Enhanced Tumor Targeting and Antitumor Activity of Methylated β-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.3. Synthesis of Methylated PRXs
2.4. Modification of cRGDfK Peptide
2.5. Binding Affinity of cRGD-Me-PRXs with Integrin αvβ3 by Surface Plasmon Resonance (SPR) Measurements
2.6. Cell Culture
2.7. In Vitro Cytotoxicity
2.8. Expression Analysis of Integrin αV and β3
2.9. Cellular Association Analysis Using Flow Cytometry
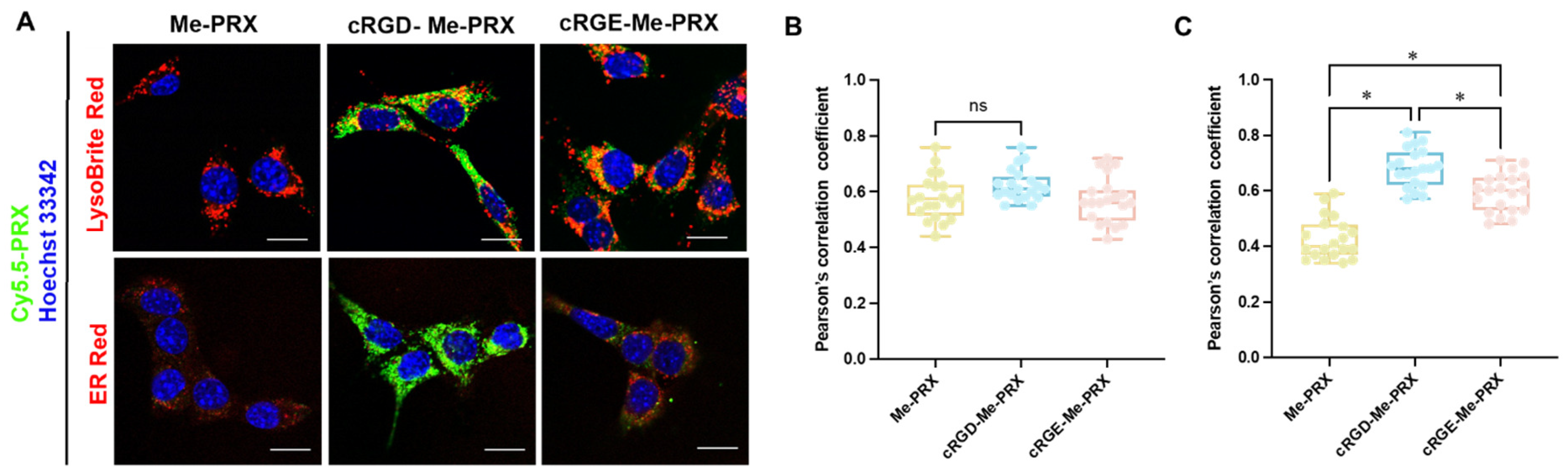
2.10. Intracellular Distribution Analysis Using Confocal Laser Scanning Microscopy
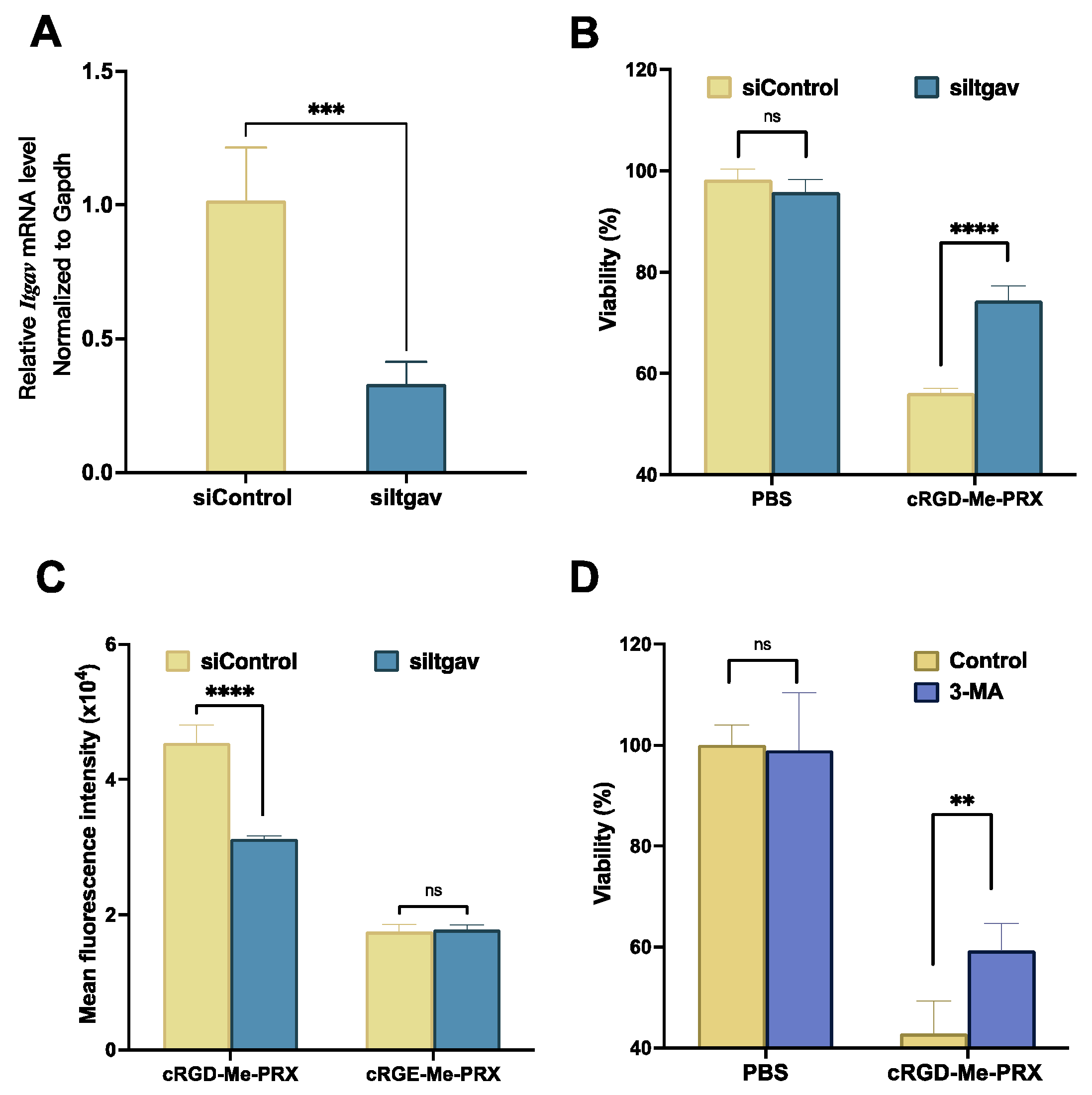
2.11. Downregulation of Integrin αV on 4T1 Cells by siRNA
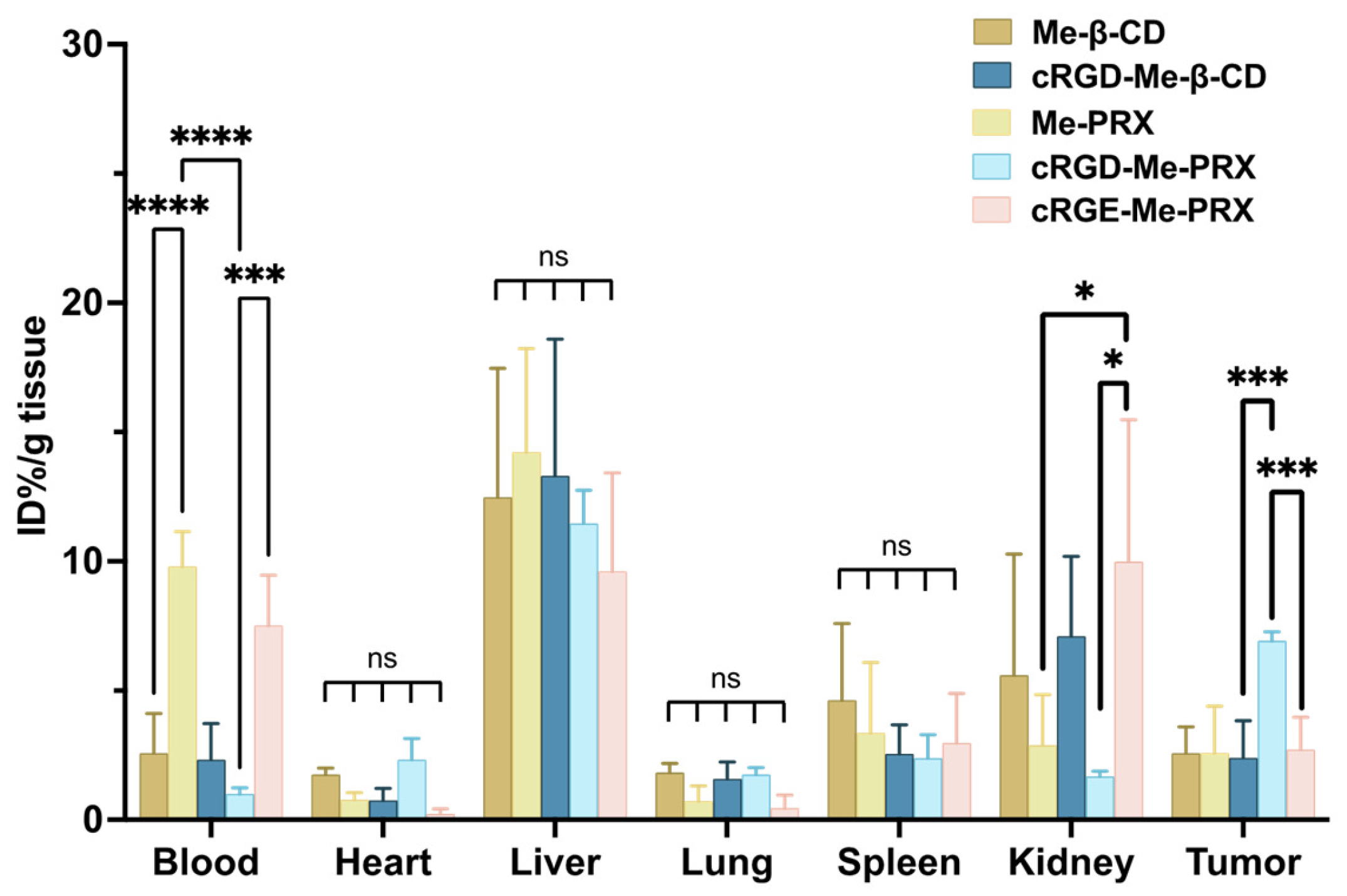
2.12. Biodistribution of Me-PRXs
2.13. Antitumor Activity Assay in 4T1 Tumor-Bearing Mice
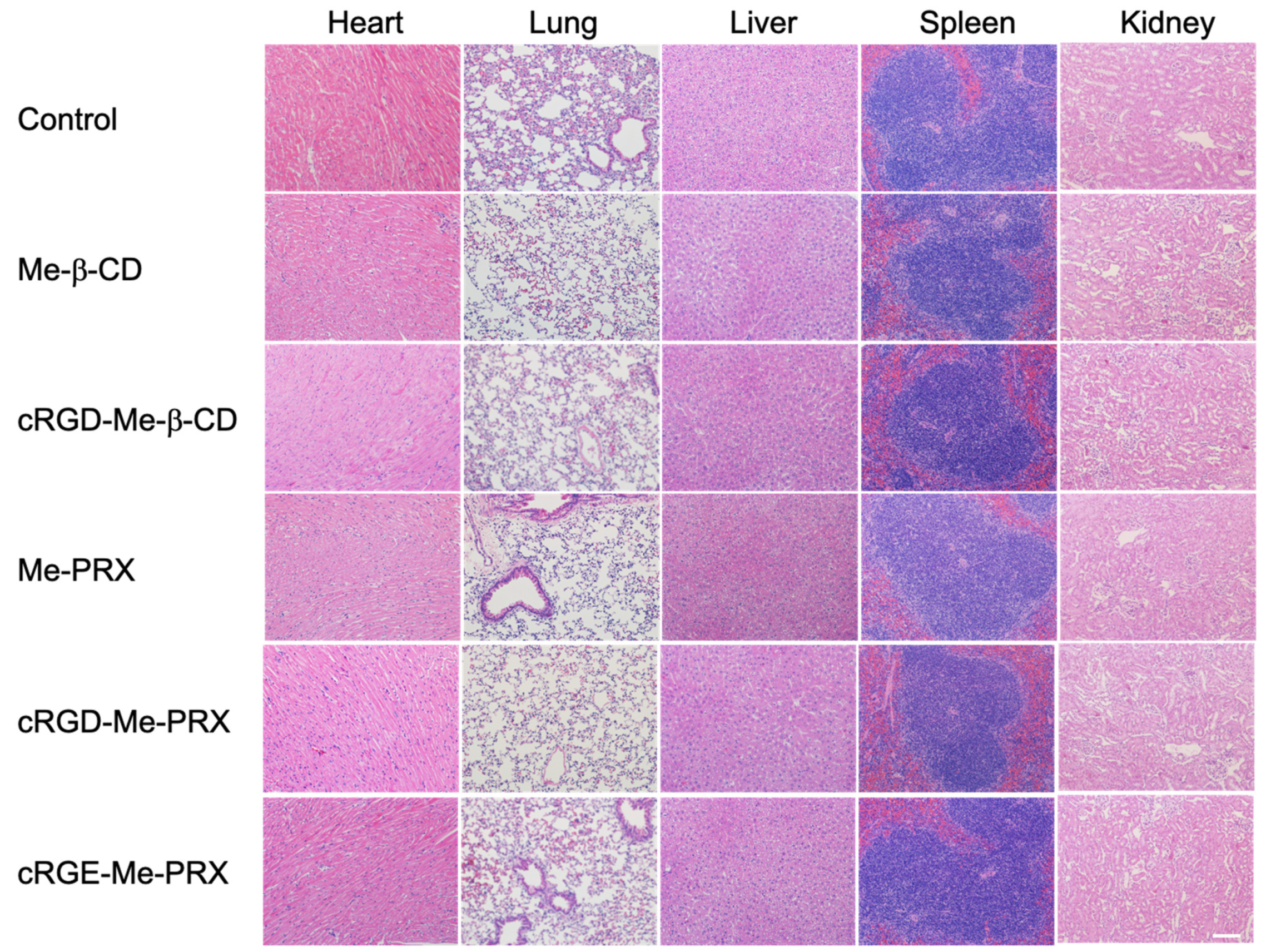
2.14. Blood Chemical Analysis
2.15. Statistical Analysis
3. Results and Discussion
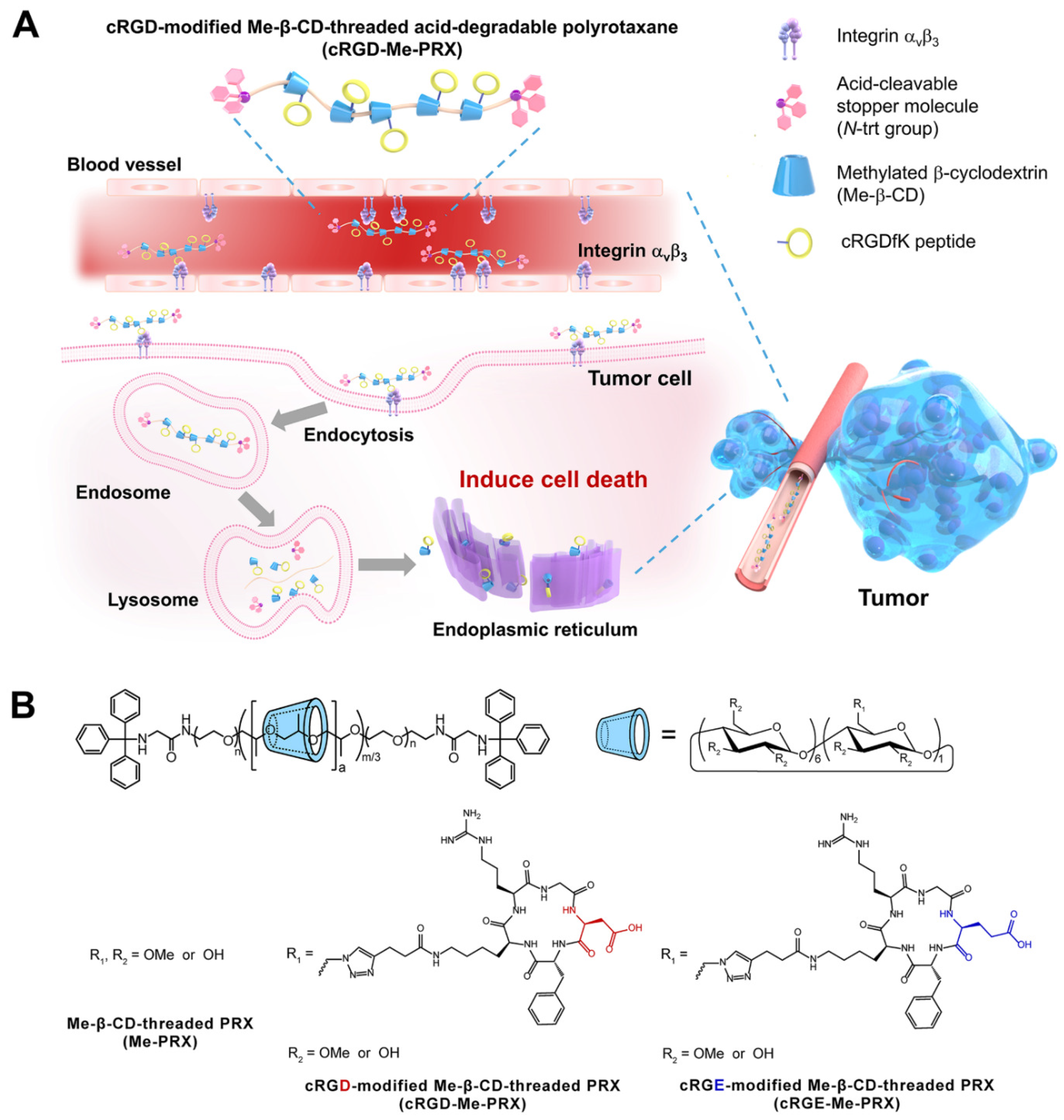
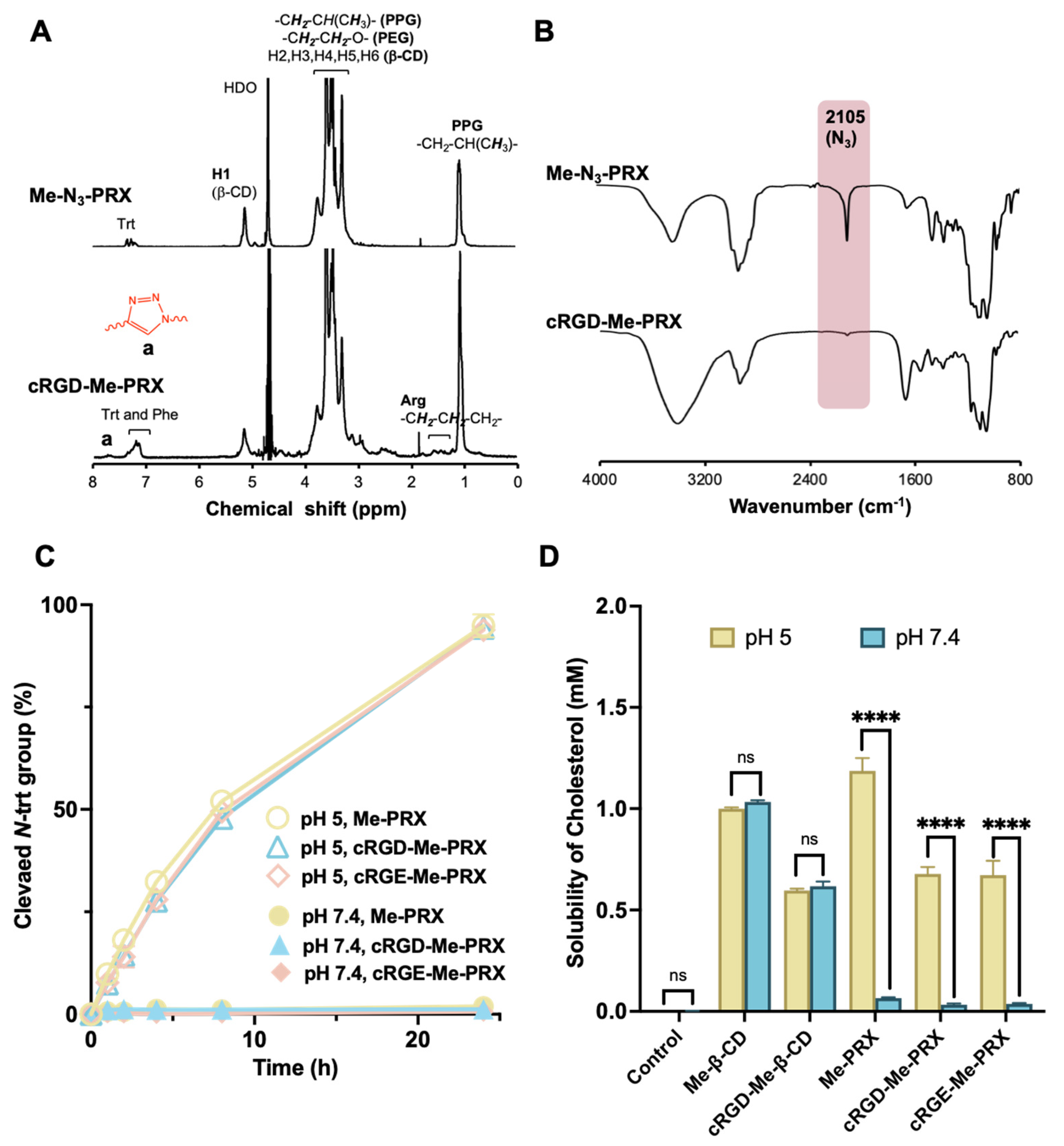
3.1. Synthesis and Characterization of cRGD-Me-PRX
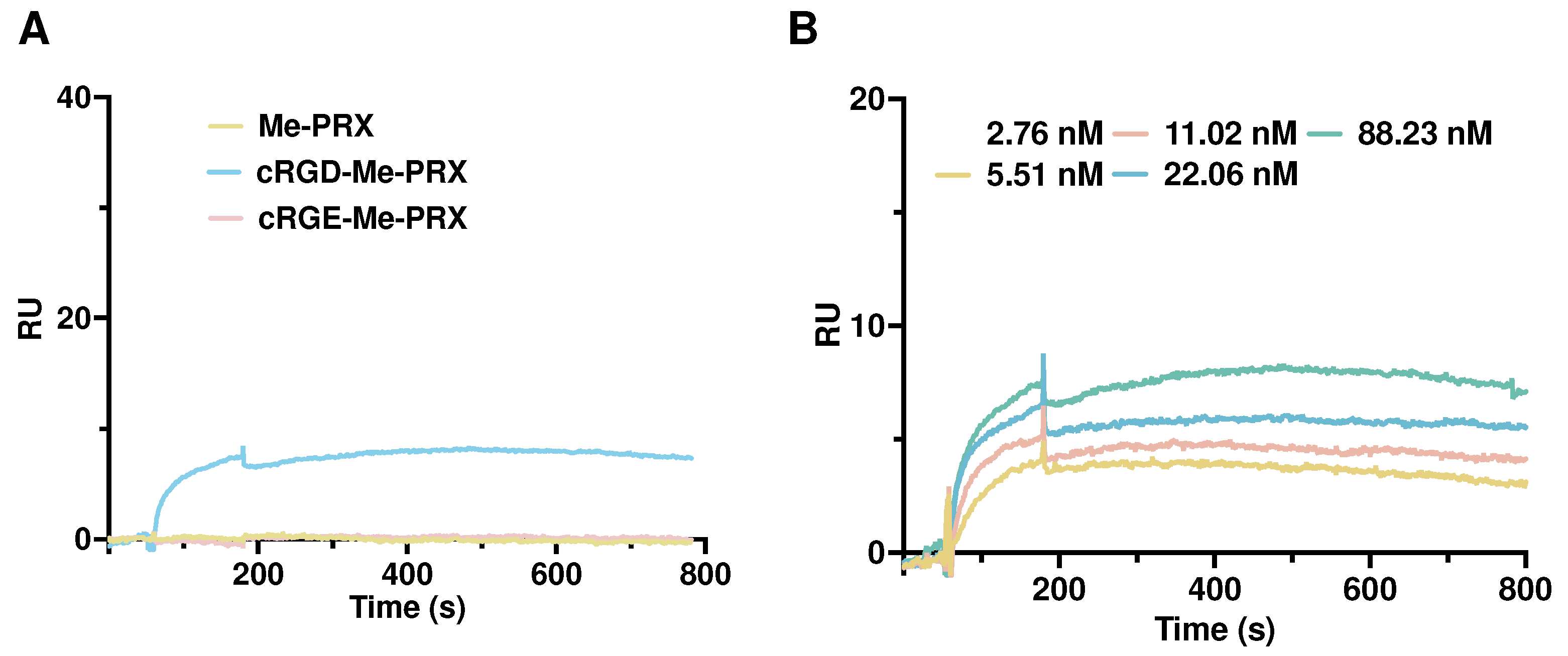
3.2. Interaction of cRGD-Me-PRX with Integrin αvβ3
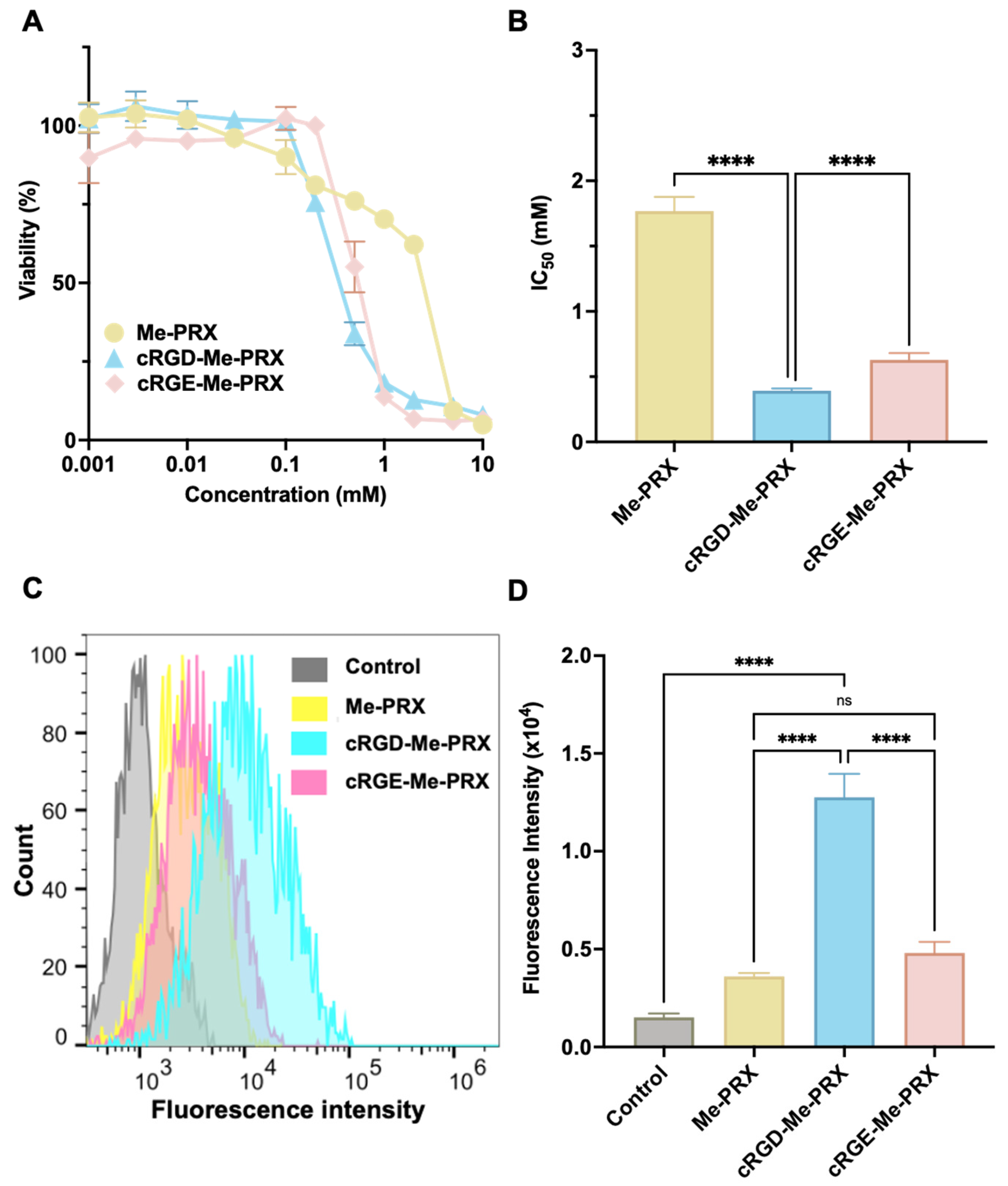
3.3. Cellular Association and Cytotoxicity of cRGD-Me-PRX
3.4. Involvement of Integrin αvβ3 in the Cellular Internalization of cRGD-Me-PRX
3.5. Biodistribution and Tumor Accumulation of cRGD-Me-PRX
3.6. Antitumor Effects of cRGD-Me-PRX in the 4T1 Tumor-Bearing Mice Model
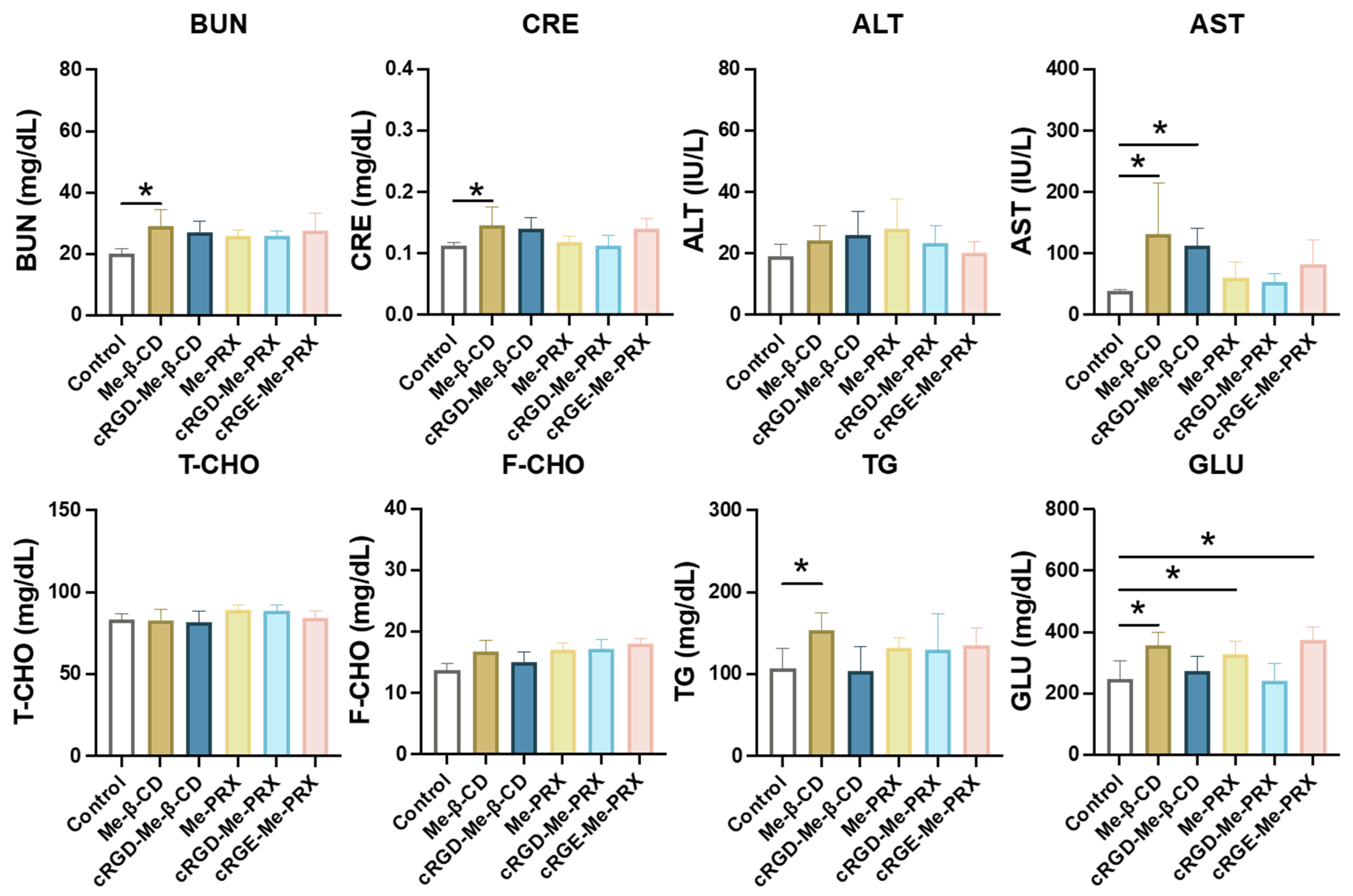
3.7. Biosafety Evaluation of Me-PRXs and Me-β-CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing Profiles of Cancer Burden Worldwide and in China: A Secondary Analysis of the Global Cancer Statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The Reversal of Chemotherapy-Induced Multidrug Resistance by Nanomedicine for Cancer Therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mishra, L.; Li, S. EMT, EMT, CTCs and CSCS in Tumor Relapse and Drug-Resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 41–160. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Turchi, J.J. Chemotherapy Induced DNA Damage Response: Convergence of Drugs and Pathways. Cancer Biol. Ther. 2013, 14, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 4. [Google Scholar]
- Okada, H.; Mak, T.W. Pathways of Apoptotic and Non-Apoptotic Death in Tumour Cells. Nat. Rev. Cancer. 2004, 4, 592–603. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Wu, F.; Zhai, L.; Wang, K.; Xiao, M.; Xie, T.; Sui, X. Non-Apoptotic Cell Death in Malignant Tumor Cells and Natural Compounds. Cancer Lett. 2018, 420, 210–227. [Google Scholar] [CrossRef]
- Kitada, M.; Koya, D. Autophagy in Metabolic Disease and Ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Mehta, V.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. Exploring the Role of Autophagy Dysfunction in Neurodegenerative Disorders. Mol. Neurobiol. 2021, 58, 4886–4905. [Google Scholar] [CrossRef]
- Udristioiu, A.; Nica-Badea, D. Autophagy Dysfunctions Associated with Cancer Cells and Their Therapeutic Implications. Biomed. Pharmacother. 2019, 115, 108892. [Google Scholar] [CrossRef]
- Kroemer, G.; Martin, S.J. Caspase-Independent Cell Death. Nat. Med. 2005, 11, 725–730. [Google Scholar] [CrossRef]
- Yoshida, G.J. Therapeutic Strategies of Drug Repositioning Targeting Autophagy to Induce Cancer Cell Death: From Pathophysiology to Treatment. J. Hematol. Oncol. 2017, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zou, Z. Targeting Autophagy to Overcome Drug Resistance: Further Developments. J. Hematol. Oncol. 2020, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug. Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Christoforides, E.; Papaioannou, A.; Bethanis, K. Crystal Structure of the Inclusion Complex of Cholesterol in β-Cyclodextrin and Molecular Dynamics Studies. Beilstein J. Org. Chem. 2018, 14, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Irie, T.; Matsuo, M. Cyclodextrins Applied to the Treatment of Lysosomal Storage Disorders. Adv. Drug Deliv. Rev. 2022, 191, 114617. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of Defective Lysosomal Transport in NPC Disease Ameliorates Liver Dysfunction and Neurodegeneration in the Npc1−/− Mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Vite, C.H.; Bagel, J.H.; Swain, G.P.; Prociuk, M.; Sikora, T.U.; Stein, V.M.; O’Donnell, P.; Ruane, T.; Ward, S.; Crooks, A.; et al. Intracisternal Cyclodextrin Prevents Cerebellar Dysfunction and Purkinje Cell Death in Feline Niemann-Pick Type C1 Disease. Sci. Transl. Med. 2015, 7, 276ra26. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-Hydroxypropyl-β-cyclodextrin Decreases Neurological Disease Progression in Niemann-Pick Disease, Type C1: A Non-Randomised, Open-Label, Phase 1−2 Trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by Cyclodextrin in Cell and Mouse Models of Alzheimer Disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin Promotes Atherosclerosis Regression via Macrophage Reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef]
- Kiss, T.; Fenyvesi, F.; Bacskay, I.; Varadi, J.; Fenyvesi, E.; Ivanyi, R.; Szente, L.; Tosaki, A.; Vecsernyes, M. Evaluation of the Cytotoxicity of β-Cyclodextrin Derivatives: Evidence for the Role of Cholesterol Extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Schönfelder, U.; Radestock, A.; Elsner, P.; Hipler, U.C. Cyclodextrin-Induced Apoptosis in Human Keratinocytes is Caspase-8 Dependent and Accompanied by Mitochondrial Cytochrome C Release. Exp. Dermatol. 2006, 15, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of Cholesterol Depletion from Lipid Rafts in Apoptosis Induced by Methyl-β-Cyclodextrin. Int. J. Pharm. 2013, 452, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Arima, H. Potential Use of Folate-Appended Methyl-β-Cyclodextrin as an Anticancer Agent. Sci. Rep. 2013, 3, 1104. [Google Scholar] [CrossRef] [PubMed]
- Onodera, R.; Motoyama, K.; Tanaka, N.; Ohyama, A.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of Autophagy in Antitumor Activity of Folate-Appended Methyl-β-Cyclodextrin. Sci. Rep. 2014, 4, 4417. [Google Scholar] [CrossRef]
- Tamura, A.; Nishida, K.; Yui, N. Lysosomal pH-Inducible Supramolecular Dissociation of Polyrotaxanes Possessing Acid-Labile N-Triphenylmethyl End Groups and Their Therapeutic Potential for Niemann-Pick Type C Disease. Sci. Technol. Adv. Mater. 2016, 17, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Tamura, A.; Yui, N. Tailoring the Temperature-Induced Phase Transition and Coacervate Formation of Methylated β-Cyclodextrins-Threaded Polyrotaxanes in Aqueous Solution. Macromolecules 2016, 49, 6021–6030. [Google Scholar] [CrossRef]
- Nishida, K.; Tamura, A.; Yui, N. ER Stress-Mediated Autophagic Cell Death Induction through Methylated β-Cyclodextrins-Threaded Acid-Labile Polyrotaxanes. J. Control. Release 2018, 275, 20–31. [Google Scholar] [CrossRef]
- Nishida, K.; Tamura, A.; Kang, T.W.; Masuda, H.; Yui, N. An Antibody–Supermolecule Conjugate for Tumor-Specific Targeting of Tumoricidal Methylated β-Cyclodextrin-Threaded Polyrotaxanes. J. Mater. Chem. B 2020, 8, 6975–6987. [Google Scholar] [CrossRef]
- Zhang, S.; Tamura, A.; Yui, N. Cothreading of Unmodified and Monoazidated β-Cyclodextrins in Polyrotaxanes for Orthogonal Modification of Cell-Penetrating Peptides via Click Chemistry. ACS Appl. Polym. Mater. 2022, 4, 3866–3876. [Google Scholar] [CrossRef]
- Alday-Parejo, B.; Stupp, R.; Rüegg, C. Are Integrins Still Practicable Targets for Anti-Cancer Therapy? Cancers 2019, 11, 978. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. RGD-Modified Polymer and Liposome Nanovehicles: Recent Research Progress for Drug Delivery in Cancer Therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17. [Google Scholar] [CrossRef]
- Ohashi, M.; Tamura, A.; Yui, N. Exploring Receptor Binding Affinities and Hepatic Cell Association of N-Acetyl-D-Galactosamine-Modified β-Cyclodextrin-Based Polyrotaxanes for Liver-Targeted Therapies. Biomacromolecules 2023, 24, 2327–2341. [Google Scholar] [CrossRef]
- Abu-El-Halawa, R.; Zabin, S.A. Removal Efficiency of Pb, Cd, Cu and Zn from Polluted Water Using Dithiocarbamate Ligands. J. Taibah Univ. Sci. 2017, 11, 57–65. [Google Scholar] [CrossRef]
- Bieri, M.; Hendrickx, R.; Bauer, M.; Yu, B.; Jetzer, T.; Dreier, B.; Mittl, P.R.E.; Sobek, J.; Plückthun, A.; Greber, U.F.; et al. The RGD-Binding Integrins αvβ6 and αvβ8 are Receptors for Mouse Adenovirus-1 and -3 Infection. PLoS Pathog. 2021, 17, e1010083. [Google Scholar] [CrossRef]
- Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; et al. A Comparative Study of the Antitumor Efficacy of Peptide-Doxorubicin Conjugates with Different Linkers. J. Control. Release 2018, 275, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Baneshi, M.; Hosseinkhani, S.; Mahmoudi, R.; Jabari Arabzadeh, A.; Akrami, M.; Mehrzad, J.; Bardania, H. Recent Progress in Biomedical Applications of RGD-Based Ligand: From Precise Cancer Theranostics to Biomaterial Engineering: A Systematic Review. J. Biomed. Mater. Res. Part A 2020, 108, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Viela, F.; Speziale, P.; Pietrocola, G.; Dufrêne, Y.F. Mechanostability of the Fibrinogen Bridge between Staphylococcal Surface Protein ClfA and Endothelial Cell Integrin αVβ3. Nano Lett. 2019, 19, 7400–7410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Ohashi, M.; Nishida, K.; Yui, N. Acid-Induced Intracellular Dissociation of β-Cyclodextrin-Threaded Polyrotaxanes Directed toward Attenuating Phototoxicity of Bisretinoids through Promoting Excretion. Mol. Pharm. 2017, 14, 4714–4724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tamura, A.; Yui, N. Weakly Acidic Carboxy Group-Grafted β-Cyclodextrin-Threaded Acid-Degradable Polyrotaxanes for Modulating Protein Interaction and Cellular Internalization. Sci. Technol. Adv. Mater. 2021, 22, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Ma, X.; Liao, X.; Yang, B. Scutellarin-Graft Cationic β-Cyclodextrin-Polyrotaxane: Synthesis, Characterization and DNA Condensation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1028–1036. [Google Scholar] [CrossRef]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual Role of 3-Methyladenine in Modulation of Autophagy via Different Temporal Patterns of Inhibition on Class I and III Phosphoinositide 3-Kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Hu, C.; Yang, X.; Liu, R.; Ruan, S.; Zhou, Y.; Xiao, W.; Yu, W.; Yang, C.; Gao, H. Coadministration of iRGD with Multistage Responsive Nanoparticles Enhanced Tumor Targeting and Penetration Abilities for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 22571–22579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; You, S.J.; Kim, H.J.; Yang, D.H.; Chun, H.J.; Lee, D.; Khang, G. Theranostic Potential of Biodegradable Polymeric Nanoparticles with Paclitaxel and Curcumin against Breast Carcinoma. Biomater. Sci. 2021, 9, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Sohma, Y.; Kanai, M. Synthesis of chemically-tethered amyloid-β segment trimer possessing amyloidogenic properties. Bioorg. Med. Chem. Lett. 2015, 25, 2976–2979. [Google Scholar] [CrossRef]
- Dai, X.; Su, Z.; Liu, J.O. An improved synthesis of a selective αvβ3-integrin antagonist cyclo (-RGDfK-). Tetrahedron Lett. 2000, 41, 6295–6298. [Google Scholar] [CrossRef]
- McCusker, C.F.; Kocienski, P.J.; Boyle, F.T.; Schätzlein, A.G. Solid-phase synthesis of c (RGDfK) derivatives: On-resin cyclisation and lysine functionalization. Bioorg. Med. Chem. Lett. 2002, 12, 547–549. [Google Scholar] [CrossRef]
- Ohashi, M.; Tamura, A.; Yui, N. Terminal structure of triethylene glycol-tethered chains on β-cyclodextrin-threaded polyrotaxanes dominates temperature responsivity and biointeractions. Langmuir 2021, 37, 11102–11114. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]

| Code a | Precursor PRXs b | Number of Threaded β-CD | Number of Methyl Groups per PRX c | Number of Peptides per PRX c | Conversion Ratio of Ligand | Mn d |
|---|---|---|---|---|---|---|
| Me-β-CD | – | – | 14.0 | – | – | 1310 |
| cRGD-Me-β-CD | – | – | 12.4 | 0.75 | – | 1840 |
| Me-PRX | PRX | 10.5 | 147.0 (14.0) | – | – | 21,500 |
| cRGE-Me-PRX | N3-PRX | 14.9 | 184.9 (12.4) | 11.1 | 76.6% | 34,600 |
| cRGD-Me-PRX | N3-PRX | 14.9 | 184.9 (12.4) | 11.3 | 77.9% | 34,600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Tamura, A.; Yui, N. Enhanced Tumor Targeting and Antitumor Activity of Methylated β-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides. Biomolecules 2024, 14, 223. https://doi.org/10.3390/biom14020223
Zhang S, Tamura A, Yui N. Enhanced Tumor Targeting and Antitumor Activity of Methylated β-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides. Biomolecules. 2024; 14(2):223. https://doi.org/10.3390/biom14020223
Chicago/Turabian StyleZhang, Shunyao, Atsushi Tamura, and Nobuhiko Yui. 2024. "Enhanced Tumor Targeting and Antitumor Activity of Methylated β-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides" Biomolecules 14, no. 2: 223. https://doi.org/10.3390/biom14020223
APA StyleZhang, S., Tamura, A., & Yui, N. (2024). Enhanced Tumor Targeting and Antitumor Activity of Methylated β-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides. Biomolecules, 14(2), 223. https://doi.org/10.3390/biom14020223










