EEG Frequency Correlates with α2-Receptor Density in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Radiochemistry
2.3. Positron Emission Tomography
2.4. Image Processing
2.5. EEG
2.6. Statistics
3. Results
4. Discussion
4.1. Encephalography
4.2. Binding of [11C]yohimbine
4.3. Dementia
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, S.; Strafella, A.P. Imaging Mild Cognitive Impairment and Dementia in Parkinson’s Disease. Front. Neurol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Hall, S.; Jalakas, M.; Grothe, M.J.; Strandberg, O.; Stomrud, E.; Westman, E.; van Westen, D.; Hansson, O. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson’s disease. Neurobiol. Dis. 2020, 139, 104831. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Sommerauer, M.; Fedorova, T.D.; Hansen, A.K.; Knudsen, K.; Otto, M.; Jeppesen, J.; Frederiksen, Y.; Blicher, J.U.; Geday, J.; Nahimi, A.; et al. Evaluation of the noradrenergic system in Parkinson’s disease: An 11C-MeNER PET and neuromelanin MRI study. Brain 2018, 141, 496–504. [Google Scholar] [CrossRef]
- Klassen, B.T.; Hentz, J.G.; Shill, H.A.; Driver-Dunckley, E.; Evidente, V.G.H.; Sabbagh, M.N.; Adler, C.H.; Caviness, J.N. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology 2011, 77, 118–124. [Google Scholar] [CrossRef]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.R.; Snaedal, J.; Wahlund, L.O.; Waldemar, G.; et al. Oscillatory connectivity as a diagnostic marker of dementia due to Alzheimer’s disease. Clin. Neurophysiol. 2019, 130, 1889–1899. [Google Scholar] [CrossRef]
- Sebban, C.; Zhang, X.Q.; Tesolin-Decros, B.; Millan, M.J.; Spedding, M. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br. J. Pharmacol. 1999, 128, 1045. [Google Scholar] [CrossRef]
- Loo, S.K.; Bilder, R.M.; Cho, A.L.; Sturm, A.; Cowen, J.; Walshaw, P.; Levitt, J.; Del’Homme, M.; Piacentini, J.; McGough, J.J.; et al. Effects of d-Methylphenidate, Guanfacine, and Their Combination on Electroencephalogram Resting State Spectral Power in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 674–682.e1. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Pliszka, S.R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Nahimi, A.; Jakobsen, S.; Munk, O.L.; Vang, K.; Phan, J.A.; Rodell, A.; Gjedde, A. Mapping α2 Adrenoceptors of the Human Brain with 11C-Yohimbine. J. Nucl. Med. 2015, 56, 392–398. [Google Scholar] [CrossRef]
- Laurencin, C.; Lancelot, S.; Brosse, S.; Mérida, I.; Redouté, J.; Greusard, E.; Lamberet, L.; Liotier, V.; Le Bars, D.; Costes, N.; et al. Noradrenergic alterations in Parkinson’s disease: A combined 11C-yohimbine PET/neuromelanin MRI study. Brain 2023, awad338. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Blankson, S.; Lees, A.J. A Clinicopathologic Study of 100 Cases of Parkinson’s Disease. Arch. Neurol. 1993, 50, 140–148. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.; Pedersen, K.; Smith, D.F.; Jensen, S.B.; Munk, O.L.; Cumming, P. Detection of alpha2-adrenergic receptors in brain of living pig with 11C-yohimbine. J. Nucl. Med. 2006, 47, 2008–2015. [Google Scholar]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolf, A.P.; Dewey, S.L.; Schlyer, D.J.; MacGregor, R.R.; Hitzemann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical Analysis of Reversible Radioligand Binding from Time—Activity Measurements Applied to [N-11C-Methyl]-(−)-Cocaine PET Studies in Human Subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef]
- Phan, J.A.; Landau, A.M.; Jakobsen, S.; Wong, D.F.; Gjedde, A. Radioligand binding analysis of α2 adrenoceptors with [11C]yohimbine in brain in vivo: Extended Inhibition Plot correction for plasma protein binding. Sci. Rep. 2017, 7, 15979. [Google Scholar] [CrossRef]
- Morita, A.; Kamei, S.; Serizawa, K.; Mizutani, T. The Relationship Between Slowing EEGs and the Progression of Parkinson’s Disease. J. Clin. Neurophysiol. 2009, 26, 426–429. [Google Scholar] [CrossRef]
- Serizawa, K.; Kamei, S.; Morita, A.; Hara, M.; Mizutani, T.; Yoshihashi, H.; Yamaguchi, M.; Takeshita, J.; Hirayanagi, K. Comparison of Quantitative EEGs Between Parkinson Disease and Age-Adjusted Normal Controls. J. Clin. Neurophysiol. 2008, 25, 361. [Google Scholar] [CrossRef]
- Caviness, J.N.; Hentz, J.G.; Evidente, V.G.; Driver-Dunckley, E.; Samanta, J.; Mahant, P.; Connor, D.J.; Sabbagh, M.N.; Shill, H.A.; Adler, C.H. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 348–354. [Google Scholar] [CrossRef]
- Bosman, C.A.; Lansink, C.S.; Pennartz, C.M.A. Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 2014, 39, 1982–1999. [Google Scholar] [CrossRef]
- Caviness, J.N.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Driver-Dunckley, E.D.; Adler, C.H. Association Between Pathology and Electroencephalographic Activity in Parkinson’s Disease. Clin. EEG Neurosci. 2018, 49, 321–327. [Google Scholar] [CrossRef]
- Eschenko, O.; Magri, C.; Panzeri, S.; Sara, S.J. Noradrenergic Neurons of the Locus Coeruleus Are Phase Locked to Cortical Up-Down States during Sleep. Cereb. Cortex 2012, 22, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Locus Coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 2015, 35, 87–94. [Google Scholar] [CrossRef]
- Le Corre, P.A.; Peskind, E.R.; Chevanne, F.; Raskind, M.A.; Le Verge, R. Cerebrospinal fluid and plasma disposition of yohimbine and 11-hydroxy-yohimbine in young and older healthy subjects, and Alzheimer’s disease patients. Eur. J. Clin. Pharmacol. 1997, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Dyve, S.; Jakobsen, S.; Alstrup, A.K.O.; Gjedde, A.; Doudet, D.J. Acute Vagal Nerve Stimulation Lowers α2 Adrenoceptor Availability: Possible Mechanism of Therapeutic Action. Brain Stimul. 2015, 8, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santos, I.; Palomero-Gallagher, N.; Zilles, K.; Cavada, C. Distribution of the Noradrenaline Innervation and Adrenoceptors in the Macaque Monkey Thalamus. Cereb. Cortex 2021, 31, 4115–4139. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J.; Bouret, S. Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kugler, J.; Seus, R.; Krauskopf, R.; Brecht, H.M.; Raschig, A. Differences in psychic performance with guanfacine and clonidine in normotensive subjects. Br. J. Clin. Pharmacol. 1980, 10 (Suppl. S1), 71S–80S. [Google Scholar] [CrossRef]
- Clark, C.R.; Geffen, G.M.; Geffen, L.B. Role of monoamine pathways in attention and effort: Effects of clonidine and methylphenidate in normal adult humans. Psychopharmacology 1986, 90, 35–39. [Google Scholar] [CrossRef]
- Coull, J.T.; Middleton, H.C.; Robbins, T.W.; Sahakian, B.J. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology 1995, 120, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Nutt, D. Noradrenaline and attention lapses. Nature 1996, 380, 291. [Google Scholar] [CrossRef] [PubMed]
- Jäkälä, P.; Riekkinen, M.; Sirviö, J.; Koivisto, E.; Riekkinen, P. Clonidine, but not Guanfacine, Impairs Choice Reaction Time Performance in Young Healthy Volunteers. Neuropsychopharmacology 1999, 21, 495. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Jones, M.E.P.; Egan, T.D.; Frith, C.D.; Maze, M. Attentional effects of noradrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. NeuroImage 2004, 22, 315–322. [Google Scholar] [CrossRef]
- Smith, A.P.; Wilson, S.J.; Glue, P.; Nutt, D.J. The effects and after effects of the α2-adrenoceptor antagonist idazoxan on mood, memory and attention in normal volunteers. J. Psychopharmacol. 1992, 6, 376–381. [Google Scholar] [CrossRef]
- Weinshenker, D. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Vermeiren, Y.; De Deyn, P.P. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem. Int. 2017, 102, 22–32. [Google Scholar] [CrossRef]
- Tully, K.; Bolshakov, V.Y. Emotional enhancement of memory: How norepinephrine enables synaptic plasticity. Mol. Brain 2010, 3, 15. [Google Scholar] [CrossRef]
- Kuwabara, H.; McCaul, M.E.; Wand, G.S.; Earley, C.J.; Allen, R.P.; Weerts, E.M.; Dannals, R.F.; Wong, D.F. Dissociative changes in Bmax and KD of dopamine D2/D3 receptors with aging observed in functional subdivisions of striatum: A revisit with an improved data analysis method. J. Nucl. Med. 2012, 53, 805–812. [Google Scholar] [CrossRef]
- Geraedts, V.J.; Marinus, J.; Gouw, A.A.; Mosch, A.; Stam, C.J.; van Hilten, J.J.; Contarino, M.F.; Tannemaat, M.R. Quantitative EEG reflects non-dopaminergic disease severity in Parkinson’s disease. Clin. Neurophysiol. 2018, 129, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
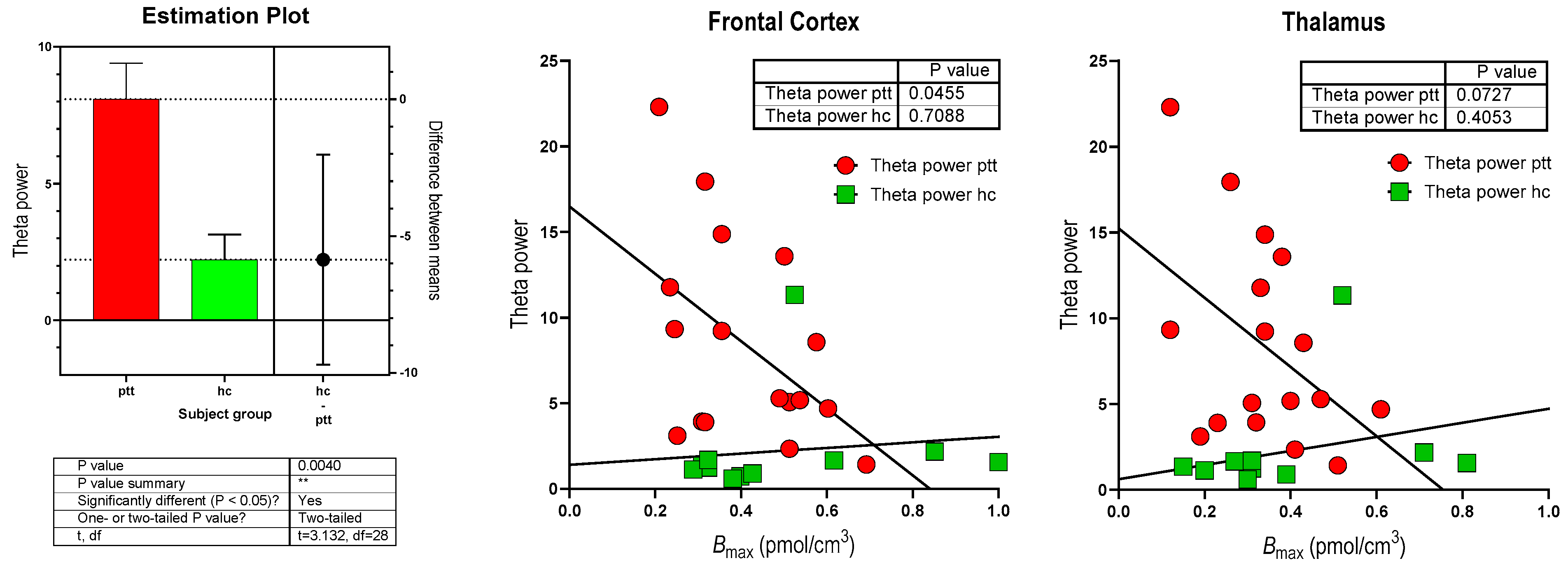
| Variables | PD Mean (Std. Deviation) | HC Mean (Std. Deviation) | p-Value |
|---|---|---|---|
| Gender | Male = 12 | Male = 6 | 0.696 |
| Female = 5 | Female = 5 | ||
| Age (years) | 65.39 (8.70) | 66.55 (8.24) | 0.726 |
| Delta Power (µV2) | 3.10 | 1.23 | 0.002 * |
| Theta Power (µV2) | 8.08 | 2.21 | 0.002 *† |
| Alpha Power (µV2) | 9.20 | 8.47 | 0.450 † |
| Beta Power (µV2) | 3.09 | 3.15 | 1.000 † |
| Total Power (µV2) | 23.47 | 15.06 | 0.128 † |
| BRF (Hz) | 7.75 (1.26) | 8.28 (1.03) | 0.252 |
| Spectral Ratio | 1.61 | 3.47 | 0.021 *† |
| Thalamus BPND (ratio) | 0.68 (0.26) | 0.78 (0.42) | 0.452 |
| Frontal Cortex BPND (ratio) | 0.82 | 0.99 | 1.000 † |
| Thalamus Bmax (pmol/cm3) | 0.34 (0.13) | 0.39 (0.21) | 0.452 |
| Frontal Cortex Bmax (pmol/cm3) | 0.41 | 0.49 | 1.000 † |
| Bmax Frontal Cortex | p-Value | Bmax Thalamus | p-Value | ||
|---|---|---|---|---|---|
| Variables | Coefficients | Variables | Coefficients | ||
| Gender | 0.232 | 0.4127 | Gender | 0.127 | 0.683 |
| Age | 0.255 | 0.290 | Age | 0.173 | 0.509 |
| BRF (Hz) | −0.466 | 0.145 | BRF (Hz) | −0.439 | 0.208 |
| Theta Power (µV2) | −0.620 | 0.022 * | Theta Power (µV2) | −0.550 | 0.055 |
| R2 = 0.426, adjusted R2 = 0.234 | |||||
| Bmax Frontal Cortex | p-Value | Bmax Thalamus | p-Value | ||
|---|---|---|---|---|---|
| Variables | Coefficients | Variables | Coefficients | ||
| Gender | 0.927 | 0.080 | Gender | 0.793 | 0.120 |
| Age | 0.640 | 0.039 * | Age | 0.540 | 0.119 |
| BRF (Hz) | −1.491 | 0.021 * | BRF (Hz) | −1.262 | 0.046 * |
| Spectral Ratio | 0.624 | 0.228 | Spectral Ratio | 0.312 | 0.483 |
| Beta (µV2) | 0.031 | 0.902 | Delta (µV2) | 0.257 | 0.479 |
| R2 = 0.687, adjusted R2 square = 0.374 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemp, A.F.; Kinnerup, M.; Johnsen, B.; Jakobsen, S.; Nahimi, A.; Gjedde, A. EEG Frequency Correlates with α2-Receptor Density in Parkinson’s Disease. Biomolecules 2024, 14, 209. https://doi.org/10.3390/biom14020209
Kemp AF, Kinnerup M, Johnsen B, Jakobsen S, Nahimi A, Gjedde A. EEG Frequency Correlates with α2-Receptor Density in Parkinson’s Disease. Biomolecules. 2024; 14(2):209. https://doi.org/10.3390/biom14020209
Chicago/Turabian StyleKemp, Adam F., Martin Kinnerup, Birger Johnsen, Steen Jakobsen, Adjmal Nahimi, and Albert Gjedde. 2024. "EEG Frequency Correlates with α2-Receptor Density in Parkinson’s Disease" Biomolecules 14, no. 2: 209. https://doi.org/10.3390/biom14020209
APA StyleKemp, A. F., Kinnerup, M., Johnsen, B., Jakobsen, S., Nahimi, A., & Gjedde, A. (2024). EEG Frequency Correlates with α2-Receptor Density in Parkinson’s Disease. Biomolecules, 14(2), 209. https://doi.org/10.3390/biom14020209







