Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
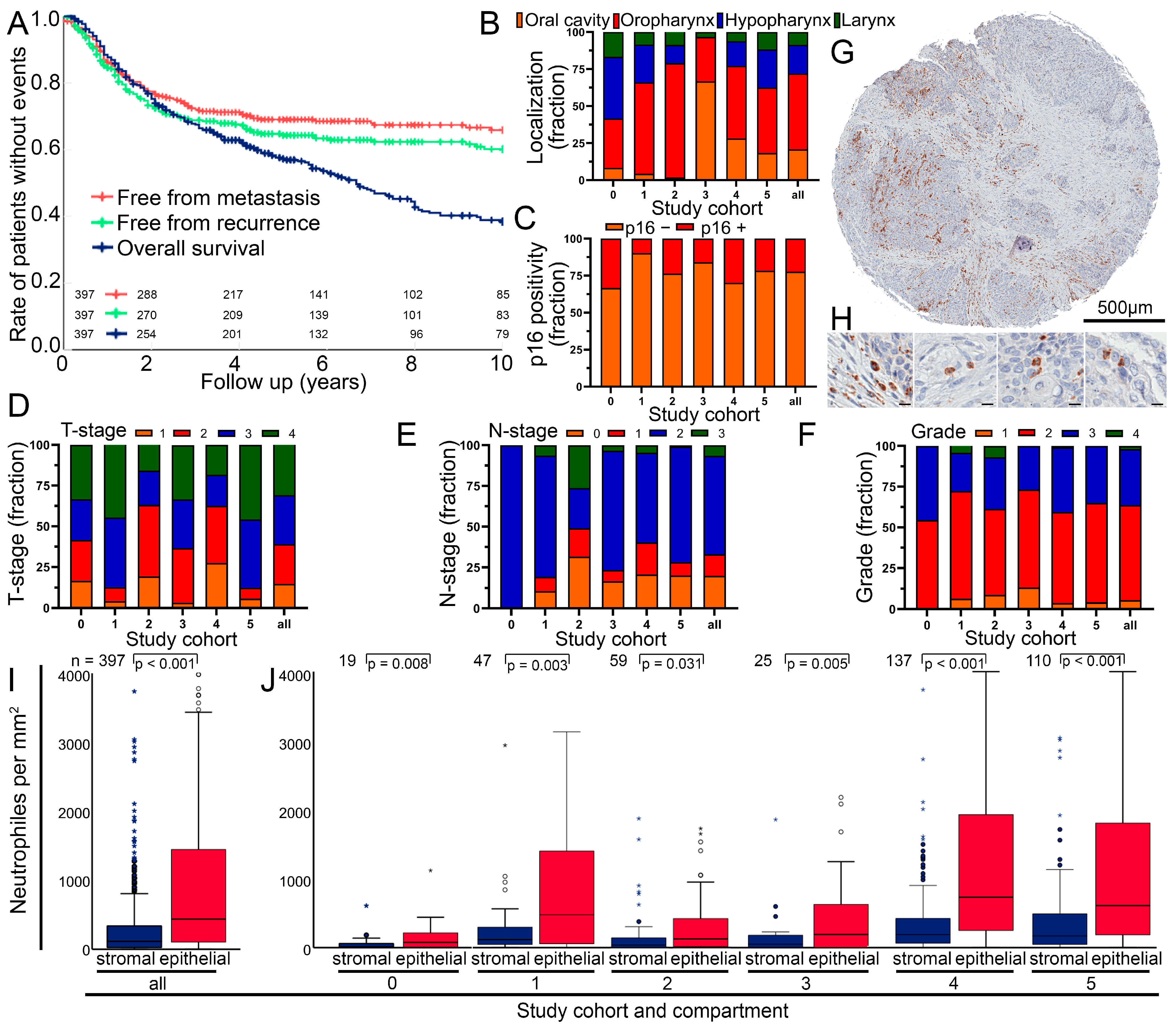
3.1. Clinical Characterization of the Head and Neck Squamous Cell Carcinoma Cohort
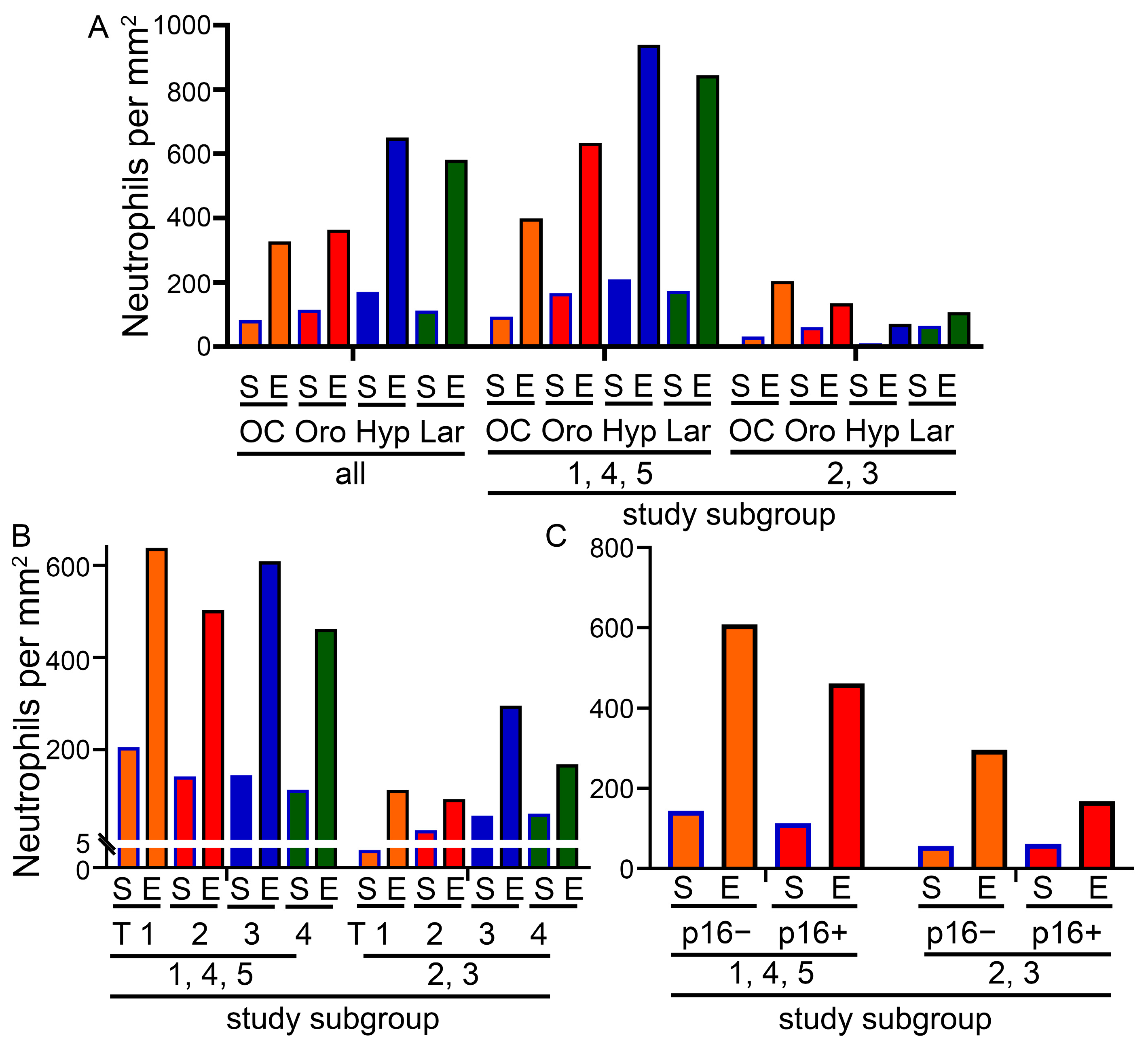
3.2. Composition of the Cohort and Cell Numbers in the Different Tumor Sides
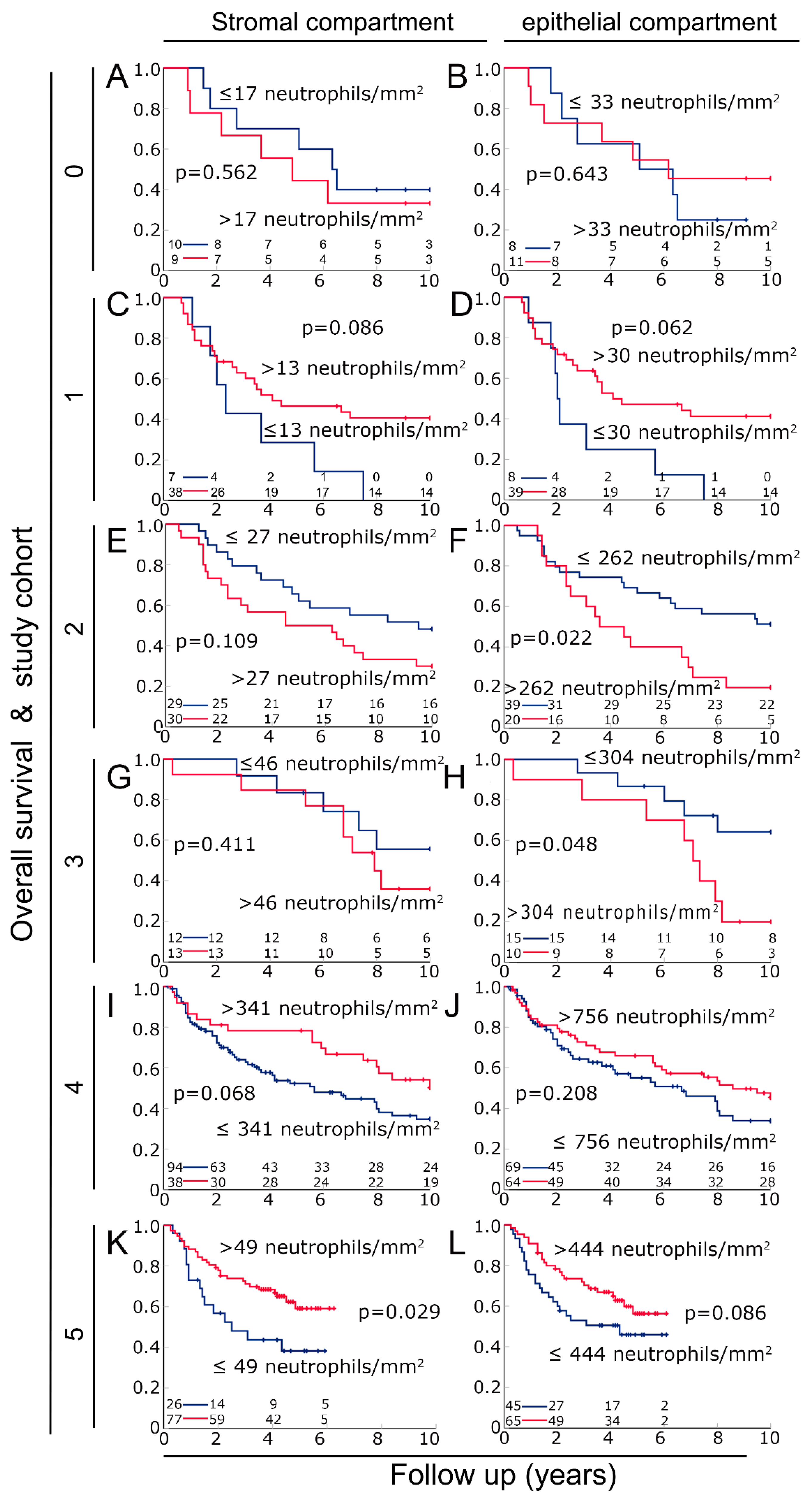
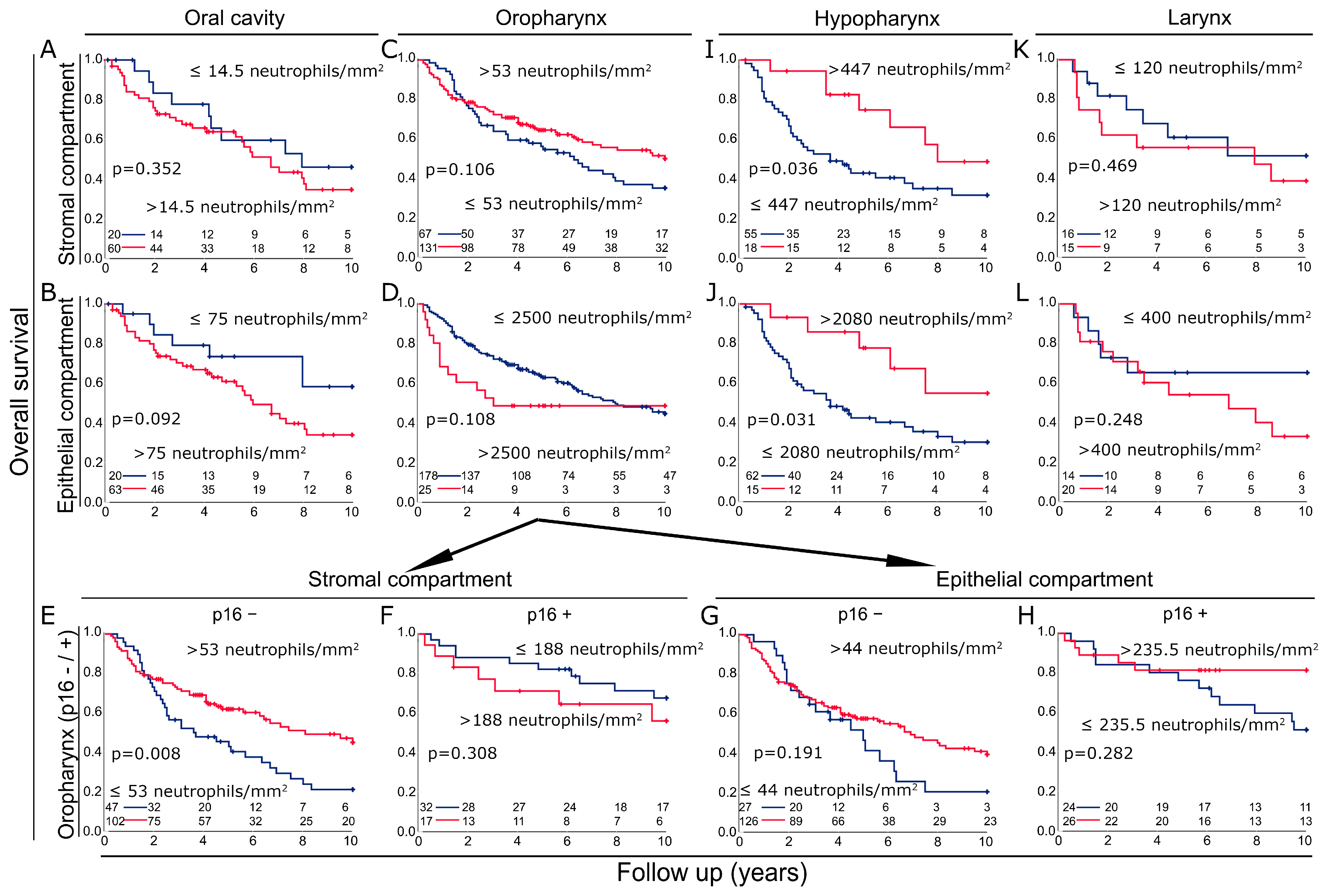
3.3. Prognosis in the Different Cohorts According to Neutrophil Density in the Stromal and the Epithelial Compartments
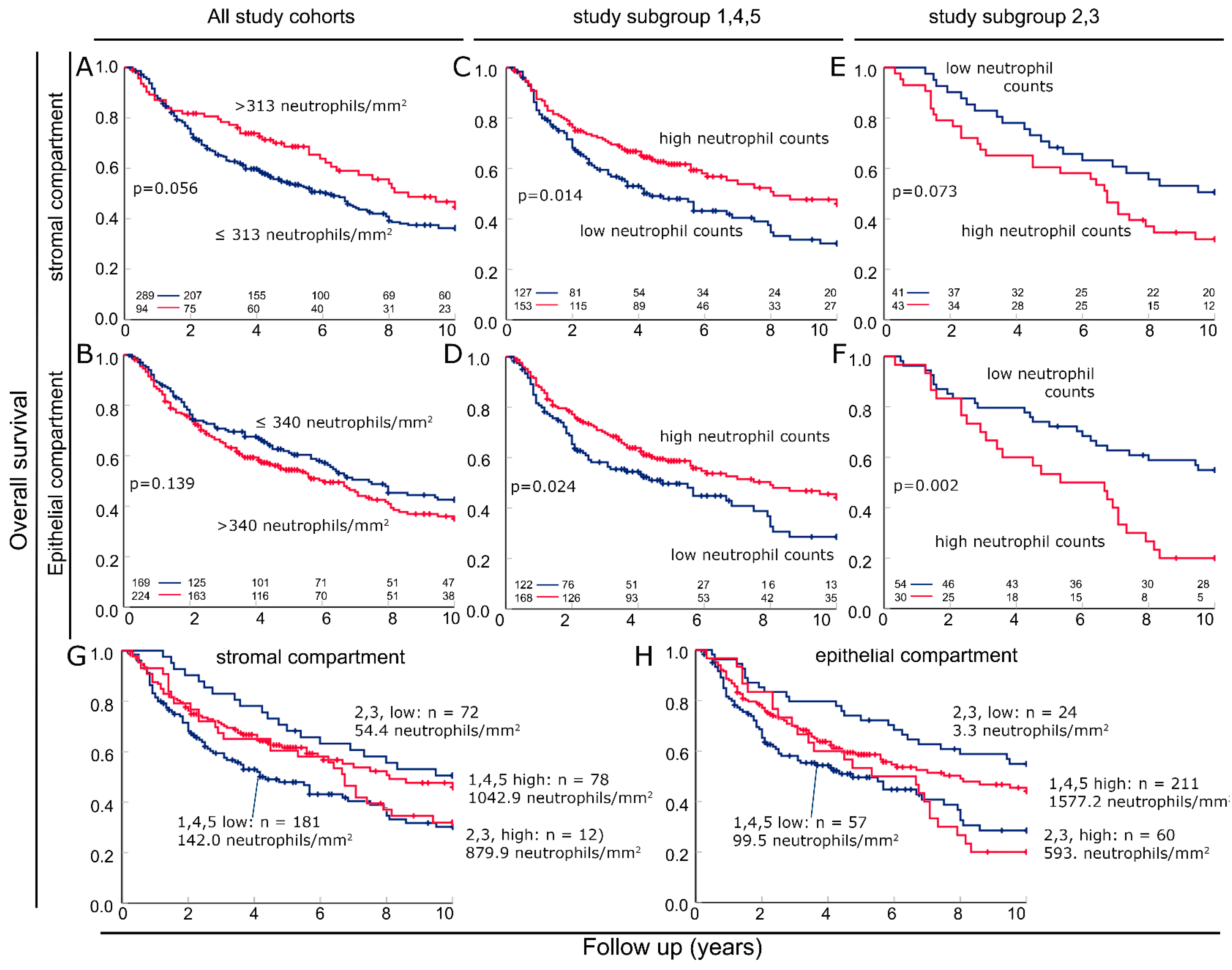
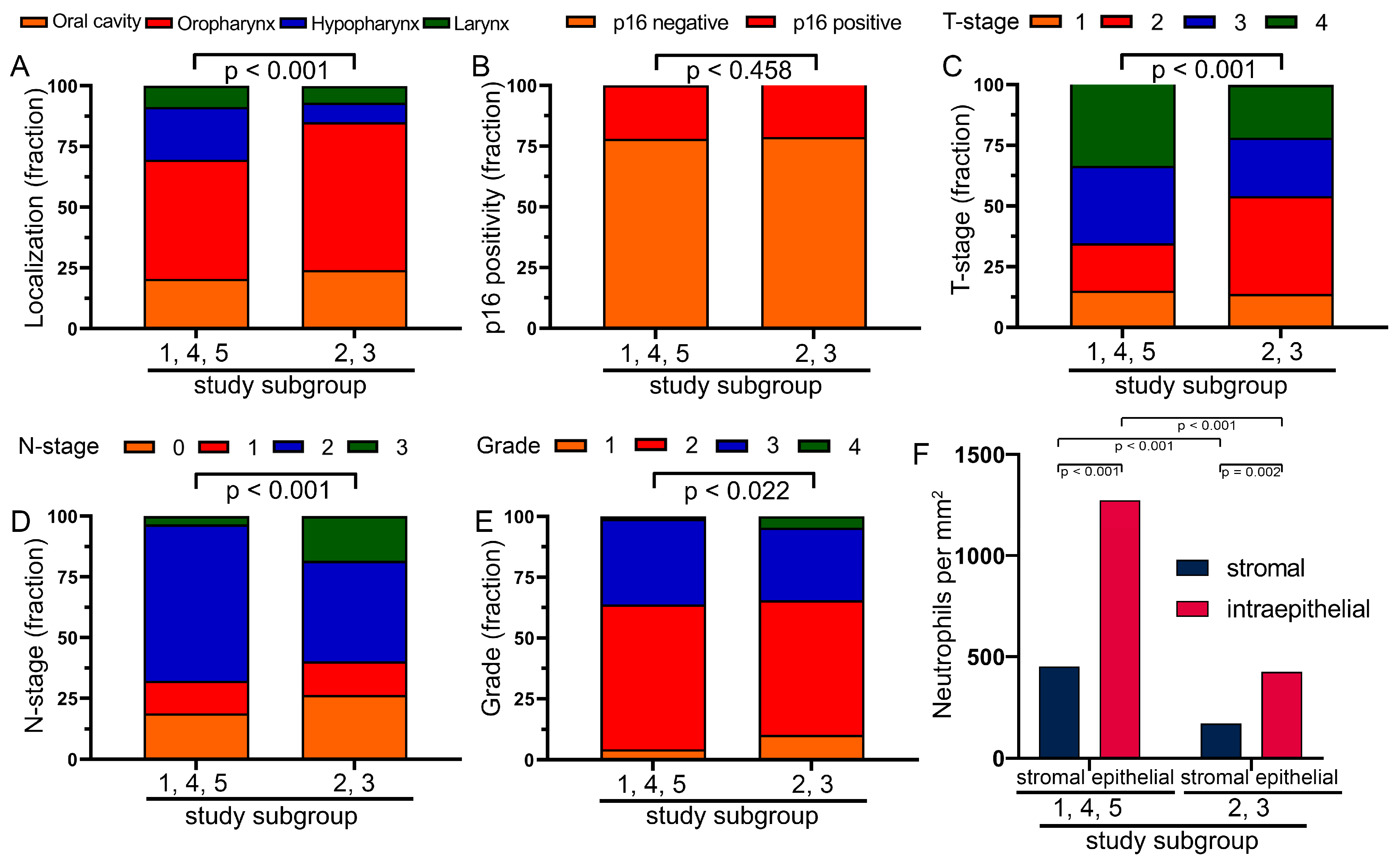
3.4. Combining Cohorts into Groups with Favorable and Unfavorable Prognostic Significance of Neutrophils
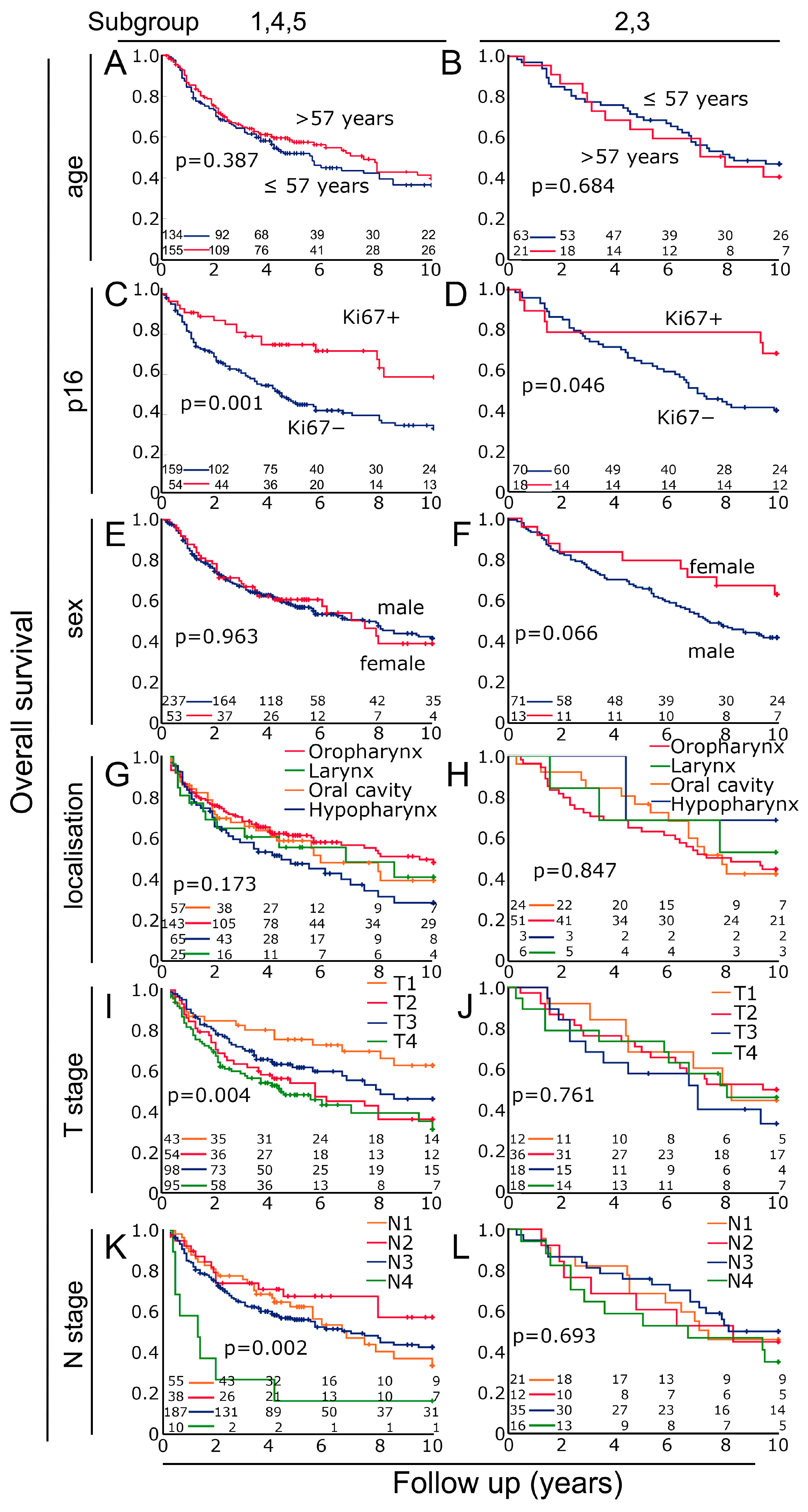
3.5. Characterization of the Two Previously Defined Subgroups
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, Y.; Shao, Y.; He, W.; Hu, W.; Xu, Y.; Chen, J.; Wu, C.; Jiang, J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: A Meta-Analysis. Cell. Physiol. Biochem. 2015, 37, 1560–1571. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mandal, R.; Senbabaoglu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, H.; Walz, K.; Buchbender, M.; Rieckmann, T.; Strasser, E.; Grottker, F.; Fietkau, R.; Frey, B.; Gaipl, U.S.; Ruckert, M. Head and neck tumor cells treated with hypofractionated irradiation die via apoptosis and are better taken up by M1-like macrophages. Strahlenther. Onkol. 2022, 198, 171–182. [Google Scholar] [CrossRef]
- Yilmaz, B.; Somay, E.; Topkan, E.; Kucuk, A.; Pehlivan, B.; Selek, U. Utility of pre-chemoradiotherapy Pan-Immune-Inflammation-Value for predicting the osteoradionecrosis rates in locally advanced nasopharyngeal cancers. Strahlenther. Onkol. 2023, 199, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.Y.; Krinsky, A.L.; Woolaver, R.A.; Wang, X.; Chen, Z.; Wang, J.H. Tumor immune microenvironment in head and neck cancers. Mol. Carcinog. 2020, 59, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zheng, X.; Gao, W.; Wang, B.; Wu, Y. Tumor microenvironment and immune-related therapies of head and neck squamous cell carcinoma. Mol. Ther. Oncolytics 2021, 20, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Echarti, A.; Hecht, M.; Buttner-Herold, M.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. CD8+ and Regulatory T cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer. Cancers 2019, 11, 1398. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef]
- Wondergem, N.E.; Nauta, I.H.; Muijlwijk, T.; Leemans, C.R.; van de Ven, R. The Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: On Subsets and Subsites. Curr. Oncol. Rep. 2020, 22, 81. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Fioretti, F.; Fradelizi, D.; Stoppacciaro, A.; Ramponi, S.; Ruco, L.; Minty, A.; Sozzani, S.; Garlanda, C.; Vecchi, A.; Mantovani, A. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: Perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J. Immunol. 1998, 161, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef] [PubMed]
- Szulc-Kielbik, I.; Klink, M. Polymorphonuclear Neutrophils and Tumors: Friend or Foe? Exp. Suppl. 2022, 113, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.Y.; Yi, H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Invest. 2010, 120, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Bankfalvi, A.; Gu, X.; Eberhardt, W.E.; Zeidler, R.; Lang, S.; Brandau, S. Neutrophils Activate Tumoral CORTACTIN to Enhance Progression of Orohypopharynx Carcinoma. Front. Immunol. 2013, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hutte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, M.A.; Glogauer, J.E.; Glogauer, M. Neutrophils and oral squamous cell carcinoma: Lessons learned and future directions. J. Leukoc. Biol. 2014, 96, 695–702. [Google Scholar] [CrossRef]
- Sounbuli, K.; Mironova, N.; Alekseeva, L. Diverse Neutrophil Functions in Cancer and Promising Neutrophil-Based Cancer Therapies. Int. J. Mol. Sci. 2022, 23, 15827. [Google Scholar] [CrossRef]
- Hartan, R.; Schnellhardt, S.; Buttner-Herold, M.; Daniel, C.; Hartmann, A.; Fietkau, R.; Distel, L. The Prognostic and Predictive Significance of Tumor-Infiltrating Memory T Cells Is Reversed in High-Risk HNSCC. Cells 2022, 11, 1960. [Google Scholar] [CrossRef]
- Tabachnyk, M.; Distel, L.V.; Buttner, M.; Grabenbauer, G.G.; Nkenke, E.; Fietkau, R.; Lubgan, D. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral. Oncol. 2012, 48, 594–601. [Google Scholar] [CrossRef]
- Rudolf, J.; Buttner-Herold, M.; Erlenbach-Wunsch, K.; Posselt, R.; Jessberger, J.; Haderlein, M.; Hecht, M.; Hartmann, A.; Fietkau, R.; Distel, L. Regulatory T cells and cytotoxic T cells close to the epithelial-stromal interface are associated with a favorable prognosis. Oncoimmunology 2020, 9, 1746149. [Google Scholar] [CrossRef]
- Garley, M.; Dziemianczyk-Pakiela, D.; Ratajczak-Wrona, W.; Pryczynicz, A.; Nowak, K.; Lazarczyk, B.; Jablonska, E. NETs biomarkers in saliva and serum OSCC patients: One hypothesis, two conclusions. Adv. Med. Sci. 2022, 67, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.U.; Varricchi, G.; Galdiero, M.R. Neutrophil extracellular traps in cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef] [PubMed]
| Variable | Cohort n (%) | |
|---|---|---|
| Sex: | Male | 330 (83%) |
| Female | 67 (17%) | |
| Age: | Median (range) | 57 (27–81) |
| Primary tumour: | T Stage 1 | 59 (14.9%) |
| 2 | 96 (24.2%) | |
| 3 | 119 (30.0%) | |
| 4 | 123 (31.0%) | |
| Lymph node status: | N Stage 0 | 76 (19.1%) |
| 1 | 52 (13.1%) | |
| 2 | 240 (60.5%) | |
| 3 | 26 (6.5%) | |
| Metastasis: | M Stage 0 | 392 (98.7%) |
| 1 | 5 (1.3%) | |
| Grade: | Grading 1 | 22 (5.5%) |
| 2 | 231 (58.2%) | |
| 3 | 136 (34.3%) | |
| 4 | 8 (2%) | |
| Stage: | UICC Stage I | 12 (3.0%) |
| II | 26 (6.5%) | |
| III | 47 (11.8%) | |
| IV | 293 (73.8%) | |
| unknown | 19 (4.8%) | |
| Location: | Oral Cavity | 83 (21%) |
| Oropharynx | 203 (51%) | |
| Oropharynx p16 pos. | 50 (13%) | |
| Oropharynx p16 neg. | 153 (38%) | |
| Hypopharynx | 77 (19%) | |
| Larynx | 34 (9%) | |
| Treatment: | definitive | 47 (11.8%) |
| adjuvant | 325 (81.9%) | |
| neoadjuvant | 25 (6.3%) | |
| Tumor: | p16− | 232 (78.1%) |
| p16+ | 65 (21.9%) |
| Study Cohort | Study From–Until | Radiation Therapy | n (397) | Median Follow-Up (Years) |
|---|---|---|---|---|
| 0 | 1998–2003 | adjuvant | 19 | 5.6 |
| 1 | 1998–2005 | definitive | 47 | 3.4 |
| 2 | 1984–1998 | adjuvant | 59 | 7.4 |
| 3 | 1997–2004 | neoadjuvant | 25 | 7.9 |
| 4 | 2002–2010 | adjuvant | 137 | 4.5 |
| 5 | 2010–2015 | adjuvant | 110 | 3.9 |
| all | 1998–2015 | --- | 397 | 4.3 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% C.I. | p | Hazard Ratio | 95% C.I. | p |
| Age, years (younger 57 years [n = 80] vs. older 57 years [n = 73]) | 1.255 | 0.799–1.973 | 0.324 | --- | --- | --- |
| Sex (male [n = 125] vs. female [n = 28]) | 0.763 | 0.438–1.33 | 0.341 | --- | --- | --- |
| p16 (negative [n = 118] vs. positive [n = 35]) | 2.093 | 1.13–3.876 | 0.019 | 2.133 | 1.169–3.89 | 0.013 |
| T category (T1/T2 [n = 100] vs. T3/T4 [n = 53]) | 0.535 | 0.329–0.868 | 0.011 | 0.557 | 0.354–0.875 | 0.011 |
| N category (N0 [n = 20] vs. N+ [n = 133]) | 1.107 | 0.596–2.056 | 0.748 | --- | --- | --- |
| Grading (1 + 2 [n = 64] vs. 3 + 4 [n = 89]) | 0.763 | 0.471–1.235 | 0.270 | 0.800 | 0.505–1.265 | 0.339 |
| Neutrophils epithelial (low [n = 63] vs. high [n = 90]) | 1.528 | 0.855–2.731 | 0.152 | 2.011 | 1.29–3.135 | 0.002 |
| Neutrophils stomal (low [n = 72] vs. high [n = 81]) | 1.415 | 0.783–2.558 | 0.250 | 1.713 | 0.978–3.001 | 0.060 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% C.I. | p | Hazard Ratio | 95% C.I. | p |
| Age, years (younger 57 years [n = 55] vs. older 57 years [n = 18]) | 1.054 | 0.498–2.23 | 0.891 | --- | --- | --- |
| Sex (male [n = 61] vs. female [n = 12]) | 2.608 | 0.737–9.232 | 0.137 | 2.929 | 0.894–9.595 | 0.076 |
| p16 (negative [n = 58] vs. positive [n = 15]) | 1.423 | 0.475–4.262 | 0.529 | 1.339 | 0.472–3.793 | 0.583 |
| T category (T1/T2 [n = 59] vs. T3/T4 [n = 14]) | 1.285 | 0.548–3.014 | 0.564 | 0.819 | 0.362–1.854 | 0.632 |
| N category (N0 [n = 17] vs. N+ [n = 56]) | 0.857 | 0.399–1.842 | 0.693 | --- | --- | --- |
| Grading (1 + 2 [n = 49] vs. 3 + 4 [n = 24]) | 0.959 | 0.481–1.91 | 0.905 | --- | --- | --- |
| Neutrophils epithelial (low [n = 46] vs. high [n = 27]) | 0.532 | 0.282–1.003 | 0.051 | 0.500 | 0.27–0.926 | 0.027 |
| Neutrophils stomal (low [n = 33] vs. high [n = 40]) | 0.537 | 0.27–1.069 | 0.077 | 0.530 | 0.281–1 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunkhorst, H.; Schnellhardt, S.; Büttner-Herold, M.; Daniel, C.; Fietkau, R.; Distel, L.V. Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis. Biomolecules 2024, 14, 205. https://doi.org/10.3390/biom14020205
Brunkhorst H, Schnellhardt S, Büttner-Herold M, Daniel C, Fietkau R, Distel LV. Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis. Biomolecules. 2024; 14(2):205. https://doi.org/10.3390/biom14020205
Chicago/Turabian StyleBrunkhorst, Hendrik, Sören Schnellhardt, Maike Büttner-Herold, Christoph Daniel, Rainer Fietkau, and Luitpold V. Distel. 2024. "Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis" Biomolecules 14, no. 2: 205. https://doi.org/10.3390/biom14020205
APA StyleBrunkhorst, H., Schnellhardt, S., Büttner-Herold, M., Daniel, C., Fietkau, R., & Distel, L. V. (2024). Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis. Biomolecules, 14(2), 205. https://doi.org/10.3390/biom14020205


_Kwok.png)