Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CMG Fractionation and Isolation
2.2.1. Polymer Removal Procedure
2.2.2. Pilot Extraction of the NF and AF of Triterpenes of CMG
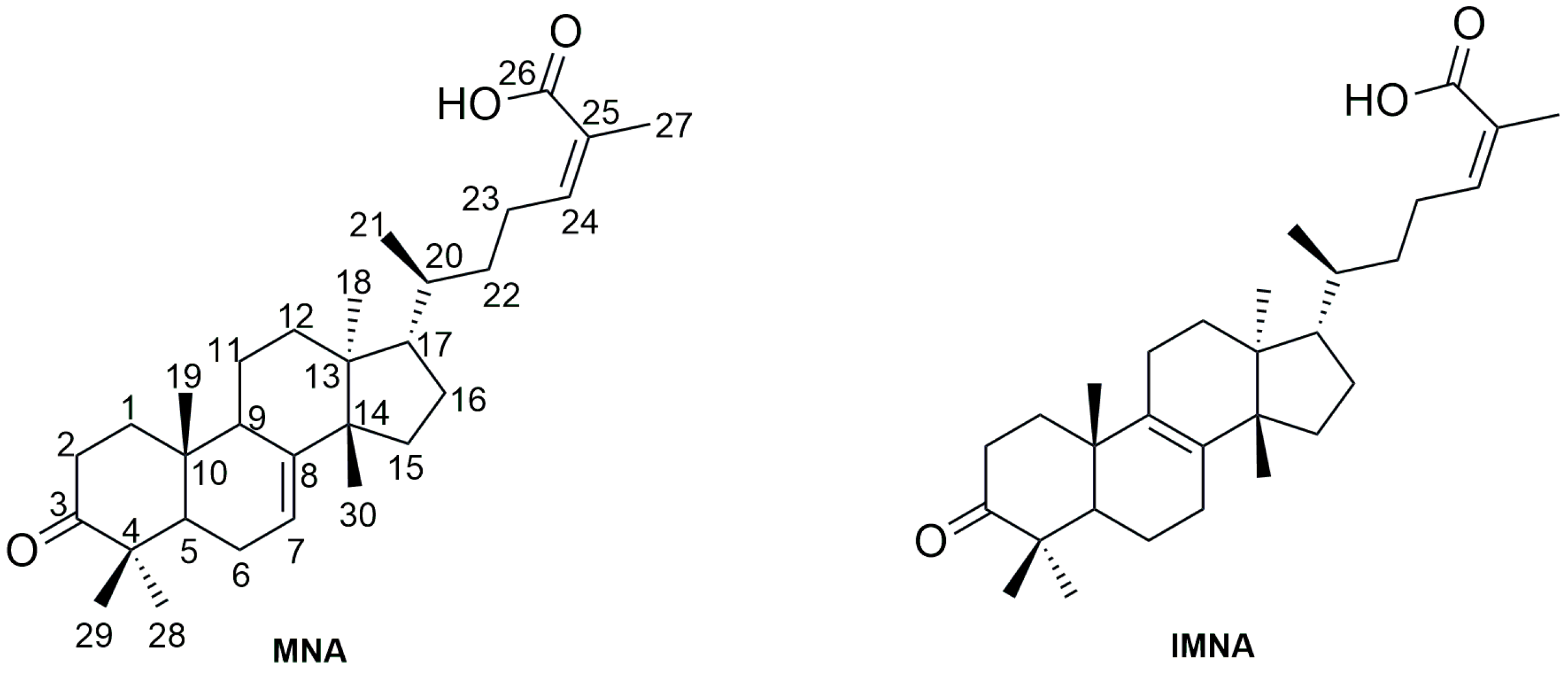
2.2.3. Isolation of the Major Triterpenic Acids
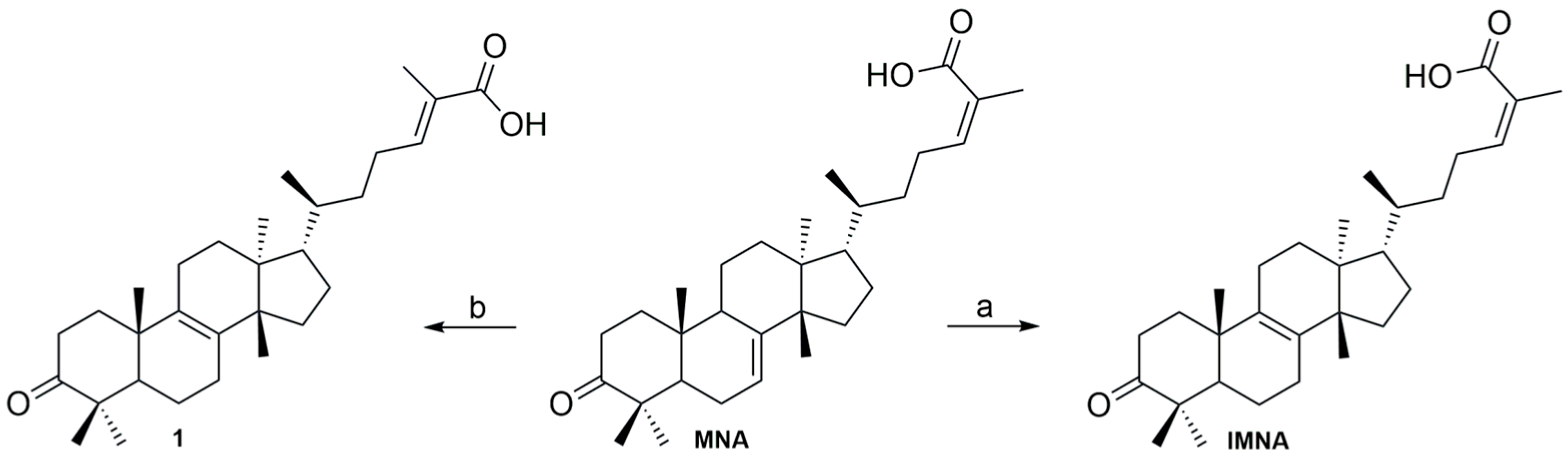
2.3. Acid-Catalyzed Synthesis of IMNA
2.3.1. Synthesis of IMNA from MNA
2.3.2. Synthesis of 24E-Isomasticadienonic Acid (1) from MNA
2.3.3. Synthesis of IMNA from MNA/IMNA Mixture
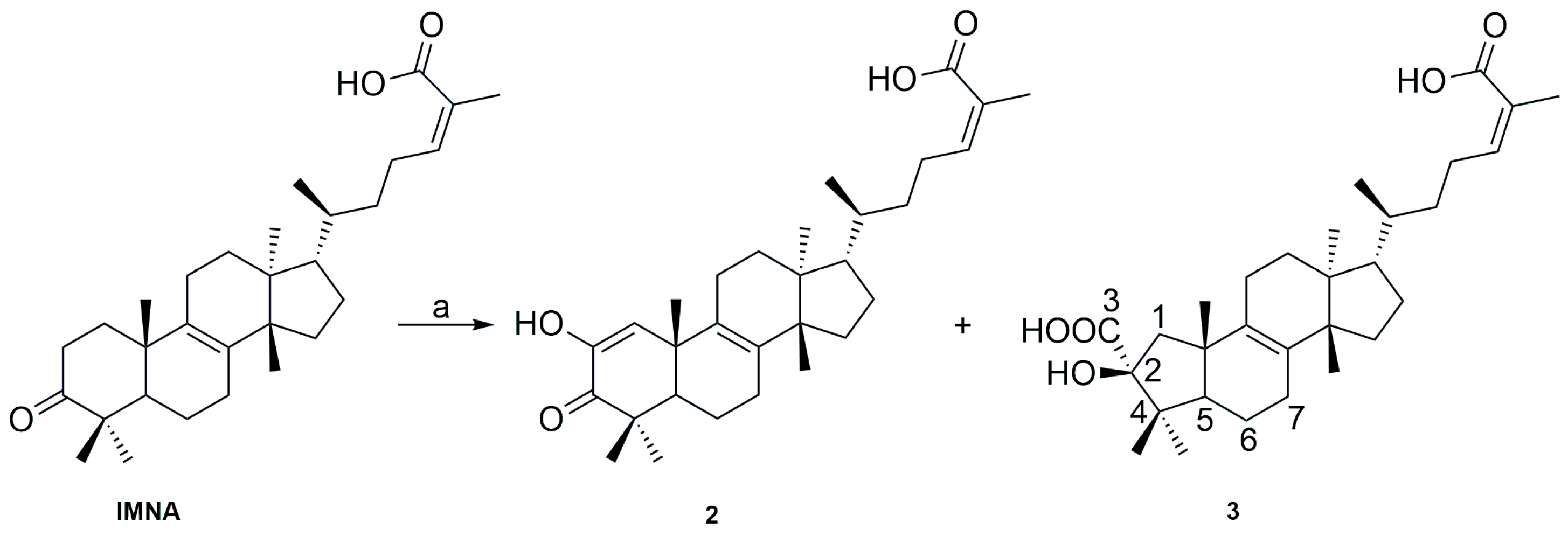
2.4. Basic Catalyzed Synthesis of IMNA Analogs
2.5. Biological Assays
2.5.1. Cell Line and Cell Culture Conditions
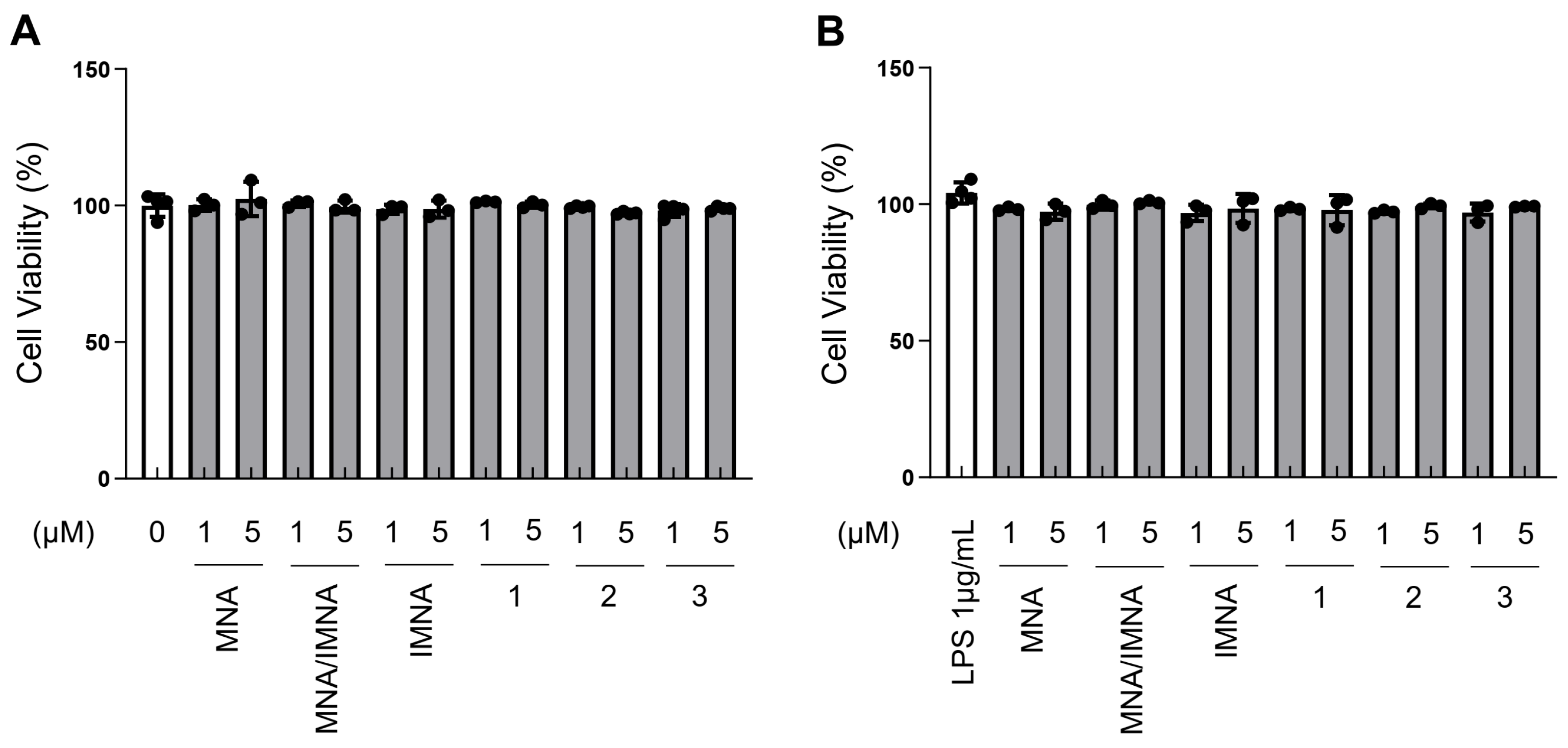
2.5.2. Cell Survival Assay
2.5.3. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (Q-RT-PCR) Analysis
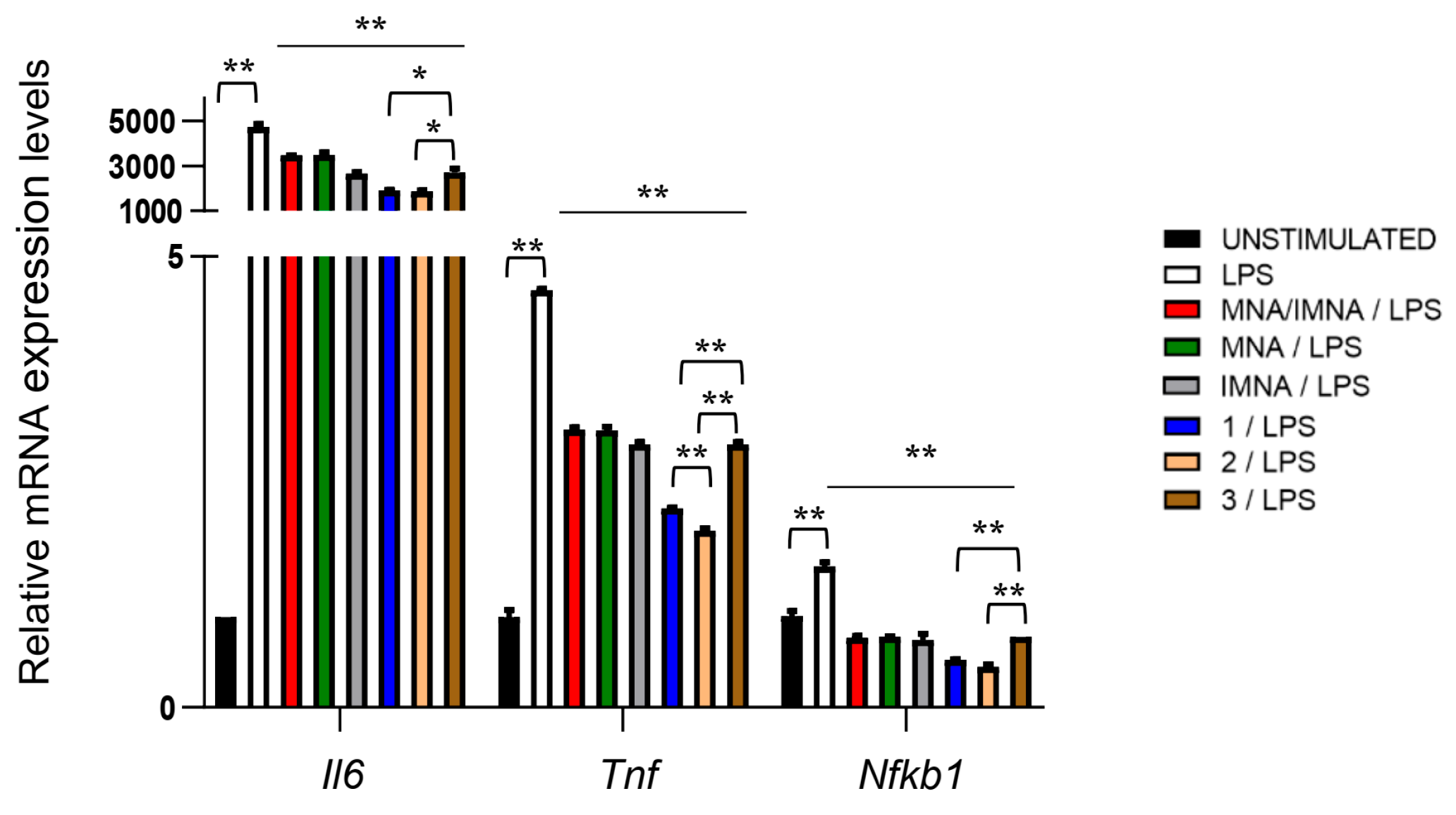
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, C. Points of Control in Inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in Lps-Induced Raw264.7 Macrophages by Inhibiting Foxo3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Gamble, C.; McIntosh, K.; Scott, R.; Ho, K.H.; Plevin, R.; Paul, A. Inhibitory Kappa B Kinases as Targets for Pharmacological Regulation. Br. J. Pharmacol. 2012, 165, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Makarov, S. NF-κB as a Therapeutic Target in Chronic Inflammation: Recent Advances. Mol. Med. Today 2000, 6, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, X.; Gao, Y.; Qi, R.; Cai, R.; Qi, Y. Isodeoxyelephantopin, a Sesquiterpene Lactone from Elephantopus Scaber Linn., Inhibits pro-Inflammatory Mediators’ Production through Both NF-κB and AP-1 Pathways in LPS-Activated Macrophages. Int. Immunopharmacol. 2020, 84, 106528. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.-L. Chios Gum Mastic: A Review of Its Biological Activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Ierapetritis, D. The Geography of the Chios Mastic Trade from the 17th through to the 19th Century. Ethnobot. Res. Appl. 2010, 8, 153–167. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and Pharmacology of Chios Mastic Gum (Pistacia lentiscus var. Chia, Anacardiaceae): A Review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on Pistacia lentiscus L., Resin (Mastix); European Medicine Agency: London, UK, 2015. [Google Scholar]
- Liu, W.; Gao, J.; Li, M.; Aisa, H.A.; Yuan, T. Tirucallane Triterpenoids from the Mastic (Pistacia Lentiscus) and Their Anti-Inflammatory and Cytotoxic Activities. Phytochemistry 2021, 182, 112596. [Google Scholar] [CrossRef]
- Giner-Larza, E.M.; Máez, S.; Recio, M.C.; Giner, R.M.; Prieto, J.M.; Cerdá-Nicolás, M.; Ríos, J.L. Oleanonic Acid, a 3-Oxotriterpene from Pistacia, Inhibits Leukotriene Synthesis and Has Anti-Inflammatory Activity. Eur. J. Pharmacol. 2001, 428, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS Analysis of Penta- and Tetra-Cyclic Triterpenes from Resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS Analysis of Penta- and Tetra-Cyclic Triterpenes from Resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Stefi, A.L.; Nikou, T.; Vassilacopoulou, D.; Skaltsounis, L.-A.; Halabalaki, M.; Christodoulakis, N.S. Structure and Organization of the Secretion Apparatus of the Mastic Tree (Pistacia lentiscus L.) and LC–HRMS Analysis of Leaf Extracts. Planta 2021, 253, 70. [Google Scholar] [CrossRef]
- Mulholland, D.; Nair, J.J. Triterpenoids from Dysoxylum pettigrewianum. Phytochemistry 1994, 37, 1409–1411. [Google Scholar] [CrossRef]
- Olivera Ortega, A.G.; Soto Hernández, M.; Martínez Vázquez, M.; Salgado, T.T.; Solares Arenas, F. Phytochemical Study of Cuachalalate (Amphiptherygium adstringens, Schiede Ex Schlecht). J. Ethnopharmacol. 1999, 68, 109–113. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of Oleanolic Acid and Ursolic Acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Prodea, A.; Racoviceanu, R.; Mioc, A.; Ghiulai, R.; Milan, A.; Voicu, M.; Mardale, G.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). Int. J. Mol. Sci. 2022, 23, 8896. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monroy, M.B.; Jacobo-Herrera, N.J.; Zentella-Dehesa, A.; Hernández-Téllez, B.; Martínez-Vázquez, M. Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo. Molecules 2017, 22, 1479. [Google Scholar] [CrossRef]
- Piñón-Zárate, G.; Reyes-Riquelme, F.; Sánchez-Monroy, M.B.; Velasco-Torrez, M.; Martínez-Vázquez, M.; Cárdenas-Monroy, C.A.; Hernandez-Téllez, B.; Jarquín-Yáñez, K.; Herrera-Enríquez, M.Á.; Castell-Rodríguez, A.E. Immunomodulatory Properties of Masticadienonic Acid and 3α-Hydroxy Masticadienoic Acid in Dendritic Cells. Molecules 2022, 27, 1451. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In Vitro and in Vivo Activities of Chios Mastic Gum Extracts and Constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Trader, D.J.; Carlson, E.E. Chemoselective Enrichment as a Tool to Increase Access to Bioactive Natural Products: Case Study Borrelidin. Bioorg. Med. Chem. Lett. 2015, 25, 4767–4769. [Google Scholar] [CrossRef]
- Hara, T.; Tainosyo, A.; Kawakami, T.; Aimoto, S.; Murata, M. Peptide Purification Using the Chemoselective Reaction between N-(Methoxy)Glycine and Isothiocyanato-Functionalized Resin. J. Pept. Sci. 2016, 22, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Trader, D.J.; Carlson, E.E. Chemoselective Hydroxyl Group Transformation: An Elusive Target. Mol. Biosyst. 2012, 8, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.; Cook, N.; Liang, A.Y.; Desai, U.R.; Sidorov, V. Chemoselective Precipitation of Lactose from a Lactose/Sucrose Mixture: Proof of Concept for a New Separation Methodology. Supramol. Chem. 2010, 22, 751–757. [Google Scholar] [CrossRef]
- Odendaal, A.Y.; Trader, D.J.; Carlson, E.E. Chemoselective Enrichment for Natural Products Discovery. Chem. Sci. 2011, 2, 760–764. [Google Scholar] [CrossRef]
- Hubert, A.J.; Reimlinger, H. The Isomerization of Olefins Part II. Thermal and Catalytic Lsomerization of Olefins Using Acids, Metals, Metal Complexes, or Boron Compounds as Catalysts. Synthesis 1970, 1970, 405–430. [Google Scholar] [CrossRef]
- Hubert, A.; Reimlinger, H. The Lsomerization of Olefins Part I. Base-Catalysed Lsomerization of Olefins. Synthesis 2002, 1969, 97–112. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Sun, T.; Lv, Z.; Zhan, Z.; Yin, G.; Chen, Z. Lewis Acid Promoted Double Bond Migration in O-Allyl to Z-Products by Ru-H Complexes. Mol. Catal. 2019, 469, 10–17. [Google Scholar] [CrossRef]
- Kacharov, A.D.; Yemets, S.V.; Nemykin, V.N.; Kacharova, L.M.; Fokin, A.A.; Krasutsky, P.A. Stereoselectivity of A-Ring Contraction for 3-Oxotriterpenoids. RSC Adv. 2013, 3, 19057–19063. [Google Scholar] [CrossRef]
- Cui, H.; Li, X.; An, X.R.; Liu, W.; Yuan, T. Masticadienonic Acid from Chios Mastic Gum Mitigates Colitis in Mice via Modulating Inflammatory Response, Gut Barrier Integrity and Microbiota. Phytomedicine 2023, 108, 154518. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Bian, M.; Gong, G.; Bai, C.; Liu, C.; Wei, C.; Quan, Z.S.; Du, H.H. Synthesis and Evaluation of Bakuchiol Derivatives as Potent Anti-Inflammatory Agents in Vitro and in Vivo. J. Nat. Prod. 2022, 85, 15–24. [Google Scholar] [CrossRef]
- Brieudes, V.; Mikropoulou, E.V.; Kallergis, E.; Kaliora, A.C.; Papada, E.; Gkiouvetidis, P.; Angelis, A.; Halabalaki, M. Development, Validation and Application of a UHPLC-MS Method for the Quantification of Chios Mastic Gum Triterpenoids in Human Plasma. Planta Med. 2021, 87, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, M.; Angelis, A.; Laskari, V.; Termentzi, A.; Hubert, J.; Fokialakis, N.; Aligiannis, N.; Renault, J.; Skaltsounis, A. Separation of Neutral and Acidic Triterpenes from Mastic Gum Using Centrifugal Partition Chromatography (CPC) and Supercritical Fluid Chromatography (SFC-CO2). Planta Med. 2015, 81, 62. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamou, P.; Gianniou, D.D.; Trougakos, I.P.; Mitakou, S.; Halabalaki, M.; Kostakis, I.K.; Skaltsounis, A.-L. Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues. Biomolecules 2024, 14, 1618. https://doi.org/10.3390/biom14121618
Stamou P, Gianniou DD, Trougakos IP, Mitakou S, Halabalaki M, Kostakis IK, Skaltsounis A-L. Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues. Biomolecules. 2024; 14(12):1618. https://doi.org/10.3390/biom14121618
Chicago/Turabian StyleStamou, Panagiota, Despoina D. Gianniou, Ioannis P. Trougakos, Sofia Mitakou, Maria Halabalaki, Ioannis K. Kostakis, and Alexios-Leandros Skaltsounis. 2024. "Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues" Biomolecules 14, no. 12: 1618. https://doi.org/10.3390/biom14121618
APA StyleStamou, P., Gianniou, D. D., Trougakos, I. P., Mitakou, S., Halabalaki, M., Kostakis, I. K., & Skaltsounis, A.-L. (2024). Anti-Inflammatory Activity of the Major Triterpenic Acids of Chios Mastic Gum and Their Semi-Synthetic Analogues. Biomolecules, 14(12), 1618. https://doi.org/10.3390/biom14121618







