0.700) | −0.334 (0.180) | 0.536 (1.653) | 0.061 |
| ß-Cl | 0.577 (1.215) | −0.437 (0.234) | −0.595 (0.084) | <0.001 |
| 6α-OH-α-Cl | 0.546 (1.222) | −0.548 (0.307) | −0.419 (0.255) | <0.001 |
| 6α-OH-ß-Cl | 0.138 (1.070) | −0.795 (0.134) | 0.601 (0.888) | <0.001 |
| 1ß-OH-ß-Cl | 0.121 (1.014) | −0.887 (0.135) | 0.732 (0.805) | <0.001 |
| A5-3ß,17α | 0.272 (1.041) | −0.722 (0.384) | 0.277 (1.044) | 0.005 |
| DHEA | 0.254 (0.886) | −0.539 (0.347) | 0.112 (1.432) | 0.052 |
| Adiol | −0.209 (0.890) | −0.233 (0.618) | 0.637 (1.282) | 0.026 |
| 16α-OH-DHEA | 0.341 (1.033) | −0.534 (0.441) | −0.055 (1.158) | 0.029 |
| 16ß-OH-DHEA | −0.064 (0.568) | −0.564 (0.144) | 0.725 (1.606) | 0.002 |
| 15ß,16α -OH-DHEA | −0.431 (0.419) | −0.540 (0.375) | 1.378 (0.953) | <0.001 |
| 16-O-A5D | 0.515 (1.035) | −0.554 (0.491) | −0.354 (0.912) | 0.001 |
| A5T-16α | 0.321 (0.839) | −0.380 (0.881) | −0.184 (1.250) | 0.082 |
| A5T-16ß | 0.163 (1.003) | −0.371 (0.334) | 0.098 (1.376) | 0.265 |
| 16α,18OH-DHEA | −0.216 (0.749) | −0.573 (0.202) | 1.015 (1.206) | <0.001 |
| A5-3ß16α17ß18-tetrol | −0.330 (0.499) | −0.527 (0.285) | 1.176 (1.250) | <0.001 |
| A5-3ß15ß16α17ß-tetrol | −0.245 (0.722) | −0.608 (0.372) | 1.107 (1.066) | <0.001 |
| 16OHP5o | −0.217 (0.604) | −0.682 (0.263) | 1.136 (1.165) | <0.001 |
| 21OHP5o | −0.023 (0.661) | −0.911 (0.450) | 1.024 (1.003) | <0.001 |
| P5-3ß20α21-triol | 0.379 (0.922) | −0.620 (0.335) | −0.031 (1.296) | 0.009 |
| P5-tetrol | 0.014 (0.723) | −0.711 (0.206) | 0.740 (1.392) | <0.001 |
| 11ß-HSD * | 0.178 (1.344) | −0.292 (0.303) | −0.013 (0.666) | 0.384 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heckmann, M.; Runkel, A.S.; Sunny, D.E.; Hartmann, M.F.; Ittermann, T.; Wudy, S.A. Steroid Metabolomic Signature in Term and Preterm Infants. Biomolecules 2024, 14, 235. https://doi.org/10.3390/biom14020235
Heckmann M, Runkel AS, Sunny DE, Hartmann MF, Ittermann T, Wudy SA. Steroid Metabolomic Signature in Term and Preterm Infants. Biomolecules. 2024; 14(2):235. https://doi.org/10.3390/biom14020235
Chicago/Turabian StyleHeckmann, Matthias, Anna S. Runkel, Donna E. Sunny, Michaela F. Hartmann, Till Ittermann, and Stefan A. Wudy. 2024. "Steroid Metabolomic Signature in Term and Preterm Infants" Biomolecules 14, no. 2: 235. https://doi.org/10.3390/biom14020235
APA StyleHeckmann, M., Runkel, A. S., Sunny, D. E., Hartmann, M. F., Ittermann, T., & Wudy, S. A. (2024). Steroid Metabolomic Signature in Term and Preterm Infants. Biomolecules, 14(2), 235. https://doi.org/10.3390/biom14020235






