Abstract
Adropin, a secreted peptide hormone identified in 2008, plays a significant role in regulating energy homeostasis, glucose metabolism, and lipid metabolism. Its expression is linked to dietary macronutrient intake and is influenced by metabolic syndrome, obesity, diabetes, and cardiovascular diseases. Emerging evidence suggests that adropin might be a biomarker for various conditions, including metabolic syndrome, coronary artery disease, and hypertensive disorders complicating pregnancy. In cerebrovascular diseases, adropin demonstrates protective effects by reducing blood–brain barrier permeability, brain edema, and infarct size while improving cognitive and sensorimotor functions in ischemic stroke models. The protective effects of adropin extend to preventing endothelial damage, promoting angiogenesis, and mitigating inflammation, making it a promising therapeutic target for cardiovascular and neurodegenerative diseases. This review provides a comprehensive overview of adropin’s multifaceted roles in physiological and pathological conditions, as well as our recent work demonstrating adropin’s role in subarachnoid hemorrhage-mediated neural injury and delayed cerebral infarction.
1. Introduction
In 2008, Kumar et al. [1] identified adropin as a secreted factor that links dietary macronutrient intake with energy homeostasis and lipid metabolism. Adropin (derived from the Latin roots “aduro” [to set fire to] and “pinquis” [fats or oils]) is encoded by the Energy Homeostasis Associated gene (gene symbol: Enho) [1]. In lean mice, high-fat diets rapidly increased liver Enho expression, while fasting led to a decrease compared to controls. However, in cases of diet-induced obesity (DIO) or genetically induced obesity, liver Enho expression declined, linking it to metabolic disorders in obesity. For mice with DIO, both transgenic overexpression and systemic treatment with adropin reduced liver fat accumulation and insulin resistance independently of reducing food intake or adiposity. Adropin influenced the expression of lipogenic genes in white adipose tissue. Thus, adropin appears to be a critical factor in managing glucose and lipid balance, protecting against metabolic syndrome related to obesity [1].
Metabolic syndrome is a cluster of disorders that increase the risk of atherosclerotic cardiovascular disease, such as heart attacks, strokes, peripheral vascular disease, insulin resistance, and type II diabetes. It is characterized by central obesity, insulin resistance, hypertension, and atherogenic dyslipidemia [2]. Currently, cardiovascular disease is identified as a major cause of mortality around the world, and this situation is estimated to remain for many years to come, thus bringing a considerable burden to the world’s health resources [3]. Endothelial dysfunction is recommended as an independent risk factor for cardiovascular disorders [4]. Endothelial cell (EC) homeostasis is maintained, in part, through the synthesis of nitric oxide (NO) from the precursor L-arginine by the enzyme endothelial NO synthase (eNOS). Aside from exerting several critical anti-inflammatory, antithrombotic, and antiatherosclerotic roles within blood vessels, NO also promotes postnatal angiogenesis and reparative vasculogenesis. Furthermore, there is good evidence that NO bioavailability serves an important role in metabolic regulation and insulin sensitivity [5,6,7]. Adropin protects endothelial cells (human umbilical vein and coronary artery endothelial cells) by stimulating the phosphorylation of eNOS at Ser1177 through the VEGFR2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal-regulated kinase 1/2 pathways in these cells [8], and also promote angiogenesis through the activation of VEGFR2 signaling pathways (Figure 1) [9].
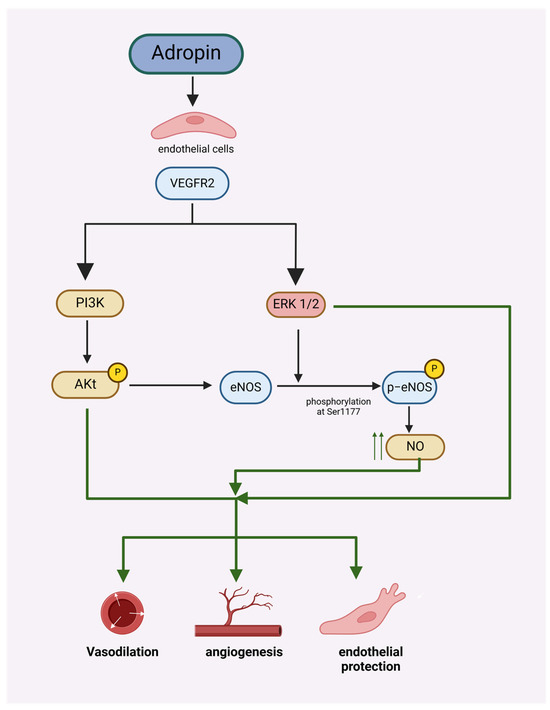
Figure 1.
Adropin protects endothelial cells and promotes angiogenesis by stimulating the phosphorylation of eNOS at Ser1177 through the VEGFR2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal-regulated kinase 1/2 pathway.
Adropin is widely expressed and has been detected in various tissues of rats, including the brain (vascular area, pia mater, neuroglial cell, and neurons), cerebellum (neuroglial cells, Purkinje cells, vascular areas, and granular layer), kidneys (glomerulus, peritubular interstitial cells, and peritubular capillary endothelial cells), heart (endocardium, myocardium, and epicardium), liver (sinusoidal cells), and pancreas (serous acini) [10]. In nonhuman primates, Enho expression is primarily found in the central nervous system, especially in the amygdala and hypothalamus, with lower levels in peripheral tissues such as the liver, kidney, lung, and adrenal glands [11]. Low plasma adropin levels were correlated with higher fasting glucose, plasma leptin, and apolipoprotein C-III levels, and during high-sugar diet consumption, lower adropin predicted greater weight gain and metabolic issues in nonhuman primates [11]. In humans, circulating adropin levels are correlated with indicators of metabolic homeostasis, including lipoprotein metabolism and insulin resistance [12].
Adropin gained attention not only as a potential biomarker for metabolic and cardiovascular conditions but also as a therapeutic agent due to its role in regulating energy homeostasis and endothelial function. In this review, we aim to explore the roles of adropin in metabolic syndrome, cerebrovascular and cardiovascular diseases, as well as our recent work demonstrating adropin’s role in subarachnoid hemorrhage-mediated neural injury and delayed cerebral infarction.
1.1. Sex Hormones and Adropin
Adropin is widely expressed, suggesting pleiotropic roles and complex regulation [1]. Given that adropin was initially studied as a “hepatokine” [1], many studies have focused on the regulation and role of adropin expressed in the liver. In a study involving C57BL/6J mice, menopause induced through ovariectomy (OVX) reduced hepatic Enho mRNA expression levels [13]. OVX is associated with visceral adiposity, hepatic steatosis, and increased risk for cardiovascular disease, which is alleviated upon adropin treatment, suggesting that adropin can improve metabolic homeostasis in the context of hypogonadism [13]. Additionally, an increase in Enho expression and elevated adropin levels in response to 17β-estradiol (E2), an estrogen steroid hormone, were observed, correlating with improved metabolic outcomes [13]. Estrogen receptor alpha (ERα) inhibits fatty acid synthesis in female mice under a high-fat diet (HFD) but not in male mice or those lacking ERα in the liver, playing a sex-specific role in regulating hepatic fat metabolism [14]. Enho expression in the liver follows a similar pattern and increases in female mice under HFD but not in male mice or those lacking the ERα receptor [15]. This study identified adropin as the hepatokine that better predicts sex-specific and hepatic ERα-dependent regulation of hepatic metabolism under different hormonal and nutritional conditions [15].
In other studies on polycystic ovary syndrome (PCOS), a relationship between sex hormones and adropin was demonstrated. PCOS, which can cause hormonal, metabolic, and reproductive abnormalities, is linked to an elevated risk of type 2 DM (T2DM) and cardiovascular disease due to atherosclerosis, metabolic syndrome, hyperlipidemia, hypertension, obesity, and oxidative stress [16]. In cases of PCOS, adropin levels are generally decreased in both serum and follicular fluid [17,18,19], though some studies have found no significant difference [20]. Notably, a meta-analysis highlighted that the decrease in adropin is more pronounced in overweight PCOS patients [21], and a meta-regression analysis suggested that factors like age, glucose ratio, and luteinizing hormone (LH) levels could contribute to the observed heterogeneity [22]. These findings underscore the importance of estrogen in modulating adropin levels and highlight its potential role in protecting against adverse metabolic conditions. However, caution is needed when interpreting these studies and other studies reporting adropin as a biomarker, as different exploratory assays were used to measure circulating adropin levels. Moreover, the relationship between circulating levels and tissue expression remains unclear.
1.2. Adropin and Hypertensive Disorders Complicating Pregnancy and Gestational Diabetes Mellitus
Hypertensive disorders complicating pregnancy (HDCP) are associated with decreased adropin levels [23]. Both maternal and umbilical cord adropin levels decrease in preeclamptic pregnancies compared to controls, with significantly lower levels observed in severe cases compared to mild ones. These findings suggest that adropin could potentially serve as a predictive marker for the presence and severity of preeclampsia [24,25]. Conversely, in another study, serum adropin levels increased in women with preeclampsia; this elevation may serve as a compensatory mechanism [26]. In cases of intrauterine growth retardation/restriction (IUGR), adropin levels decrease in both cord and maternal blood. However, in severe IUGR cases, maternal adropin levels increase, possibly as a regulatory mechanism against placental dysfunction [27,28]. Gestational diabetes mellitus (GDM) presents a complex relationship with adropin levels, with increases observed in both GDM 1 and GDM 2 during different trimesters, while decreases are noted in maternal and cord serum levels during specific gestational weeks [29,30,31,32,33]. Additionally, cord blood adropin levels increase in infants of GDM mothers [34]. These changes in adropin levels in different disorders during pregnancy should be adjusted based on the gestational age, as in the context of preterm delivery, cord blood adropin levels decrease and are positively correlated with gestational age and placental weight [35].
Conversely, in investigations focusing on pregnancy, a pivotal period demanding intricate cellular adaptations, the epidermal growth factor domain-specific O-linked GlcNAc transferase–adropin axis in decidual cells was found impaired, potentially linking metabolic disorders like obesity to adverse pregnancy outcomes [23].
1.3. Adropin and Coronary Artery Diseases
Lower serum adropin levels have been consistently associated with various cardiac conditions, serving as potential biomarkers for disease onset and severity. For instance, in patients with microvascular angina (cardiac syndrome X, CSX), serum adropin levels were significantly reduced, with adropin identified as an independent risk factor for CSX [36]. Similarly, in individuals with acute myocardial infarction (AMI), decreased serum adropin levels were linked to the presence of AMI in Coronary Artery Disease (CAD) patients, with adropin serving as an independent predictor for AMI [37], a possible role of adropin in CAD prevention [38], and the presence of good coronary collateral circulation [39]. Low adropin levels are correlated with adverse outcomes in CAD patients, such as the increased risk of long-term recurrent myocardial infarction and higher severity of CAD, as assessed by the SYNTAX score [40]. Additionally, investigations into the genetic landscape of CAD have identified polymorphisms in genes related to adropin production, further implicating adropin in the pathogenesis of CAD [41]. A study found that adropin enhanced the survival and therapeutic potential of mesenchymal stem cells (MSCs) in myocardial infarction by activating the Akt and ERK1/2 pro-survival pathways, reducing inflammation, and improving cardiac function while reducing myocardial fibrosis [42].
Studies on Coronary Artery Ectasia (CAE) found that lower serum adropin levels are associated with CAE, suggesting that adropin could be a significant predictor and independent risk factor for the condition [43,44]. Studies on heart failure (HF) have shown that plasma adropin levels increase with the severity of HF, are higher in patients with cardiac cachexia, and correlate with brain natriuretic peptide (BNP) and NYHA class, suggesting a role for adropin in the pathogenesis and severity of HF [45,46,47]. Studies on cardiomyopathies have shown that adropin regulated mitochondrial fuel substrate utilization in cardiac cells, protected against myocardial fibrosis, and improved diastolic function, suggesting its potential role in treating conditions like diabetic cardiomyopathy and doxorubicin-induced cardiomyopathy [48,49,50]. Serum adropin levels were elevated in Kawasaki disease patients, particularly those with coronary artery lesions [51].
1.4. Adropin’s Role in Hypertension
Adropin protects endothelial cells by stimulating the phosphorylation of eNOS in these cells [8]. Endothelial dysfunction in hypertension may play a role in triggering and advancing vascular inflammation, vascular remodeling, and atherosclerosis, and it is independently linked to a higher risk of cardiovascular events [52]. Adropin level has been shown to correlate with hypertension. Observational studies indicate that individuals with hypertension exhibit significantly lower plasma adropin levels compared to normotensive controls [40,53]. Antihypertensive medications such as amlodipine and valsartan have been demonstrated to increase adropin levels after 12 weeks of treatment [54]. However, the predictive value of adropin for target organ damage in hypertensive patients remains controversial [40]. Moreover, no studies have determined whether adropin affected blood pressure in animal models. These results are correlative and do not indicate causality.
1.5. Adropin’s Role in Atherosclerosis
Experimental studies highlight adropin’s ability to mitigate the progression of this cardiovascular disorder through several mechanisms. One study demonstrated that the induction of lipoprotein lipase (LPL) by the non-coding RNA HDAC11-AS1 may be mediated by adropin, leading to a reduction in atherosclerosis in mice [55]. This effect is attributed to adropin-mediated histone deacetylation, which enhances LPL expression by activating AMP-activated protein kinase (AMPK) [55]. Another study underscores adropin’s role in suppressing inflammatory responses, inhibiting foam cell formation, and reducing the migration and proliferation of vascular smooth muscle cells [56]. These actions collectively contribute to the attenuation of atherosclerotic lesion development [56]. Moreover, studies have delved into the complex interplay between lifestyle factors and vascular health, revealing that short-term adverse lifestyle changes induce vascular insulin resistance, accompanied by decreased plasma adropin levels, particularly in young, healthy men [57]. The anti-inflammatory and lipid-regulating properties of adropin suggest its potential as a therapeutic target for atherosclerosis, offering a novel approach to reducing the burden of this disease. However, adropin transgenic mice do not prevent atherosclerosis, as observed with low-density lipoprotein receptor (LDLR) deficiency [58]. The results of those studies suggest that cholesterol and intermediates in cholesterol metabolism inhibit adropin/Enho expression. However, overexpression did not affect cholesterol levels.
1.6. Adropin’s Role in Obesity
The link between obesity and glucose and lipid metabolism disturbances is well established [59]. Peptide hormones play a key role in regulating overall energy balance in the body, and adropin is also associated with this process [1,59]. In children, higher plasma adropin levels have been associated with lower waist-to-hip ratios, lower insulin resistance, and lower body fat percentage in both girls and boys [60]. However, no significant differences were observed between genders or in lipid profiles among high versus low adropin subjects, suggesting a complex interaction with body composition [59,60,61,62,63]. Similarly, observational studies highlight a decrease in adropin levels with obesity and aging, with significant differences observed between prepubertal and pubertal children and varying associations with obesity depending on developmental stage [64], with higher concentrations found in males [65].
In adolescents, lower adropin levels have been associated with high-density lipoprotein cholesterol (HDL-C) levels and have been shown to increase with exercise [66]. Bariatric surgery has been observed to increase circulating adropin levels, peaking 3 months after surgery [65], which may contribute to the long-term metabolic improvements seen post-surgery through enhanced secretion of anti-inflammatory and insulin-sensitizing factors [67,68]. In obese children with metabolic syndrome, lower levels of adropin were noted, and adropin was identified as a protective factor against metabolic syndrome, indicating its potential as a biomarker for this condition [69]. Interestingly, in conditions such as Prader–Willi syndrome (PWS), characterized by severe obesity, adropin levels were found to be higher compared to controls [70]. White adipose tissue regulates lipid and carbohydrate metabolism by storing triacylglycerol and releasing various hormones and metabolic factors that interact with peripheral tissues and the brain [71,72]. In adipocytes, the expression of Gpr19 and Enho genes follows a similar trend, suggesting that Gpr19 could be a potential receptor for adropin [73]. However, no studies have directly confirmed this relationship. Collectively, these findings suggest that adropin may serve as a biomarker for obesity and related metabolic syndromes, with potential implications for therapeutic interventions targeting metabolic dysregulation. Worth mentioning is the fact that the studies cited used different assays to measure adropin levels. While some assays, such as ELISA, are widely validated and commonly used in research, others may require more cautious interpretation. This variability in assay choice could influence the comparability of the results, and readers should consider these differences when drawing conclusions across studies.
1.7. Adropin’s Role in Obstructive Sleep Apnea (OSA)
Obstructive sleep apnea (OSA) is a prevalent sleep-related breathing disorder resulting from partial or complete upper airway obstruction [74]. OSA has been independently linked to endothelial dysfunction, inflammation, hypertension, dysregulation of the hypothalamic–pituitary–adrenal axis, increased arterial stiffness, impaired glucose metabolism, and cognitive and psychomotor deficits [74].
Severe OSA in adults is associated with markedly lower adropin levels compared to moderate OSA, healthy controls [74,75], and obese males without OSA [76]. In OSA, decreased adropin levels suggest its utility as an early biomarker for predicting endothelial dysfunction prior to the manifestation of clinical symptoms. However, serum NO levels do not significantly differ between OSA patients and healthy controls [77]. In pediatric OSA patients, adropin levels are significantly reduced but return to normal values following adenotonsillectomy [78,79]. These findings underscore the complex relationship between adropin and OSA, emphasizing its potential role in both the diagnosis and management of this condition.
1.8. Adropin’s Role in DM
Research using different animal models, spanning multiple studies, sheds light on adropin’s multifaceted role, particularly focusing on its molecular mechanisms within the context of diabetes mellitus (DM) [80,81,82,83,84,85,86,87]. Studies have shown adropin’s involvement in glucose and lipid metabolism regulation [80,81]. For instance, adropin treatment has been shown to enhance insulin sensitivity, ameliorate insulin resistance, and promote glucose utilization while suppressing fatty acid oxidation, thus favoring a metabolic shift toward carbohydrate metabolism [80,82,88]. Mechanistically, adropin treatment activates key molecules in insulin signaling pathways, such as Akt and GLUT4, sensitizing tissues to insulin action [81]. Additionally, adropin affects glucose homeostasis by modulating enzymes and transcription factors involved in glucose and lipid metabolism, such as pyruvate dehydrogenase and PPARα [81]. Furthermore, adropin has been found to regulate hepatic glucose production by inhibiting gluconeogenic enzymes and the modulation of AMPK signaling [88].
Observational studies in clinical settings have revealed elevated serum adropin levels in T2DM patients compared to healthy controls, suggesting a potential association with disease pathogenesis [89]. However, interventional studies have provided contrasting insights, demonstrating that treatment with sitagliptin, a common antidiabetic medication, could significantly increase adropin levels in newly diagnosed T2DM patients, possibly contributing to improved glucose metabolism [90]. Adropin also plays a crucial role in metabolic dysfunction-associated fatty liver disease (MAFLD) in patients with T2D, as highlighted in a study examining the impact of liraglutide treatment. Results revealed that liraglutide not only increased serum adropin levels but also correlated with a significant reduction in liver fat content [91]. Training, particularly high-intensity interval training, has been shown to significantly elevate adropin levels in patients with T2DM, potentially contributing to improved insulin sensitivity and endothelial function [92,93]. Cross-sectional investigations have further explored the relationship between dietary factors and metabolic health in T2DM, highlighting the potential impact of dietary insulin index on metabolic profiles, albeit without significant associations with adropin levels [94].
1.9. Endothelial Dysfunction in T2DM
Adropin plays a significant role in T2DM and associated cardiovascular disorders by influencing endothelial function and atherosclerosis, independently associated with angiographic severity of coronary atherosclerosis, suggesting that serum adropin serves as a novel predictor of coronary atherosclerosis [95]. Studies have shown that lower plasma adropin levels are associated with endothelial dysfunction in T2DM patients, indicating its potential as a biomarker for vascular health [95,96,97]. Specifically, T2DM patients with lower adropin levels exhibited worse endothelial function and higher hemoglobin A1C levels, suggesting an independent risk factor for vascular complications [97]. Additionally, higher serum adropin levels were correlated with a reduced risk of carotid atherosclerosis and a decrease in carotid intimal–medial thickness, underscoring its protective role against atherosclerotic cardiovascular disease in T2DM patients [98]. Adropin levels also showed potential as a risk indicator for ischemic heart disease in T2DM, with significantly lower levels observed in patients with ischemic heart disease compared to those without [99]. Furthermore, although adropin levels did not significantly vary with glycemic control in chronic heart failure patients, they were associated with favorable hemodynamic changes during treatment with SGLT2 inhibitors, indicating a beneficial role in cardiac remodeling and function [100,101].
1.10. DM Nephropathy and Retinopathy
Serum adropin concentrations exhibit a consistent negative correlation with renal function, particularly in patients with T2DM. Notably, lower adropin levels are associated with an increased risk of developing both T2DM and diabetic nephropathy, suggesting its pivotal role in the disease progression [102,103,104]. Moreover, adropin’s role extends beyond mere association, as experimental studies demonstrate its therapeutic potential [105]. Adropin, encapsulated in ROS-responsive nanocapsules, showcases promising effects in improving renal function, mitigating oxidative stress, and regulating lipid metabolism in models of diabetic kidney disease [105]. Furthermore, clinical observations underscore adropin’s prognostic value, with low levels serving as an independent predictor of chronic kidney disease stages 1–3 in T2DM patients with chronic heart failure [106].
Recent studies have established a negative correlation between adropin levels and the severity of diabetic retinopathy (DR), with lower adropin concentrations associated with increased risk and severity of DR [107,108].
1.11. Adropin and Hepatic Diseases
Since adropin was originally investigated as a “hepatokine” [1], numerous studies have concentrated on the regulation and function of adropin in the liver. Adropin has been shown to correlate with dyslipidemia and related metabolic disorders. Observational human studies have revealed that plasma adropin levels were inversely related to atherogenic LDL-C levels, suggesting a potential regulatory role in hepatic lipid metabolism [58]. Research on mice indicates that the interaction between adropin and cholesterol metabolism is mostly one-way, with cholesterol and seven-oxygenated sterols primarily inhibiting adropin expression in cultured cells [109]. The concentration of angiopoietin-like protein 3, a promising target for managing cardiovascular disease, which inhibits lipid clearance, is inversely related to adropin levels in fructose-induced dyslipidemia in rhesus macaques [110]. Therefore, this study implicates the role of adropin in the complex regulatory network of insulin resistance and hypertriglyceridemia [110]. In obese adolescents with non-alcoholic fatty liver disease (NAFLD), adropin levels are significantly decreased compared to both healthy controls and patients without fatty liver disease [111]. Additionally, adropin levels are inversely correlated with oxidative stress and histological severity in NAFLD, suggesting a potential role as a predictor of coronary artery disease [112,113]. Conversely, in alcoholic liver cirrhosis, adropin concentrations are increased, and this elevation correlates with disease severity, as indicated by the Child–Pugh score [114]. Moreover, in cirrhotic patients, higher adropin levels are associated with shorter survival within 180 days, and combining serum adropin with Child–Pugh and MELD–Na scores enhances mortality prediction [115]. Furthermore, in chronic hepatitis B or C, adropin levels increase with disease progression, offering insights into fibrosis staging and monitoring [116]. However, in MAFLD, adropin levels are decreased, particularly in patients with T2DM, highlighting its potential as a marker for metabolic dysfunction [117]. In preclinical models, adropin demonstrates a protective role in diet-induced nonalcoholic steatohepatitis (NASH) by activating Nrf2 signaling and inhibiting NLRP3 inflammasome activation, suggesting its therapeutic potential in NASH prevention and treatment [118,119]. Notably, adropin regulation in the liver appears to be sex-specific and dependent on estrogen receptor alpha (ERα) in mice on an HFD [15]. These findings underscore the multifaceted involvement of adropin in liver pathophysiology and its potential as a diagnostic and therapeutic target in liver diseases.
1.12. Adropin and Renal Diseases
Chronic kidney disease (CKD) is linked to an age-related decline in kidney function, which is further exacerbated by conditions such as hypertension, diabetes, obesity, and primary renal disorders [120]. Cardiovascular disease is the leading cause of illness and death, with CKD recognized as both a risk factor for cardiovascular events and a condition that accelerates cardiovascular disease risk [120]. Adropin has been increasingly studied for its role in renal diseases. In a rat model of adenine-induced chronic kidney disease (CKD), adropin was found to reduce urine protein levels and 24-hour urine volume and downregulate inflammatory markers such as TIMP-1, IL-33, and MMP-2, suggesting a protective effect against renal damage and inflammation [121]. In patients undergoing hemodialysis (HD), lower serum adropin levels were significantly associated with malnutrition, inflammation, and longer HD duration, highlighting its potential involvement in the pathophysiology of CKD and its complications [122]. Studies also showed that genetic polymorphisms affecting adropin levels were associated with dyslipidemia and cardiovascular risks, further emphasizing the hormone’s role in metabolic and cardiovascular health in kidney disease patients [123,124,125]. Additionally, adropin levels were found to be inversely correlated with body composition parameters in end-stage renal disease patients, suggesting its utility as a marker for nutritional status [126]. Overall, these findings indicate that adropin plays a significant role in modulating inflammatory responses, renal function, and metabolic health in various stages of kidney disorders [127,128]. Adropin could also positively serve as a marker for metabolic health and recovery in kidney transplant patients [119,129].
1.13. Nutrition and Exercise Effects on Adropin
Plasma adropin concentrations strongly correlate with the intake of saturated fat [130]. Levels of adropin were found to be higher in cheese whey compared to those in the corresponding milk peptides and plasma of dairy cows [131]. Consumption of fructose, probiotic yogurt, a low-calorie diet, dietary fats, legumes, and nuts increases adropin levels, while carbohydrate-rich diets decrease them [130,132,133,134,135].
Adropin promotes lipolysis, suppresses lipogenesis, and modulates adipokine expression in white rodent adipocytes, suggesting its pivotal role in regulating metabolic processes within adipose tissue [136]. Adropin appears to exert significant effects on skeletal muscle metabolism and function, as evidenced by several studies. Research indicates that adropin promotes carbohydrate oxidation over fat oxidation in muscle tissue, suggesting a role in regulating substrate preferences [137]. Additionally, alterations in adropin levels, such as downregulation observed in response to dietary changes, may impact gene expression related to lipid metabolism and oxidative stress in skeletal muscle [138]. Exercise interventions, particularly aerobic training, have been associated with increases in adropin levels, correlating with improvements in arterial stiffness and adiposity in obese adults [139]. Furthermore, various modes of exercise, including descending stair walking and elastic band resistance training, have demonstrated significant elevations in adropin levels, suggesting a potential role in enhancing vascular function and cardiometabolic health [140,141].
1.14. Adropin and Neurological Disorders
High levels of adropin peptide have been detected in the mouse brain [142], and Enho mRNA is abundant in both human and nonhuman primate brains [143], suggesting its relevance in regulating brain functions. Studies in nonhuman primates indicate that adropin expression follows a circadian rhythm, with transcriptional activation by RORα/γ playing a role in this gene’s transcription [58]. This could indicate a role for adropin in mediating circadian processes in the nervous system [58]. Disruptions in circadian regulation, potentially involving adropin, have been linked to neurological disorders [144,145]. In depression, decreased adropin levels have been observed, though depression subtypes do not show differences in these levels [146]. Similarly, reduced adropin levels are noted in individuals with bipolar disorders [147]. Experimental studies on Wistar albino rats indicate that adropin has a beneficial antiepileptic effect during penicillin-induced epileptiform activity [148]. In multiple sclerosis, patients exhibit decreased adropin levels, but these levels do not correlate with pituitary diameters [149,150,151]. In experimental Wistar rats subjected to chronic stress, Enho gene expression increased with chronic stress [152]. Adropin exerts its physiological effects through direct postsynaptic actions on neurons in the paraventricular nucleus, implicating it as a potential modulator of fluid balance, energy homeostasis, and cardiovascular regulation [153]. Adropin levels are elevated in acute hypoxia, leading to reduced water intake by modulating the TRPV4-CamKK-AMPK signaling pathway in the SFO. These findings suggest that adropin is a key mediator of the Water intake reduction (anti-dipsogenic) effects observed under hypoxic conditions [154]. Adropin may serve as a novel local stimulator for growth hormone gene expression in tilapia pituitary [155].
In a study using in silico expression profiling in a nonhuman primate diurnal transcriptome atlas, it was found that Enho expression followed a dynamic, diurnal pattern and was abundant in brain regions regulating appetite and autonomic function, with lower levels in the liver, lung, kidney, ileum, and some endocrine glands. Hierarchical clustering identified 426 genes co-regulated with Enho, enriched for epigenetic silencing and neural functions [11]. In a study, adropin knockout mice exhibited decreased locomotor activity and impaired motor coordination coupled with defective synapse formation, a phenotype similar to NB-3 (contactin-6) knockout mice [142]. However, no studies have directly demonstrated that adropin acted through NB-3.
1.15. Adropin and Neurodegenerative Diseases
Adropin has been shown to play a significant role in cognitive function across various models of metabolic and neurological diseases. In LDLR-deficient (low-density lipoprotein receptor) mice with diet-induced obesity, adropin overexpression improved cognitive performance by attenuating the negative effects of metabolic dysregulation on neuronal signaling pathways [156]. In rats, adropin administration enhanced spatial memory performance through the activation of the Akt/CREB/BDNF signaling pathway, demonstrating its potential to improve learning and memory [157]. Human studies revealed that higher adropin levels are associated with better cognitive outcomes and reduced risk of cognitive decline in older adults, suggesting a protective role against age-related cognitive impairment [143,158,159]. Furthermore, adropin’s positive correlations with mitochondrial processes and synapse function, particularly in individuals without dementia, indicate its involvement in maintaining neural health [143].
Potent neurotrophic responses in primary cultured neurons are consistent with adropin, supporting the development and function of neural networks. Increasing adropin expression using transgenesis improved spatial learning and memory, novel object recognition, resilience to exposure to new environments, and reduced mRNA markers of inflammation in old mice. Treatment with synthetic adropin peptide also reversed age-related declines in cognitive functions and affected the expression of genes involved in morphogenesis and cellular metabolism [143].
Adropin potentially plays a role in neurodegenerative conditions such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) [160]. PD ranks as the second most prevalent neurodegenerative disorder and is often linked with gastric ulcers [160]. In an experimental model combining indomethacin-induced gastric ulcers with rotenone-induced PD [160], adropin effectively restored dopamine levels in the striatum and ameliorated rotenone-induced motor impairments. Additionally, it demonstrated significant gastroprotective effects, likely mediated by its antioxidant properties, evidenced by a reduction in malondialdehyde levels and increased activities in superoxide dismutase, catalase, and serum ferric reducing antioxidant power (assessing the serum’s ability to neutralize oxidative stress by measuring its capacity to reduce ferric iron to ferrous iron). Adropin reinstated the delicate equilibrium between the compromised pro-survival PI3K/Akt/murine double minute 2 pathways and the apoptotic P53/Puma pathways. Thus, adropin emerges as a promising therapeutic target for PD and its associated gastric ulcers [160]. In a separate clinical investigation [161], plasma levels of adropin were found to be diminished in the AD group and reduced in the MOTS-c (mitochondrial open reading frame of the 12S rRNA-c), acute ischemic stroke, and AD groups compared to the control. Similarly, comparable values were observed in the MS group relative to its control. Correlation analyses revealed platelet and cholesterol levels to be negatively associated with adropin levels, while platelet, lymphocyte, and triglyceride levels exhibited positive correlations with MOTS-c. This study provides novel insights, indicating that adropin may serve as a potentially significant marker in AD and MOTS-C in acute ischemic stroke and AD contexts [161].
1.16. Adropin and Ischemic Stroke
Adropin has demonstrated significant protective effects on the BBB in various models of neurological disorders. In ischemic conditions, adropin treatment reduced endothelial permeability by inhibiting the ROCK-MLC2 (Rho-associated kinase-myosin light chain 2)-signaling pathway, even though it did not protect junction proteins or reduce VEGF levels [162]. In aged mice subjected to ischemic stroke, adropin treatment decreased infarct volume and brain edema and improved sensorimotor and cognitive functions by reducing Matrix metalloproteinase-9 (MMP-9) activity and preserving tight junction proteins [163]. Further studies indicated that adropin reduced BBB damage by decreasing oxidative stress and neutrophil infiltration, with its protective effects dependent on eNOS activation [164]. Adropin serum levels are associated with poor clinical outcomes and greater infarcted areas in acute ischemic stroke patients [165]. Aging reduces adropin levels in the brain, which correlates with reduced eNOS and increased oxidative stress associated with age-related endothelial dysfunction and the development of aging-associated cerebrovascular dysfunction in Sprague-Dawley naïve rats [166]. In aged mice subjected to ischemic stroke, adropin markedly decreased infarct volume and brain edema and improved both sensorimotor and cognitive functions, with effects linked to reduced MMP-9 activity and preservation of tight junction proteins [163].
1.17. Adropin and Subarachnoid Hemorrhage
Our group has investigated the role of adropin in subarachnoid hemorrhage-mediated neural injury and delayed cerebral infarction. Current understanding of the pathophysiology of post-SAH cerebral infarction points to injury cascades involving decreased nitric oxide (NO) bioavailability and oxidative stress [167,168,169,170,171,172]. Under normal conditions, NO signaling pathways regulate cerebral blood flow (CBF) by mediating cerebral vasodilation and inhibiting platelet adhesion. However, with SAH, red blood cells lyse and release hemoglobin, which is spasmogenic. Hemoglobin scavenges NO, stimulates the production of a nitric oxide synthase (NOS) inhibitor (ADMA), and generates reactive oxygen species (ROS) and nitrogen species (RNS) [167].
We showed that in endothelial cells exposed to cell-free hemoglobin as a model of subarachnoid hemorrhage, adropin prevented increased permeability and reduced macrophage migration across the endothelial monolayer [173]. In our SAH mouse model, we showed that adropin was effective against subarachnoid hemorrhage-mediated neural injury and delayed cerebral infarction through eNOS-dependent mechanisms [174]. We demonstrated that synthetic adropin treatment reduced cerebral edema, preserved tight junction proteins, and prevented microthrombosis shortly after SAH [174]. Furthermore, adropin prevents cerebral vasospasm, decreases neuronal apoptosis, and improves sensorimotor function up to seven days post-SAH, even with a 24-hour delay in administration [174]. Moreover, in intracerebral hemorrhage (ICH) models, adropin reduced brain water content and improved neurological outcomes by preserving BBB integrity [175]. Taken together, we believe that adropin has a role in protecting against subarachnoid hemorrhage-mediated neural injury and delayed cerebral infarction via a nitric oxide pathway and reduction in oxidative stress (Figure 2).
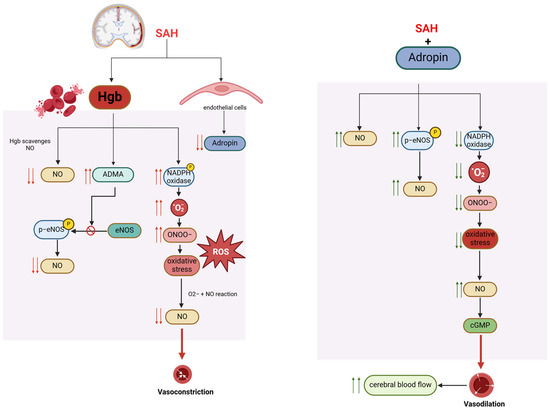
Figure 2.
Proposed mechanisms of adropin-mediated protection in post-SAH cerebral infarction.
2. Conclusions
Adropin has emerged as a crucial regulator of metabolic, cardiovascular, and cerebrovascular physiology, demonstrating protective effects across a wide range of conditions, including metabolic syndrome, diabetes, cardiovascular diseases, and stroke. Its ability to influence energy homeostasis, lipid metabolism, and endothelial function underscores its potential as both a biomarker and a therapeutic target. In the context of cerebrovascular diseases, adropin reduces blood–brain barrier damage, brain edema, and oxidative stress while preserving cognitive and sensorimotor functions after ischemic stroke. Its therapeutic benefits extend to models of subarachnoid hemorrhage, where it decreases neuronal apoptosis and improves recovery. The inverse relationship between adropin levels and disease severity in conditions such as coronary artery disease, hypertension, and neurodegenerative diseases highlights its diagnostic significance. Future research should focus on elucidating the molecular mechanisms underlying adropin’s effects, as well as its therapeutic potential in preventing and treating cardiometabolic, cerebrovascular, and neurological disorders.
Author Contributions
Conceptualization, Z.H.-S. and B.L.H.; methodology, Z.H.-S.; validation, Z.H.-S., A.A.B., E.C.-J. and B.L.H.; writing—original draft preparation, Z.H.-S.; writing—review and editing, A.A.B., E.C.-J. and B.L.H.; visualization, Z.H.-S.; supervision, A.A.B., E.C.-J. and B.L.H.; project administration, Z.H.-S.; funding acquisition, B.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH, grant number “R01NS124620”, “Eblen Research Endowment”, “Christine Desmond Fund”, “James and Brigette Marino Family Professorship Endowment”, and “St. George Family Fund”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of Adropin as a Secreted Factor Linking Dietary Macronutrient Intake with Energy Homeostasis and Lipid Metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Guo, S.; Niu, Y.; Yang, L.; Liu, B.; Jiang, N.; Su, M.; Wang, L. Heat-shock protein 60 of Porphyromonas gingivalis may induce dysfunction of human umbilical endothelial cells via regulation of endothelial-nitric oxide synthase and vascular endothelial-cadherin. Biomed. Rep. 2016, 5, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D. Insulin resistance and vascular function. J. Diabetes Complicat. 2002, 16, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.J.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Interactions Between Endothelin and Nitric Oxide in the Regulation of Vascular Tone in Obesity and Diabetes. Diabetes 2004, 53, 2060–2066. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Baron, A.D. Vascular function, insulin resistance and fatty acids. Diabetologia 2002, 45, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin Is a Novel Regulator of Endothelial Function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, Q.; Ding, Q.; Wu, W.; Li, Q.; Zheng, Z. High Level of Adropin Promotes the Progression of Pancreatic Ductal Adenocarcinoma. Curr. Cancer Drug Targets 2024, 24, 629–641. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Kocaman, N.; Citil, C.; Kendir, Y. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol. Cell. Biochem. 2013, 380, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Price, C.A.; Stevens, J.R.; Graham, J.L.; Stanhope, K.L.; King, S.; Krauss, R.M.; Bremer, A.A.; Havel, P.J. Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J. Biol. Chem. 2019, 294, 9706–9719. [Google Scholar]
- Mierzwicka, A.; Bolanowski, M. New peptides players in metabolic disorders. Postepy Hig. Med. Dosw. 2016, 70, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Stokar, J.; Gurt, I.; Cohen-Kfir, E.; Yakubovsky, O.; Hallak, N.; Benyamini, H.; Lishinsky, N.; Offir, N.; Tam, J.; Dresner-Pollak, R. Hepatic adropin is regulated by estrogen and contributes to adverse metabolic phenotypes in ovariectomized mice. Mol. Metab. 2022, 60, 101482. [Google Scholar] [CrossRef]
- Meda, C.; Barone, M.; Mitro, N.; Lolli, F.; Pedretti, S.; Caruso, D.; Maggi, A.; Della Torre, S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 2020, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Dolce, A.; Vegeto, E.; Maggi, A.; Della Torre, S. ERα-Dependent Regulation of Adropin Predicts Sex Differences in Liver Homeostasis during High-Fat Diet. Nutrients 2022, 14, 3262. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Conigliaro, R.L. Polycystic Ovarian Syndrome. Med. Clin. N. Am. 2023, 107, 227–234. [Google Scholar] [CrossRef]
- Kume, T.; Calan, M.; Yilmaz, O.; Kocabas, G.U.; Yesil, P.; Temur, M.; Bicer, M.; Calan, O.G. A possible connection between tumor necrosis factor alpha and adropin levels in polycystic ovary syndrome. J. Endocrinol. Investig. 2016, 39, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Inal, Z.O.; Erdem, S.; Gederet, Y.; Duran, C.; Kucukaydin, Z.; Kurku, H.; Sakarya, D.K. The impact of serum adropin and ischemia modified albumin levels based on BMI in PCOS. Endokrynol. Pol. 2018, 69, 135–141. [Google Scholar] [CrossRef]
- Bousmpoula, A.; Kouskouni, E.; Benidis, E.; Demeridou, S.; Kapeta-Kourkouli, R.; Chasiakou, A.; Baka, S. Adropin levels in women with polycystic ovaries undergoing ovarian stimulation: Correlation with lipoprotein lipid profiles. Gynecol. Endocrinol. 2018, 34, 153–156. [Google Scholar] [CrossRef]
- Kuliczkowska-Płaksej, J.; Mierzwicka, A.; Jończyk, M.; Stachowska, B.; Urbanovych, A.; Bolanowski, M. Adropin in women with polycystic ovary syndrome. Endokrynol. Pol. 2019, 70, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ziarniak, K.; Dudek, M.; Matuszewska, J.; Bijoch, Ł.; Skrzypski, M.; Celichowski, J.; Sliwowska, J.H. Two weeks of moderate intensity locomotor training increased corticosterone concentrations but did not alter the number of adropin-immunoreactive cells in the hippocampus of diabetic type 2 and control rats. Acta Histochem. 2021, 123, 151751. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Hu, J.; Zhu, Y.; Wang, Y.; Chen, S.; Liu, S. Correlation Between Circulating Adropin Levels and Patients with PCOS: An Updated Systematic Review and Meta-analysis. Reprod. Sci. 2022, 29, 3295–3310. [Google Scholar] [CrossRef] [PubMed]
- Muter, J.; Alam, M.T.; Vrljicak, P.; Barros, F.S.V.; Ruane, P.T.; Ewington, L.J.; Aplin, J.D.; Westwood, M.; Brosens, J.J. The Glycosyltransferase EOGT Regulates Adropin Expression in Decidualizing Human Endometrium. Endocrinology 2018, 159, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, B.D.; Dundar, B.; Acikgoz, A.S.; Ozgen, G.; Cift, T.; Ahmedian, R.; Altekin, Y. The relationship between maternal and umbilical cord adropin levels with the presence and severity of preeclampsia. J. Perinat. Med. 2017, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.; Ercan, C.C.; Akdemir, C.; Sivrikoz, T.S.; Salmaslioglu, A.; Verit, F.F.; Gurdol, F.; Omer, B. The Evaluation of Adropin and Autotaxin as Potential Markers of Endothelial Dysfunction in Preeclampsia. Angiology 2023, 17, 33197231183228. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wu, Z.; Wang, H.; Dong, M. Alteration of serum adropin level in preeclampsia. Pregnancy Hypertens. 2017, 8, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.I.; Eser, A.; Kaygusuz, I.; Yildirim, S.; Celik, T.; Gunduz, S.; Kalman, S. Adipokine, adropin and endothelin-1 levels in intrauterine growth restricted neonates and their mothers. J. Perinat. Med. 2016, 44, 669–676. [Google Scholar] [CrossRef]
- Baka, S.; Malamitsi-Puchner, A.; Briana, D.D.; Boutsikou, M.; Marmarinos, A.; Gourgiotis, D.; Boutsikou, T. Adropin concentrations in term pregnancies with normal, restricted and increased fetal growth. J. Matern. Neonatal Med. 2016, 29, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, Y.; Zhang, J. Hepatokine levels during the first or early second trimester of pregnancy and the subsequent risk of gestational diabetes mellitus: A systematic review and meta-analysis. Biomarkers 2021, 26, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Vivek, K.; Reddy, E.P.; Thangappazham, B.; Raj, H.; Pérez-López, F.R.; Varikasuvu, S.R. Maternal adropin levels in patients with gestational diabetes mellitus: A systematic review and meta-analysis. Gynecol. Endocrinol. 2022, 38, 105–109. [Google Scholar] [CrossRef]
- Adamczak, L.; Mantaj, U.; Gutaj, P.; Skrypnik, D.; Ozegowski, S.; Bogdanski, P.; Wender-Ozegowska, E. Adropin as a potential protective factor of metabolic complications in obese pregnant women with hyperglycaemia diagnosed in early pregnancy. J. Physiol. Pharmacol. 2023, 74, 11–19. [Google Scholar]
- Celik, E.; Yilmaz, E.; Celik, O.; Ulas, M.; Turkcuoglu, I.; Karaer, A.; Simsek, Y.; Minareci, Y.; Aydin, S. Maternal and fetal adropin levels in gestational diabetes mellitus. J. Perinat. Med. 2013, 41, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Beigi, A.; Shirzad, N.; Nikpour, F.; Esfahani, E.N.; Emamgholipour, S.; Bandarian, F. Association between serum adropin levels and gestational diabetes mellitus; a case–control study. Gynecol. Endocrinol. 2015, 31, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Dincer, E.; Topçuoğlu, S.; Arman, D.; Kaya, A.; Yavuz, T.; Karatekin, G. Inflammation Markers in Infants of Mothers with Gestational Diabetes. Fetal Pediatr. Pathol. 2022, 41, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; He, J.R.; Zhao, M.G.; Kuang, Y.S.; Xu, S.Q.; Zhang, H.Z.; Hu, S.P.; Chen, J.; Xia, H.M. Relationship between human cord blood adropin levels and fetal growth. Peptides 2014, 52, 19–22. [Google Scholar] [CrossRef]
- Celik, A.; Balin, M.; Kobat, M.A.; Erdem, K.; Baydas, A.; Bulut, M.; Altas, Y.; Aydin, S.; Aydin, S. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc. Ther. 2013, 31, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Zhao, P.; Wu, M.-C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, L.; Xu, W.; Li, J.; Wang, B.; Gu, X.; Chen, J. Correlation of serum adropin level with coronary artery disease. Zhonghua Yi Xue Za Zhi 2014, 94, 1255–1257. [Google Scholar] [PubMed]
- Akkaya, H.; Güntürk, E.E.; Akkaya, F.; Karabıyık, U.; Güntürk, İ.; Yılmaz, S. Assessment of the Relationship Between the Adropin Levels and the Coronary Collateral Circulation in Patients wıth Chronic Coronary Syndrome. Arq. Bras. Cardiol. 2022, 119, 402–410. [Google Scholar] [PubMed]
- Gulen, B.; Eken, C.; Kucukdagli, O.T.; Serinken, M.; Kocyigit, A.; Kılıc, E.; Uyarel, H. Adropin levels and target organ damage secondary to high blood pressure in the ED. Am. J. Emerg. Med. 2016, 34, 2061–2064. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Cao, Y.; Han, W.; Guo, Y.; Yang, G.; Zhang, J.; Jiang, P. Association of polymorphisms of preptin, irisin and adropin genes with susceptibility to coronary artery disease and hypertension. Medicine 2020, 99, e19365. [Google Scholar] [CrossRef]
- Li, H.; Hu, D.; Chen, G.; Zheng, D.; Li, S.; Lin, Y.; Hong, H.; Luo, Y.; Ke, Y.; Huang, Y.; et al. Adropin-based dual treatment enhances the therapeutic potential of mesenchymal stem cells in rat myocardial infarction. Cell Death Dis. 2021, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.A.; Kuyumcu, M.S. Serum irisin and adropin levels may be predictors for coronary artery ectasia. Clin. Exp. Hypertens. 2022, 44, 223–227. [Google Scholar] [CrossRef]
- Özkan, B.; Örsçelik, Ö.; Yaroğlu, H.Y.; Balcı, Ş.; Özcaıı, M.K.; Çelik, A.; Özcaıı, İ.T. Association between serum adropin levels and isolated coronary artery ectasia in patients with stable angina pectoris. Anatol. J. Cardiol. 2019, 22, 250–255. [Google Scholar] [PubMed]
- Lian, W.; Gu, X.; Qin, Y.; Zheng, X. Elevated Plasma Levels of Adropin in Heart Failure Patients. Intern. Med. 2011, 50, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, A.K.; Cakmak, H.A.; Erturk, M.; Kalkan, K.E.; Uzun, F.; Tasbulak, O.; Diker, V.O.; Aydin, S.; Celik, A. Adropin and Irisin in Patients with Cardiac Cachexia. Arq. Bras. de Cardiol. 2018, 111, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, L.; Yuan, X.; Lu, Y. Combined effects of hydralazine and nitrate on serum biochemistry and left ventricular remodeling in chronic heart failure patients. Pak. J. Pharm. Sci. 2021, 34, 381–386. [Google Scholar]
- Thapa, D.; Stoner, M.W.; Zhang, M.; Xie, B.; Manning, J.R.; Guimaraes, D.; Shiva, S.; Jurczak, M.J.; Scott, I. Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway. Redox Biol. 2018, 18, 25–32. [Google Scholar] [CrossRef]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism 2019, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ai, J.; Shuai, Z.; Tang, K.; Li, Z.; Huang, Y. Adropin Alleviates Myocardial Fibrosis in Diabetic Cardiomyopathy Rats: A Preliminary Study. Front. Cardiovasc. Med. 2021, 8, 688586. [Google Scholar] [CrossRef]
- Yang, M.; Pei, Q.; Zhang, J.; Weng, H.; Jing, F.; Yi, Q. Association between adropin and coronary artery lesions in children with Kawasaki disease. Eur. J. Pediatr. 2021, 180, 2253–2259. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, H.; Zhu, X.; Gu, H.; Chen, J.; Wang, L.; Harding, P.; Xu, W. Inverse Correlation Between Plasma Adropin and ET-1 Levels in Essential Hypertension: A Cross-Sectional Study. Medicine 2015, 94, e1712. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.T.; Akkaya, N.; Erdamar, H.; Gok, S.; Kazanci, F.; Demircelik, B.; Cakmak, M.; Yigitoglu, R. The Effects of Valsartan and Amlodipine on the Levels of Irisin, Adropin, and Perilipin. Clin. Lab. 2015, 61, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, W. LncRNA HDAC11-AS1 Suppresses Atherosclerosis by Inhibiting HDAC11-Mediated Adropin Histone Deacetylation. J. Cardiovasc. Transl. Res. 2022, 15, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Soares, R.N.; McMillan, N.J.; Jurrissen, T.J.; Martinez-Lemus, L.A.; Padilla, J.; Manrique-Acevedo, C. Young Women Are Protected Against Vascular Insulin Resistance Induced by Adoption of an Obesogenic Lifestyle. Endocrinology 2022, 163, bqac137. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Stevens, J.R.; Billon, C.; Girardet, C.; Sitaula, S.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Rankinen, T.; Bouchard, C.; et al. Adropin: An endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 2018, 8, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-M.; Yuan, X.; Ouyang, Q.; Lin, X.-Q.; Ai, Z.-Z.; Zhang, Y.; Yang, X.-H. Adropin and glucagon-like peptide-2 are associated with glucose metabolism in obese children. World J. Pediatr. 2019, 15, 565–571. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Ouyang, Q.; Lin, X.; Ai, Z.; Zhang, Y.; Yang, X. Novel associations of serum adropin and lipopolysaccharide-binding protein versus lipid profiles in childhood obesity. J. Pediatric. Endocrinol. Metab. 2020, 33, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-B.; Chu, N.-F.; Lin, F.-H.; Hsu, J.-T.; Chen, P.-Y. Relationship between plasma adropin levels and body composition and lipid characteristics amongst young adolescents in Taiwan. Obes. Res. Clin. Pract. 2018, 12 (Suppl. S2), 101–107. [Google Scholar] [CrossRef] [PubMed]
- Altincik, A.; Sayin, O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Erman, H.; Ozdemir, A.; Sitar, M.E.; Cetin, S.I.; Boyuk, B. Role of serum adropin measurement in the assessment of insulin resistance in obesity. J. Investig. Med. 2021, 69, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; de Dios, O.; Gavela-Pérez, T.; Riestra, P.; Jois, A.; Soriano-Guillén, L.; Garcés, C. Opposite Association of Adropin Concentrations with Obesity in Prepubertal Children Compared with Adolescents. Obesity 2020, 28, 1736–1741. [Google Scholar] [CrossRef]
- Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.-P.; Ravussin, E.; Havel, P.J. Low Circulating Adropin Concentrations with Obesity and Aging Correlate with Risk Factors for Metabolic Disease and Increase after Gastric Bypass Surgery in Humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Yang, Y.-J.; Ge, R.-K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.-L.; Dong, Y.-F.; et al. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef] [PubMed]
- Faramia, J.; Ostinelli, G.; Drolet-Labelle, V.; Picard, F.; Tchernof, A. Metabolic adaptations after bariatric surgery: Adipokines, myokines and hepatokines. Curr. Opin. Pharmacol. 2020, 52, 67–74. [Google Scholar] [CrossRef]
- Glück, M.; Glück, J.; Wiewióra, M.; Rogala, B.; Piecuch, J. Serum Irisin, Adropin, and Preptin in Obese Patients 6 Months After Bariatric Surgery. Obes. Surg. 2019, 29, 3334–3341. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, H.; Zhang, M.; Xiao, Y. Adropin and apelin-12 efficiently predict metabolic syndrome in obese children. Pediatr. Diabetes 2020, 21, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Butler, A.A.; Muehlbauer, M.J.; Cui, H.N.; Rubin, D.A. Obestatin and adropin in Prader-Willi syndrome and nonsyndromic obesity: Associations with weight, BMI-z, and HOMA-IR. Pediatr. Obes. 2019, 14, e12493. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Mori, M. The brain–adipose axis: A review of involvement of molecules. Nutr. Neurosci. 2005, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Jasaszwili, M.; Wojciechowicz, T.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Effects of adropin on proliferation and differentiation of 3T3-L1 cells and rat primary preadipocytes. Mol. Cell. Endocrinol. 2019, 496, 110532. [Google Scholar] [CrossRef]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients With Obstructive Sleep Apnea: A Link With Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, Y. Soluble Vascular Adhesion Protein-1 Level Correlates With Adropin and Inflammatory Biomarkers in Patients With Obstructive Sleep Apnea. Ear Nose Throat J. 2022, 9, 1455613221074147. [Google Scholar] [CrossRef] [PubMed]
- Celikhisar, H.; Ilkhan, G.D. Alterations in Serum Adropin, Adiponectin, and Proinflammatory Cytokine Levels in OSAS. Can. Respir. J. 2020, 2020, 2571283. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Y.; Zou, F.; Xu, T.; Pan, P.; Hu, C.; Su, X. Serum adropin level is associated with endothelial dysfunction in patients with obstructive sleep apnea and hypopnea syndrome. Sleep Breath. 2020, 25, 117–123. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Molero-Ramirez, H.; Tan, H.-L.; Bandla, H.P. Circulating Adropin Concentrations in Pediatric Obstructive Sleep Apnea: Potential Relevance to Endothelial Function. J. Pediatr. 2013, 163, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gileles-Hillel, A.; Alonso-Álvarez, M.L.; Peris, E.; Bhattacharjee, R.; Terán-Santos, J.; Duran-Cantolla, J.; Gozal, D. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: A community-based study. Int. J. Obes. 2015, 39, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef]
- Thapa, D.; Xie, B.; Manning, J.R.; Zhang, M.; Stoner, M.W.; Huckestein, B.R.; Edmunds, L.R.; Zhang, X.; Dedousis, N.L.; O’Doherty, R.M.; et al. Adropin reduces blood glucose levels in mice by limiting hepatic glucose production. Physiol. Rep. 2019, 7, e14043. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, F.-J.; Li, H.-Y.; Li, L.; Song, L.-G.; Mao, Y.; Li, J.; Liu, H.-M.; Li, F.-L.; Xu, L.-Y.; et al. Anti-diabetic Role of Adropin in Streptozotocin Induced Diabetic Rats via Alteration of PI3K/Akt and Insulin Signaling Pathway. J. Oleo Sci. 2021, 70, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Cheng, K.-C.; Liu, I.-M.; Niu, H.-S. Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats. Pharmaceuticals 2022, 15, 173. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Wojciechowicz, T.; Janicka, P.; Krążek, M.; Małek, E.; Strowski, M.Z.; Nowak, K.W. Daily Treatment of Mice with Type 2 Diabetes with Adropin for Four Weeks Improves Glucolipid Profile, Reduces Hepatic Lipid Content and Restores Elevated Hepatic Enzymes in Serum. Int. J. Mol. Sci. 2022, 23, 9807. [Google Scholar] [CrossRef] [PubMed]
- Jurrissen, T.J.; Ramirez-Perez, F.I.; Cabral-Amador, F.J.; Soares, R.N.; Pettit-Mee, R.J.; Betancourt-Cortes, E.E.; McMillan, N.J.; Sharma, N.; Rocha, H.N.M.; Fujie, S.; et al. Role of adropin in arterial stiffening associated with obesity and type 2 diabetes. Am. J. Physiol. Circ. Physiol. 2022, 323, H879–H891. [Google Scholar] [CrossRef] [PubMed]
- Rizk, F.H.; El-Saka, M.H.; Ibrahim, R.R.; El-Deeb, O.S.; Ibrahim, H.A.; El Saadany, A.A.; Mashal, S.S.; Ammar, L.; Abdelsattar, A.M.; Barhoma, R.A. Possible mitigating effect of adropin on lung injury in diabetic rats: Targeting the role of Rho A/Rho-associated kinase pathway. Biofactors 2023, 49, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ghoshal, S.; Zhang, L.; Stevens, J.R.; McCommis, K.S.; Finck, B.N.; Lopaschuk, G.D.; Butler, A.A. The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J. Biol. Chem. 2019, 294, 13366–13377. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Shen, T.; Yang, W.; Chen, Q.; Zhang, P.; You, Y.; Sun, X.; Xu, H.; Tang, Y.; et al. Adropin regulates hepatic glucose production via PP2A/AMPK pathway in insulin-resistant hepatocytes. FASEB J. 2020, 34, 10056–10072. [Google Scholar] [CrossRef] [PubMed]
- Palizban, A.-A.; Yazdani, A.-H.; Jahanbani-Ardakani, H. Role of rs7903146 polymorphism and adropin serum level in patients with diabetes mellitus; a case–control study from Isfahan, Iran. Arch. Physiol. Biochem. 2022, 128, 378–381. [Google Scholar] [CrossRef]
- Wang, Q.; An, Y.; Zhang, L.; Zhang, Y.; Wang, G.; Liu, J. Regulation of Adropin by Sitagliptin monotherapy in participants with newly diagnosed type 2 Diabetes. BMC Endocr. Disord. 2022, 22, 306. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Li, X.; Chang, X.; Ding, X.; Wang, Q.; Jiang, T.; Wang, G.; Liu, J. Longitudinal changes in serum adropin levels and liver fat content during liraglutide treatment in newly diagnosed patients with type 2 diabetes mellitus and metabolic dysfunction-associated fatty liver disease. Acta Diabetol. 2023, 60, 971–979. [Google Scholar] [CrossRef]
- Rezaeinezhad, N.; Alizadeh, R.; Ghanbari-Niaki, A. Short-term circuit resistance training improves insulin resistance probably via increasing circulating Adropin. J. Diabetes Metab. Disord. 2022, 21, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, M.; Hesamabadi, B.K.; Ariabood, E.; Izadi, M.R.; Ghardashi-Afousi, A.; Bigi, M.A.B.; Asvadi-Fard, M.; Gaeini, A.A. Improved blood pressure and flow-mediated dilatation via increased plasma adropin and nitrate/nitrite induced by high-intensity interval training in patients with type 2 diabetes. Exp. Physiol. 2022, 107, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, R.; Hajhashemy, Z.; Lotfi, K.; Shahdadian, F.; Rouhani, P.; Saneei, P. Association between dietary insulin index and load with brain derived neurotrophic factor, adropin and metabolic health status in Iranian adults. Sci. Rep. 2023, 13, 20540. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Oruc, C.U.; Akpinar, Y.E.; Dervisoglu, E.; Amikishiyev, S.; Salmaslıoglu, A.; Gurdol, F.; Omer, B. Low concentrations of adropin are associated with endothelial dysfunction as assessed by flow-mediated dilatation in patients with metabolic syndrome. Clin. Chem. Lab. Med. 2017, 55, 139–144. [Google Scholar] [CrossRef]
- Topuz, M.; Celik, A.; Aslantas, T.; Demir, A.K.; Aydin, S.; Aydin, S. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J. Investig. Med. 2013, 61, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, H.; Qiu, X.; Zhang, J.; Huang, J.; Chen, H.; Qiu, S.; Lin, R.; Li, S.; Tu, M. The association between serum adropin and carotid atherosclerosis in patients with type 2 diabetes mellitus: A cross-sectional study. Diabetol. Metab. Syndr. 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Makboul, K.M.; Elhalawany, S.H.; Mansour, H.K.; Marwan, D.A.; Ibrahim, B.E. A Study of the Assessment of Serum Adropin Level as a Risk Factor of Ischaemic Heart Disease in Type 2 Diabetes Mellitus Cases. Georgian Med. News 2022, 328–329, 115–117. [Google Scholar] [CrossRef]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef]
- Berezin, A.A.; Obradovic, Z.; Fushtey, I.M.; Berezina, T.A.; Novikov, E.V.; Schmidbauer, L.; Lichtenauer, M.; Berezin, A.E. The Impact of SGLT2 Inhibitor Dapagliflozin on Adropin Serum Levels in Men and Women with Type 2 Diabetes Mellitus and Chronic Heart Failure. Biomedicines 2023, 11, 457. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L. Association of Serum Adropin Concentrations with Diabetic Nephropathy. Mediat. Inflamm. 2016, 2016, 6038261. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, X.; Guo, S.; Zhang, M.; Li, J.; Zhai, N.; Wang, H.; Zhang, Y. Pentraxin-3 and adropin as inflammatory markers of early renal damage in type 2 diabetes patients. Int. Urol. Nephrol. 2020, 52, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Es-Haghi, A.; Al-Abyadh, T.; Mehrad-Majd, H. The Clinical Value of Serum Adropin Level in Early Detection of Diabetic Nephropathy. Kidney Blood Press. Res. 2021, 46, 734–740. [Google Scholar] [CrossRef]
- Yu, M.; Wang, D.; Zhong, D.; Xie, W.; Luo, J. Adropin Carried by Reactive Oxygen Species-Responsive Nanocapsules Ameliorates Renal Lipid Toxicity in Diabetic Mice. ACS Appl. Mater. Interfaces 2022, 14, 37330–37344. [Google Scholar] [CrossRef] [PubMed]
- Berezina, T.A.; Obradovic, Z.; Boxhammer, E.; Berezin, A.A.; Lichtenauer, M.; Berezin, A.E. Adropin Predicts Chronic Kidney Disease in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J. Clin. Med. 2023, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, J.; Hu, W.; Liu, Y.; Lin, D.; Duan, H.; Liu, F. The association of serum and vitreous adropin concentrations with diabetic retinopathy. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2018, 56, 253–258. [Google Scholar] [CrossRef]
- Li, B.; Li, N.; Guo, S.; Zhang, M.; Li, J.; Zhai, N.; Wang, H.; Zhang, Y. The changing features of serum adropin, copeptin, neprilysin and chitotriosidase which are associated with vascular endothelial function in type 2 diabetic retinopathy patients. J. Diabetes Its Complicat. 2020, 34, 107686. [Google Scholar] [CrossRef]
- Wang, Y.; Kumar, N.; Solt, L.A.; Richardson, T.I.; Helvering, L.M.; Crumbley, C.; Garcia-Ordonez, R.D.; Stayrook, K.R.; Zhang, X.; Novick, S. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J. Biol. Chem. 2010, 285, 5013–5025. [Google Scholar] [CrossRef]
- Graham, J.L.; Stanhope, K.L.; Wong, S.; King, S.; Bremer, A.A.; Krauss, R.M.; Hamilton, J.; Havel, P.J. Role of angiopoietin-like protein 3 in sugar-induced dyslipidemia in rhesus macaques: Suppression by fish oil or RNAi. J. Lipid Res. 2020, 61, 376–386. [Google Scholar]
- Sayın, O.; Tokgöz, Y.; Arslan, N. Investigation of adropin and leptin levels in pediatric obesity-related nonalcoholic fatty liver disease. J. Pediatr. Endocrinol. Metab. 2014, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, O.; Altun, Ö.; Dikker, O.; Aktaş, Ö.; Özsoy, N.; Arman, Y.; Çil, E.; Özcan, M.; Yoldemir, A.; Akarsu, M.; et al. Serum Adropin Levels Are Reduced in Adult Patients with Nonalcoholic Fatty Liver Disease. Med. Princ. Pract. 2019, 28, 463–469. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Shen, T.; Chen, Q.; Chen, S.; Pang, J.; Mi, J.; Tang, Y.; You, Y.; Xu, H.; et al. Lower adropin expression is associated with oxidative stress and severity of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 160, 191–198. [Google Scholar] [CrossRef]
- Prystupa, A.; Kiciński, P.; Luchowska-Kocot, D.; Sak, J.; Prystupa, T.; Chen, K.-H.; Chen, Y.-C.; Panasiuk, L.; Załuska, W. Afamin and adropin in patients with alcohol-induced liver cirrhosis. Ann. Agric. Environ. Med. 2018, 25, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kolben, Y.; Kenig, A.; Kessler, A.; Ishay, Y.; Weksler-Zangen, S.; Eisa, M.; Ilan, Y. Serum Levels of Adropin Improve the Predictability of MELD and Child-Pugh Score in Cirrhosis: Results of Proof-of-Concept Clinical Trial. Transpl. Int. 2023, 36, 11176. [Google Scholar] [CrossRef] [PubMed]
- Eser Karlidag, G.; Arslan Solmaz, O. Are adropin, apelin, elabela, asprosin and betatrophin biomarkers for chronic hepatitis and staging of fibrosis? Biotech. Histochem. 2020, 95, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xie, G.; Zhou, B.; Qu, A.; Meng, H.; Liu, J.; Wang, G. Serum Adropin as a Potential Biomarker for Predicting the Development of Type 2 Diabetes Mellitus in Individuals With Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Physiol. 2021, 12, 696163. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xue, H.; Fang, W.; Chen, K.; Chen, S.; Yang, W.; Shen, T.; Chen, X.; Zhang, P.; Ling, X.; et al. Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 2019, 21, 101068. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Li, Y.; Zhao, G.; Guo, Z.; Luo, Y.; et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: A plausible role of adropin. Lab. Investig. 2021, 101, 369–380. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Yazgan, B.; Avcı, F.; Memi, G.; Tastekin, E. Inflammatory response and matrix metalloproteinases in chronic kidney failure: Modulation by adropin and spexin. Exp. Biol. Med. 2021, 246, 1917–1927. [Google Scholar] [CrossRef]
- Boric-Skaro, D.; Mizdrak, M.; Luketin, M.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Kurir, T.T.; Bozic, J. Serum Adropin Levels in Patients on Hemodialysis. Life 2021, 11, 337. [Google Scholar] [CrossRef]
- Grzegorzewska, A.E.; Niepolski, L.; Mostowska, A.; Warchoł, W.; Jagodziński, P.P. Involvement of adropin and adropin-associated genes in metabolic abnormalities of hemodialysis patients. Life Sci. 2016, 160, 41–46. [Google Scholar] [CrossRef]
- Liu, F.; Cui, B.; Zhao, X.; Wu, Y.; Qin, H.; Guo, Y.; Wang, H.; Lu, M.; Zhang, S.; Shen, J.; et al. Correlation of Serum Adropin Levels with Risk Factors of Cardiovascular Disease in Hemodialysis Patients. Metab. Syndr. Relat. Disord. 2021, 19, 401–408. [Google Scholar] [CrossRef]
- Grzegorzewska, A.E.; Niepolski, L.; Świderska, M.K.; Mostowska, A.; Stolarek, I.; Warchoł, W.; Figlerowicz, M.; Jagodziński, P.P. ENHO, RXRA, and LXRA polymorphisms and dyslipidaemia, related comorbidities and survival in haemodialysis patients. BMC Med. Genet. 2018, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, M.; Hoppe, K.; Schwermer, K.; Ibrahim, A.Y.; Pawlaczyk, K.; Ziemnicka, K. Adropin and irisin levels in relation to nutrition, body composition, and insulin resistance in patients with end-stage renal disease on chronic hemodialysis and peritoneal dialysis. Pol. Arch. Intern. Med. 2016, 126, 474–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Memi, G.; Yazgan, B. Adropin and spexin hormones regulate the systemic inflammation in adenine-induced chronic kidney failure in rat. Chin. J. Physiol. 2021, 64, 194–201. [Google Scholar] [CrossRef]
- Kaur, R.; Krishan, P.; Kumari, P.; Singh, T.; Singh, V.; Singh, R.; Ahmad, S.F. Clinical Significance of Adropin and Afamin in Evaluating Renal Function and Cardiovascular Health in the Presence of CKD-MBD Biomarkers in Chronic Kidney Disease. Diagnostics 2023, 13, 3158. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Lovrić Kojundžić, S.; Gelemanović, A.; Vučković, M.; Budimir Mršić, D.; Šupe Domić, D.; Novaković, M.D.; Radić, M. Serum Adropin Levels and Body Mass Composition in Kidney Transplant Recipients-Are There Sex Differences? Diagnostics 2023, 13, 2768. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.; Shechter, A.; Shlisky, J.; Tam, C.S.; Gao, S.; Ravussin, E.; Butler, A.A. Fasting plasma adropin concentrations correlate with fat consumption in human females. Obesity 2014, 22, 1056–1063. [Google Scholar] [CrossRef]
- Aydin, S. Presence of adropin, nesfatin-1, apelin-12, ghrelins and salusins peptides in the milk, cheese whey and plasma of dairy cows. Peptides 2013, 43, 83–87. [Google Scholar] [CrossRef]
- Butler, A.A.; St-Onge, M.-P.; Siebert, E.A.; Medici, V.; Stanhope, K.L.; Havel, P.J. Differential Responses of Plasma Adropin Concentrations To Dietary Glucose or Fructose Consumption In Humans. Sci. Rep. 2015, 5, 14691. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Kearney, M.L.; St-Onge, M.P.; Stanhope, K.L.; Havel, P.J.; Kanaley, J.A.; Thyfault, J.P.; Weiss, E.P.; Butler, A.A. Inverse association between carbohydrate consumption and plasma adropin concentrations in humans. Obesity 2016, 24, 1731–1740. [Google Scholar] [CrossRef]
- Zarrati, M.; Lahiji, M.R.; Salehi, E.; Yazdani, B.; Razmpoosh, E.; Shoormasti, R.S.; Shidfar, F. Effects of Probiotic Yogurt on Serum Omentin-1, Adropin, and Nesfatin-1 Concentrations in Overweight and Obese Participants Under Low-Calorie Diet. Probiotics Antimicrob. Proteins 2019, 11, 1202–1209. [Google Scholar] [CrossRef]
- Assi, M.J.; Poursalehi, D.; Tirani, S.A.; Shahdadian, F.; Hajhashemy, Z.; Mokhtari, E.; Mohammadi, S.; Saneei, P. Legumes and nuts intake in relation to metabolic health status, serum brain derived neurotrophic factor and adropin levels in adults. Sci. Rep. 2023, 13, 16455. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Pruszyńska-Oszmałek, E.; Wojciechowicz, T.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin Slightly Modulates Lipolysis, Lipogenesis and Expression of Adipokines but Not Glucose Uptake in Rodent Adipocytes. Genes 2021, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; McMillan, R.P.; Jacas, J.; Zhu, Q.; Li, X.; Kumar, G.K.; Casals, N.; Hegardt, F.G.; Robbins, P.D.; Lopaschuk, G.D.; et al. Regulation of Substrate Oxidation Preferences in Muscle by the Peptide Hormone Adropin. Diabetes 2014, 63, 3242–3252. [Google Scholar] [CrossRef] [PubMed]
- Skugor, A.; Kjos, N.P.; Sundaram, A.Y.M.; Mydland, L.T.; Ånestad, R.; Tauson, A.-H.; Øverland, M. Effects of long-term feeding of rapeseed meal on skeletal muscle transcriptome, production efficiency and meat quality traits in Norwegian Landrace growing-finishing pigs. PLoS ONE 2019, 14, e0220441. [Google Scholar] [CrossRef] [PubMed]
- Fujie, S.; Hasegawa, N.; Kurihara, T.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Association between aerobic exercise training effects of serum adropin level, arterial stiffness, and adiposity in obese elderly adults. Appl. Physiol. Nutr. Metab. 2017, 42, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, S.; Ravasi, A.A.; Soori, R.; Aydin, S.; Choobineh, S.; Aydin, S. High-intensity interval training ameliorates endothelial dysfunction through adropin, nitric oxide, MR-proADM, and copeptin changes in overweight subjects. Hormones 2022, 21, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Huang, T.-H.; Tseng, W.-C.; Tseng, K.-W.; Hsieh, C.-C.; Chen, M.-Y.; Chou, T.-Y.; Huang, Y.-C.; Chen, H.-L.; Nosaka, K. Changes in plasma C1q, apelin and adropin concentrations in older adults after descending and ascending stair walking intervention. Sci. Rep. 2021, 11, 17644. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wang, Y.; Lee JT, H.; Huang, Z.; Wu, D.; Xu, A.; Lam, K.S.L. Adropin is a brain membrane-bound protein regulating physical activity via the NB-3/Notch signaling pathway in mice. J. Biol. Chem. 2014, 289, 25976–25986. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghoshal, S.; Girardet, C.; De Mars, K.M.; Yang, C.; Niehoff, M.L.; Nguyen, A.D.; Jayanth, P.; Hoelscher, B.A.; Xu, F.; et al. Adropin correlates with aging-related neuropathology in humans and improves cognitive function in aging mice. NPJ Aging Mech. Dis. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Kolben, Y.; Weksler-Zangen, S.; Ilan, Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes. Rev. 2021, 22, e13108. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.A.; Tuygar Okutucu, F.; Ozturk, N. Irisin, adropin, and preptin as biomarkers of energy dysregulation in depressive disorder. Curr. Med. Res. Opin. 2023, 39, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Atwoli, L.; Baqui, A.H.; Benfield, T.; Bosurgi, R.; Godlee, F.; Hancocks, S.; Horton, R.; Laybourn-Langton, L.; Monteiro, C.A.; Norman, I. Call for Emergency Action to Limit Global Temperature Increases, Restore Biodiversity, and Protect Health. Alpha Psychiatry 2021, 22, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dogru, Y.Z.; Nacar, T.; Erat, M. Effect of adropin on seizure activity in rats with penicillin-induced epilepsy. Epilepsy Res. 2023, 194, 107170. [Google Scholar] [CrossRef]
- Demirdöğen, F.; Akdağ, T.; Gündüz, Z.B.; Odabaş, F.Ö. Investigation of serum adropin levels and its relationship with hypothalamic atrophy in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 66, 103948. [Google Scholar] [CrossRef] [PubMed]
- Cinkir, U.; Bir, L.S.; Topsakal, S.; Cicek, E.A.; Tekin, S. Investigation of blood leptin and adropin levels in patients with multiple sclerosis: A CONSORT-clinical study. Medicine 2021, 100, e27247. [Google Scholar] [CrossRef]
- Algul, S.; Ozcelik, O. Evaluating the energy regulatory hormones of nesfatin-1, irisin, adropin and preptin in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104221. [Google Scholar] [CrossRef]
- Abulmeaty, M.M.; Almajwal, A.M.; Razak, S.; Al-Ramadhan, F.R.; Wahid, R.M. Energy Homeostasis-Associated (Enho) mRNA Expression and Energy Homeostasis in the Acute Stress Versus Chronic Unpredictable Mild Stress Rat Models. Biomedicines 2023, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Loewen, S.P.; Ferguson, A.V. Adropin acts in the rat paraventricular nucleus to influence neuronal excitability. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R511–R519. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, L.; Qian, X.; Wang, D.; He, W.J.; Tang, Z.W.; Yin, J.; Huang, Q. Adropin Is a Key Mediator of Hypoxia Induced Anti-Dipsogenic Effects via TRPV4-CamKK-AMPK Signaling in the Circumventricular Organs of Rats. Front. Mol. Neurosci. 2017, 10, 105. [Google Scholar] [CrossRef]
- Peng, J.; Yang, P.; Zhang, Q.; Jiang, Q. Tilapia adropin: The localization and regulation of growth hormone gene expression in pituitary cells. Peptides 2017, 97, 1–7. [Google Scholar] [CrossRef]
- Ghoshal, S.; Banerjee, S.; Zhang, J.; Niehoff, M.L.; Farr, S.A.; Butler, A.A. Adropin transgenesis improves recognition memory in diet-induced obese LDLR-deficient C57BL/6J mice. Peptides 2021, 146, 170678. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Aslan, M.A.; Sinen, O.; Munzuroglu, M.; Derin, N.; Parlak, H.; Bulbul, M.; Agar, A. Effects of adropin on learning and memory in rats tested in the Morris water maze. Hippocampus 2022, 32, 253–263. [Google Scholar] [CrossRef]
- Aggarwal, G.; Morley, J.E.; Vellas, B.; Nguyen, A.D.; Butler, A.A. Low circulating adropin concentrations predict increased risk of cognitive decline in community-dwelling older adults. GeroScience 2024, 46, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Malmstrom, T.K.; Morley, J.E.; Miller, D.K.; Nguyen, A.D.; Butler, A.A. Low circulating adropin levels in late-middle aged African Americans with poor cognitive performance. NPJ Aging 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- El Gheit, R.E.A.; Atef, M.M.; El Deeb, O.S.; Badawi, G.A.; Alshenawy, H.A.; Elwan, W.M.; Arakeep, H.M.; Emam, M.N. Unique Novel Role of Adropin in a Gastric Ulcer in a Rotenone-Induced Rat Model of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Saçmacı, H.; Çakır, M.; Özcan, S.S. Adropin and MOTS-c as new peptides: Do levels change in neurodegenerative diseases and ischemic stroke? J. Biochem. Mol. Toxicol. 2023, 37, e23246. [Google Scholar] [CrossRef]
- Yang, C.; DeMars, K.M.; Hawkins, K.E.; Candelario-Jalil, E. Adropin reduces paracellular permeability of rat brain endothelial cells exposed to ischemia-like conditions. Peptides 2016, 81, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, L.; Lavayen, B.P.; Larochelle, J.; Gunraj, R.E.; Butler, A.A.; Candelario-Jalil, E. Therapeutic Benefits of Adropin in Aged Mice After Transient Ischemic Stroke via Reduction of Blood-Brain Barrier Damage. Stroke 2023, 54, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lavayen, B.P.; Liu, L.; Sanz, B.D.; DeMars, K.M.; Larochelle, J.; Pompilus, M.; Febo, M.; Sun, Y.-Y.; Kuo, Y.-M.; et al. Neurovascular protection by adropin in experimental ischemic stroke through an endothelial nitric oxide synthase-dependent mechanism. Redox Biol. 2021, 48, 102197. [Google Scholar] [CrossRef] [PubMed]
- Kadirhan, O.A.; Kucukdagli, O.T.; Gulen, B. The effectiveness of serum S100B, TRAIL, and adropin levels in predicting clinical outcome, final infarct core, and stroke subtypes of acute ischemic stroke patients. Biomédica 2022, 42, 55–63. [Google Scholar] [CrossRef]
- Yang, C.; DeMars, K.M.; Candelario-Jalil, E. Age-Dependent Decrease in Adropin is Associated with Reduced Levels of Endothelial Nitric Oxide Synthase and Increased Oxidative Stress in the Rat Brain. Aging Dis. 2018, 9, 322–330. [Google Scholar] [CrossRef]
- Siuta, M.; Zuckerman, S.L.; Mocco, J. Nitric Oxide in Cerebral Vasospasm: Theories, Measurement, and Treatment. Neurol. Res. Int. 2013, 2013, 972417. [Google Scholar] [CrossRef]
- Suhardja, A. Mechanisms of disease: Roles of nitric oxide and endothelin-1 in delayed cerebral vasospasm produced by aneurysmal subarachnoid hemorrhage. Nat. Clin. Pract. Cardiovasc. Med. 2004, 1, 110–116; quiz 2 p following 116. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.M. Delayed cerebral vasospasm and nitric oxide: Review, new hypothesis, and proposed treatment. Pharmacol. Ther. 2005, 105, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Hänggi, D.; Steiger, H.-J. Nitric oxide in subarachnoid haemorrhage and its therapeutics implications. Acta Neurochir. 2006, 148, 605–613; discussion 613. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.M. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochir. Suppl. 2008, 104, 139–147. [Google Scholar] [PubMed]
- Yang, Y.; Chen, S.; Zhang, J.-M. The Updated Role of Oxidative Stress in Subarachnoid Hemorrhage. Curr. Drug Deliv. 2017, 14, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Dodd, W.S.; Patel, D.; Lucke-Wold, B.; Hosaka, K.; Chalouhi, N.; Hoh, B.L. Adropin decreases endothelial monolayer permeability after cell-free hemoglobin exposure and reduces MCP-1-induced macrophage transmigration. Biochem. Biophys. Res. Commun. 2021, 582, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dodd, W.S.; Patel, D.; Laurent, D.; Lucke-Wold, B.; Hosaka, K.; Johnson, R.D.; Chalouhi, N.; Butler, A.A.; Candelario-Jalil, E.; Hoh, B.L. Subarachnoid hemorrhage-associated brain injury and neurobehavioral deficits are reversed with synthetic adropin treatment through sustained Ser1179 phosphorylation of endothelial nitric oxide synthase. Front. Stroke 2024, 3, 1371140. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lu, Z.; Burchell, S.; Nowrangi, D.; Manaenko, A.; Li, X.; Xu, Y.; Xu, N.; Tang, J.; Dai, H.; et al. Adropin preserves the blood-brain barrier through a Notch1/Hes1 pathway after intracerebral hemorrhage in mice. J. Neurochem. 2017, 143, 750–760. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).