Recycling of Uridylated mRNAs in Starfish Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Oocyte Preparation
2.2. RT-PCR with 3′ Adaptor Ligation and Tail Sequence
2.3. 5′-Rapid Amplification of cDNA
2.4. DNA Cloning and Plasmids for In Vitro RNA Synthesis
2.5. Microinjection
2.6. In Vitro Transcription
2.7. Real-Time qPCR
2.8. Translation Assay
2.9. Luciferase Assay
2.10. Illumina MiSeq Sequencing for Targeted TAIL-Seq
2.11. Correction of mRNA (cDNA) Sequences for Mapping of Targeted TAIL-Seq Reads
- (1)
- To prevent the misalignments of reads, a poly(A) sequence 50 nt in length was added to the 3′ end of the mRNA in silico.
- (2)
- Reads of the GV sample from the oocytes before hormonal stimulation were randomly downsampled to a coverage depth of 1000×.
- (3)
- Downsampled reads were mapped to the mRNA sequence using BWA-MEM [36] (version 0.7.12-r1044) with the default parameters.
- (4)
- Mapped reads were piled up using SAMtools [36] (version 1.3.1). The commands were ‘sort’, ‘index’, and ‘mpileup’ with the parameters, and the output was the text file of the pileup result.
- (5)
- For each position in the mRNA sequence, a base (A, T (U), G, or C) was changed to the majority position using the pileup result and the in-house program.
- (6)
- The poly(A) sequence added to (1) was removed.
2.12. Extraction of Valid 3′ Reads from the Targeted TAIL-Seq
- (1)
- The 3′ adapters in the reads were searched for using BLASTN (Camacho et al., 2009 [37]) (version 2.6.0) with the option of ‘-task blastn-short -word_size 6 -window_size 0 -dust no’ to handle short sequences. Here, the query and database inputs were reads and adapters, respectively (‘-query reads.fa -db adapter’).
- (2)
- Reads in which 3′ adapters were aligned with identity ≥ 90%, in which alignment-coverage of the adapter was 100%, and in which alignment positions were the heads of reads were extracted. Read pairs without the 3′ adapter were discarded. For each extracted read pair, the reads with and without the 3′ adapter were treated as 3′ and 5′ reads, respectively.
- (3)
- The 5′ reads were aligned to the targeted mRNA using BLASTN with the options of ‘task blastn -word_size 10 -window_size 0 -dust no’ and read pairs in which 5′ reads were aligned with identity ≥ 90% and alignment length ≥ 200 nt were extracted, and the others were discarded to exclude contamination.
2.13. Analysis of Poly(A) and 3′ Modifications in Targeted TAIL-Seq Reads
- (1)
- The 3′ reads of the extracted pairs (see ‘Extraction of 3′ reads of targeted mRNAs’) were aligned to the targeted mRNA using BLASTN with the options of ‘-task blastn -word_size 10 -window_size 0 -dust no’.
- (2)
- If an alignment with a length ≥ 30 nt was detected in a 3′ read, the aligned region was treated as a 3′ UTR (not a 3′ tail region). To handle low-quality reads associated with poly(A), a sequence identity cut-off was not applied.
- (3)
- To detect poly(A) regions, a score was calculated based on the composition of bases as follows: A, +1; N, −1; T, G, or C, −2. If the score was higher, it was considered that the region was more likely to comprise poly(T). Although the concept of this score was introduced in a previous study [20], our calculation method differed from that originally published (A, +1; N, −2; T, G, or C, −10) to detect non-canonical poly(A) regions. For each 3′ read, the scores were calculated for all regions (substrings), and the region that satisfied the following conditions was determined as a poly(A): maximum score throughout the read, score > 0, and distance to the 3′ end of the read ≤ 15 nt.
- (4)
- Using the results of (2) and (3), regions in each 3′ read were classified as 3′ UTR, poly(A), 3′ end modification (a region between poly(A) and the 3′ end of a read), and other. Statistics, such as length, were calculated for each class. When calculating the length distributions of poly(A)s, the regions with lengths ≥ 40 nt were treated as the same category (‘≥40 nt’) because the lengths of the long poly(A)s tended to be overestimated due to systematic base-calling errors [20].
- (5)
- For each 3′-end modification, classification was based on the major base (T, G, or C). If multiple bases occurred at the same time, the modification was classified as ‘≥2′.
- (6)
- To reduce the influence of sequencing errors, bases in the 3′ reads with quality values of <20 were masked, and the composition of the bases (rates of A, T, G, and C) was calculated using unmasked reads.
2.14. Analysis of TAIL-Seq Data of Xenopus Laevis
- (1)
- The data set was generated and pre-processed in a previous study [4] using Tailseeker (version 3.1.7; https://github.com/hyeshik/tailseeker, accessed on 13 September 2019); the pre-processing steps included the removal of adaptors and PCR duplicates. Intermediate FASTQ files of paired reads were obtained through personal communication with Dr. Hyeshik Chang (an author of the previous study).
- (2)
- The 5′ reads were mapped to the X. laevis RNA sequence set (RefSeq accession, GCF_001663975.1; file name, GCF_001663975.1_Xenopus_laevis_v2_rna_from_genomic.fna) using BWA-MEM (Li and Durbin, 2009 [36]) (version 0.7.12-r1044) with the default parameters.
- (3)
- The 5′ reads that were primarily mapped to the mRNA of rps29 (40S ribosomal protein; accession, NM_001171730.1) were detected and the corresponding 3′ reads extracted.
- (4)
- For the extracted 3′ reads, the same procedures were applied as for the starfish (see ‘Detection and analysis of 3′ ends of mRNA including poly(A)’).
3. Results
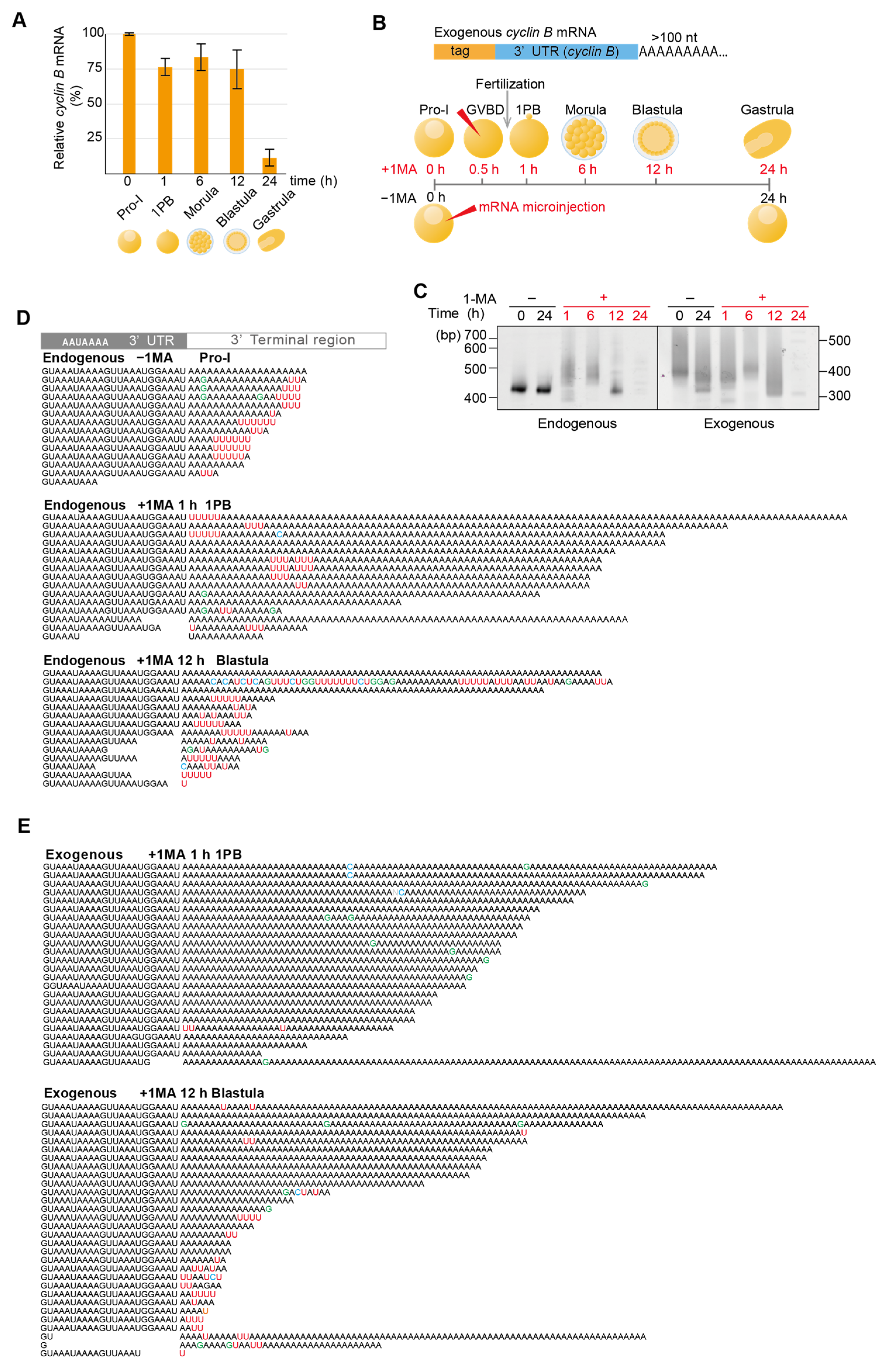
3.1. Cylin B mRNA Decay Occurs After Deadenylation and Uridylation at MZT
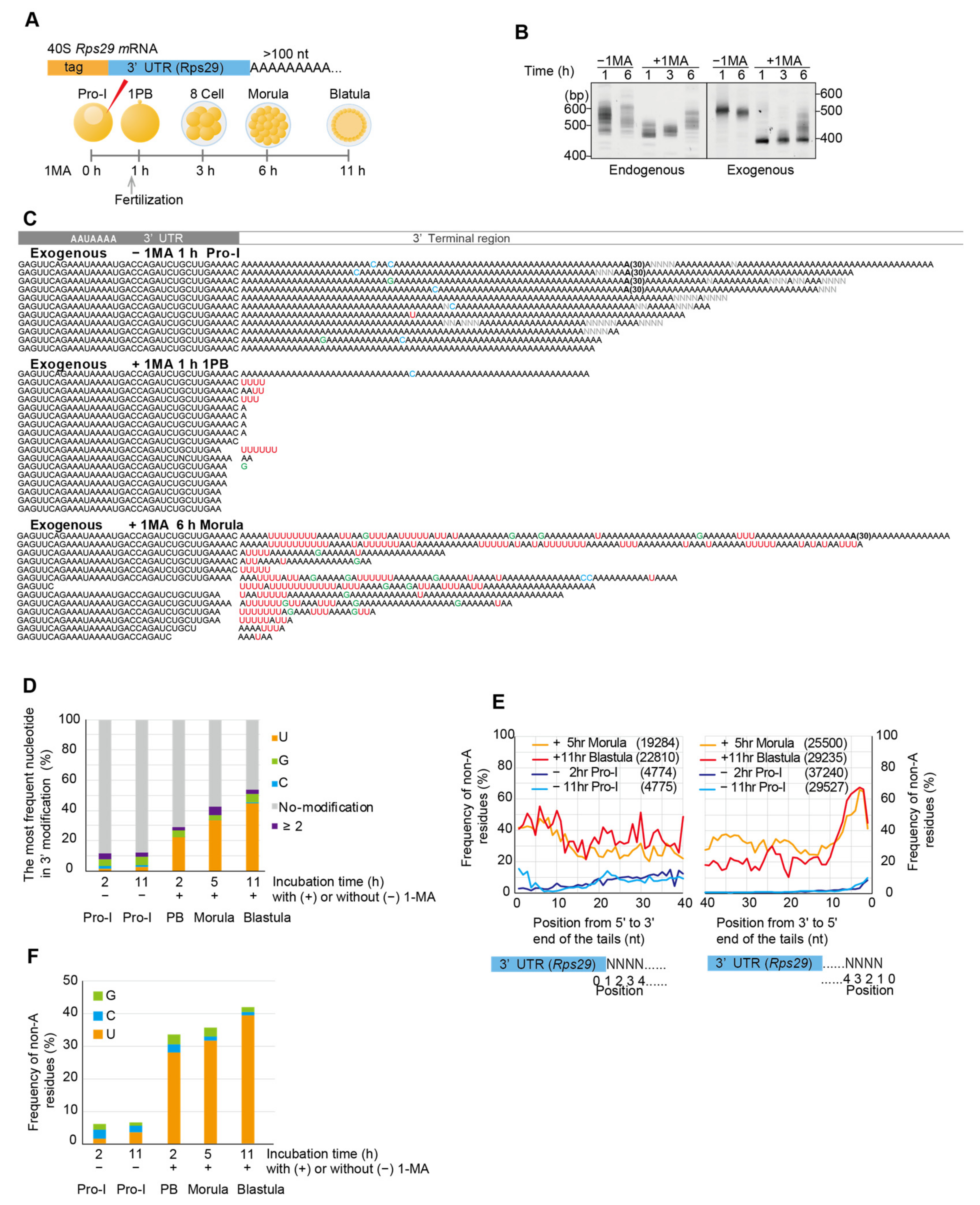
3.2. Ribosomal Protein mRNAs Are Uridylated After 1-MA Stimulation and Fertilization, Followed by Non-Canonical Poly(A) Tail Formation in Embryos at the Blastula Stage
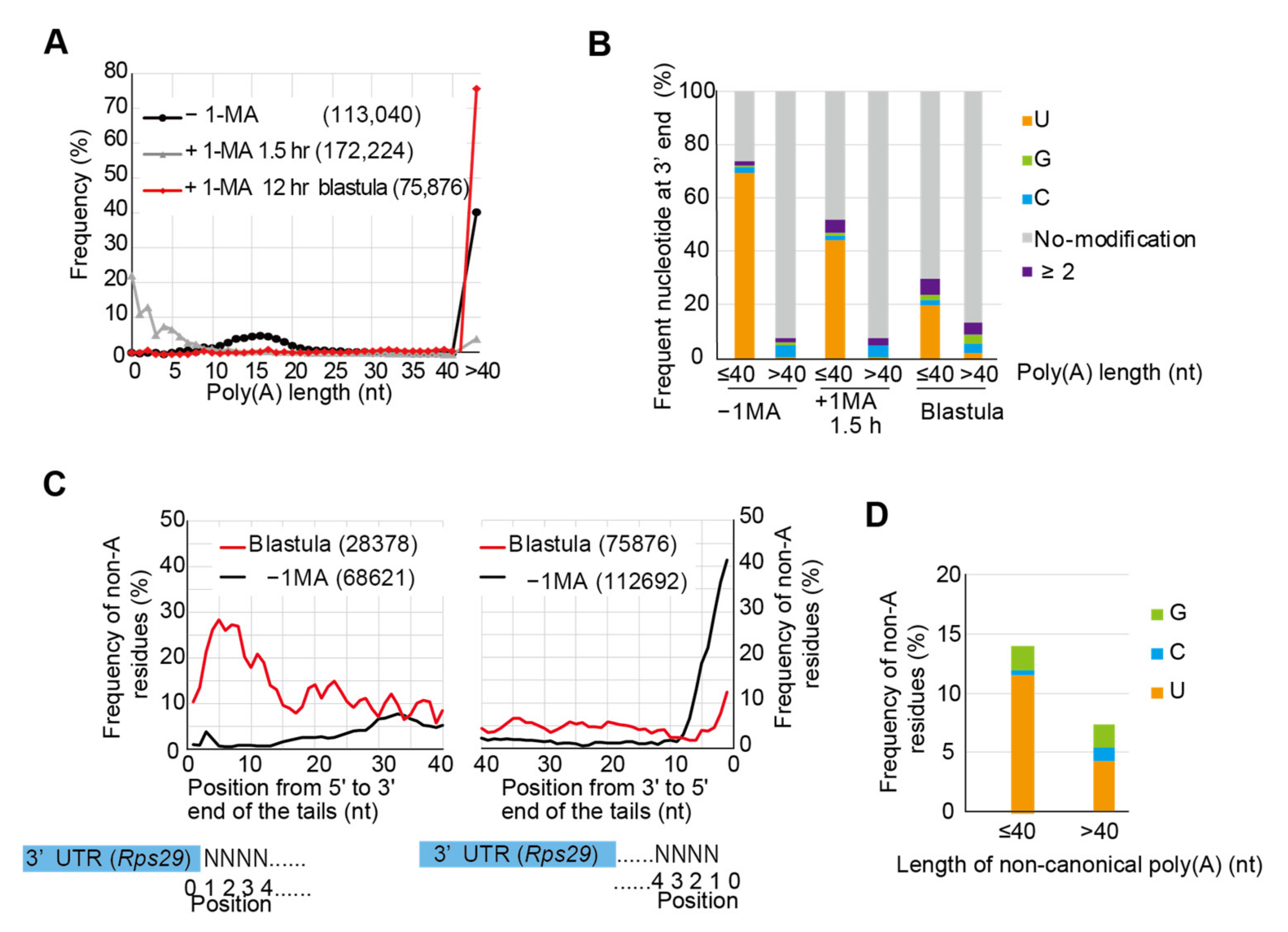
3.3. Targeted TAIL-Seq of Rps29 mRNA
3.4. Injected 3′ UTR of Rps29 mRNA Behaves Similarly to Endogenous mRNA
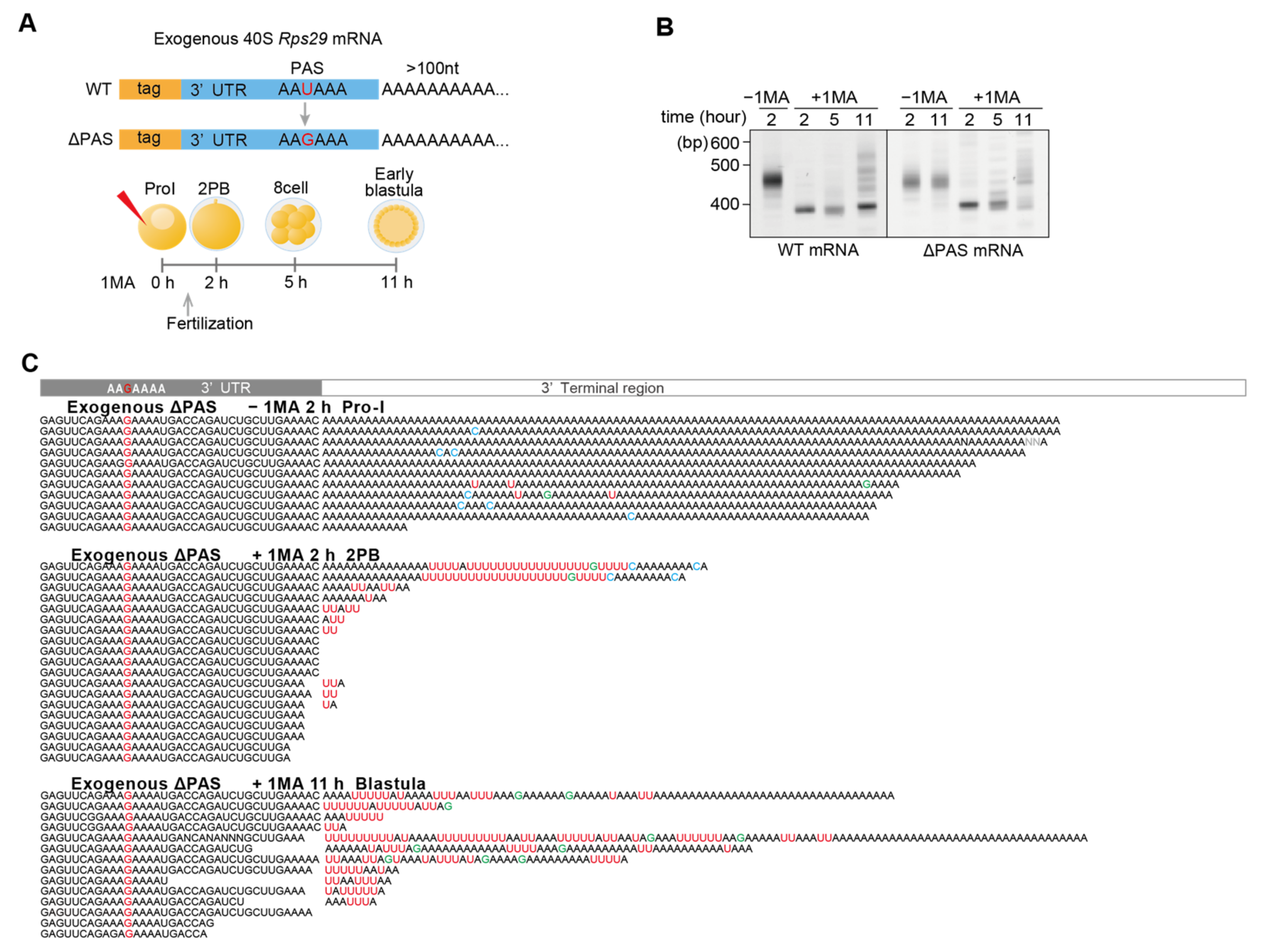
3.5. AAUAAA Cleavage Recognition Site and Polyadenylation Specificity Factor (CPSF) Are Not Required for Re-Polyadenylation
3.6. Non-Canonical Poly(A) Tailed mRNA Is Translationally Active
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brook, M.; Gray, N.K. The Role of Mammalian Poly(A)-Binding Proteins in Co-Ordinating mRNA Turnover. Biochem. Soc. Trans. 2012, 40, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Passmore, L.A.; Coller, J. Roles of mRNA Poly(A) Tails in Regulation of Eukaryotic Gene Expression. Nat. Rev. Mol. Cell Biol. 2022, 23, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ha, M.; Chang, H.; Kwon, S.C.; Simanshu, D.K.; Patel, D.J.; Kim, V.N. Uridylation by TUT4 and TUT7 Marks mRNA for Degradation. Cell 2014, 159, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yeo, J.; Kim, J.-G.; Kim, H.; Lim, J.; Lee, M.; Kim, H.H.; Ohk, J.; Jeon, H.-Y.; Lee, H.; et al. Terminal Uridylyltransferases Execute Programmed Clearance of Maternal Transcriptome in Vertebrate Embryos. Mol. Cell 2018, 70, 72–82.e7. [Google Scholar] [CrossRef]
- Sha, Q.-Q.; Zhu, Y.-Z.; Li, S.; Jiang, Y.; Chen, L.; Sun, X.-H.; Shen, L.; Ou, X.-H.; Fan, H.-Y. Characterization of Zygotic Genome Activation-Dependent Maternal mRNA Clearance in Mouse. Nucleic Acids Res. 2020, 48, 879–894. [Google Scholar] [CrossRef]
- Song, M.-G.; Kiledjian, M. 3′ Terminal Oligo U-Tract-Mediated Stimulation of Decapping. RNA 2007, 13, 2356–2365. [Google Scholar] [CrossRef]
- Lubas, M.; Damgaard, C.K.; Tomecki, R.; Cysewski, D.; Jensen, T.H.; Dziembowski, A. Exonuclease hDIS3L2 Specifies an Exosome-independent 3′-5′ Degradation Pathway of Human Cytoplasmic mRNA. EMBO J. 2013, 32, 1855–1868. [Google Scholar] [CrossRef]
- Viegas, S.C.; Silva, I.J.; Apura, P.; Matos, R.G.; Arraiano, C.M. Surprises in the 3′-End: ‘U’ Can Decide Too! FEBS J. 2015, 282, 3489–3499. [Google Scholar] [CrossRef]
- Morgan, M.; Much, C.; DiGiacomo, M.; Azzi, C.; Ivanova, I.; Vitsios, D.M.; Pistolic, J.; Collier, P.; Moreira, P.N.; Benes, V.; et al. mRNA 3′ Uridylation and Poly(A) Tail Length Sculpt the Mammalian Maternal Transcriptome. Nature 2017, 548, 347–351. [Google Scholar] [CrossRef]
- Malecki, M.; Viegas, S.C.; Carneiro, T.; Golik, P.; Dressaire, C.; Ferreira, M.G.; Arraiano, C.M. The Exoribonuclease Dis3L2 Defines a Novel Eukaryotic RNA Degradation Pathway. EMBO J. 2013, 32, 1842–1854. [Google Scholar] [CrossRef]
- Zhao, Q.; Pavanello, L.; Bartlam, M.; Winkler, G.S. Structure and Function of Molecular Machines Involved in Deadenylation-Dependent 5′-3′ mRNA Degradation. Front. Genet. 2023, 14, 1233842. [Google Scholar] [CrossRef]
- Almeida, C.D.; Scheer, H.; Zuber, H.; Gagliardi, D. RNA Uridylation: A Key Posttranscriptional Modification Shaping the Coding and Noncoding Transcriptome. WIREs RNA 2018, 9, e1440. [Google Scholar] [CrossRef] [PubMed]
- Pierandrei-Amaldi, P.; Campioni, N.; Beccari, E.; Bozzoni, I.; Amaldi, F. Expression of Ribosomal-Protein Genes in Xenopus Laevis Development. Cell 1982, 30, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.E.; Wormington, W.M. Translational Inactivation of Ribosomal Protein mRNAs during Xenopus Oocyte Maturation. Genes Dev. 1988, 2, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Bouvet, P.; Omilli, F.; Roghi, C.; Dorel, C.; LeGuellec, R.; Paris, J.; Osborne, H.B. Stability of Maternal mRNA in Xenopus Embryos: Role of Transcription and Translation. Mol. Cell. Biol. 1990, 10, 4123–4129. [Google Scholar] [CrossRef]
- Gillian-Daniel, D.L.; Gray, N.K.; Aström, J.; Barkoff, A.; Wickens, M. Modifications of the 5′ Cap of mRNAs during Xenopus Oocyte Maturation: Independence from Changes in Poly(A) Length and Impact on Translation. Mol. Cell. Biol. 1998, 18, 6152–6163. [Google Scholar] [CrossRef]
- Voeltz, G.K.; Steitz, J.A. AUUUA Sequences Direct mRNA Deadenylation Uncoupled from Decay during Xenopus Early Development. Mol. Cell. Biol. 1998, 18, 7537–7545. [Google Scholar] [CrossRef]
- Despic, V.; Neugebauer, K.M. RNA Tales–How Embryos Read and Discard Messages from Mom. J. Cell Sci. 2018, 131, jcs.201996. [Google Scholar] [CrossRef]
- Eisen, T.J.; Eichhorn, S.W.; Subtelny, A.O.; Lin, K.S.; McGeary, S.E.; Gupta, S.; Bartel, D.P. The Dynamics of Cytoplasmic mRNA Metabolism. Mol. Cell 2020, 77, 786–799.e10. [Google Scholar] [CrossRef]
- Chang, H.; Lim, J.; Ha, M.; Kim, V.N. TAIL-Seq: Genome-Wide Determination of Poly(A) Tail Length and 3′ End Modifications. Mol. Cell 2014, 53, 1044–1052. [Google Scholar] [CrossRef]
- Fox, C.A.; Wickens, M. Poly(A) Removal during Oocyte Maturation: A Default Reaction Selectively Prevented by Specific Sequences in the 3′ UTR of Certain Maternal mRNAs. Genes Dev. 1990, 4, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Hake, L.E.; Richter, J.D. CPEB Is a Specificity Factor That Mediates Cytoplasmic Polyadenylation during Xenopus Oocyte Maturation. Cell 1994, 79, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Copeland, P.R.; Wormington, M. The Mechanism and Regulation of Deadenylation: Identification and Characterization of Xenopus PARN. RNA 2001, 7, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.E.; Wang, L.; Ballantyne, S.; Kimble, J.; Wickens, M. Mammalian GLD-2 Homologs Are Poly(A) Polymerases. Proc. Natl. Acad. Sci. USA 2004, 101, 4407–4412. [Google Scholar] [CrossRef]
- Kim, J.H.; Richter, J.D. Opposing Polymerase-Deadenylase Activities Regulate Cytoplasmic Polyadenylation. Mol. Cell 2006, 24, 173–183. [Google Scholar] [CrossRef]
- Ochi, H.; Chiba, K. Hormonal Stimulation of Starfish Oocytes Induces Partial Degradation of the 3′ Termini of Cyclin B mRNAs with Oligo(U) Tails, Followed by Poly(A) Elongation. RNA 2016, 22, 822–829. [Google Scholar] [CrossRef]
- Hiraoka, D.; Hosoda, E.; Chiba, K.; Kishimoto, T. SGK Phosphorylates Cdc25 and Myt1 to Trigger Cyclin B-Cdk1 Activation at the Meiotic G2/M Transition. J. Cell Biol. 2019, 218, 3597–3611. [Google Scholar] [CrossRef]
- Hosoda, E.; Hiraoka, D.; Hirohashi, N.; Omi, S.; Kishimoto, T.; Chiba, K. SGK Regulates pH Increase and Cyclin B–Cdk1 Activation to Resume Meiosis in Starfish Ovarian Oocytes. J. Cell Biol. 2019, 218, 3612–3629. [Google Scholar] [CrossRef]
- McCauley, B.S.; Akyar, E.; Saad, H.R.; Hinman, V.F. Dose-Dependent Nuclear β-Catenin Response Segregates Endomesoderm along the Sea Star Primary Axis. Development 2015, 142, 207–217. [Google Scholar] [CrossRef]
- Gildor, T.; Hinman, V.; Ben-Tabou-De-Leon, S. Regulatory Heterochronies and Loose Temporal Scaling between Sea Star and Sea Urchin Regulatory Circuits. Int. J. Dev. Biol. 2017, 61, 347–356. [Google Scholar] [CrossRef]
- Vastenhouw, N.L.; Cao, W.X.; Lipshitz, H.D. The Maternal-to-Zygotic Transition Revisited. Dev. Camb. Engl. 2019, 146, dev161471. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hara, M.; Taneda, T.; Qingfu, C.; Takata, R.; Moro, K.; Takeda, K.; Kishimoto, T.; Handa, H. Antisense Morpholino Targeting Just Upstream from a Poly(A) Tail Junction of Maternal mRNA Removes the Tail and Inhibits Translation. Nucleic Acids Res. 2012, 40, e173. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Okumura, E.-I.; Hinkle, B.; Kishimoto, T. Localization and Dynamics of Cdc2-Cyclin B during Meiotic Reinitiation in Starfish Oocytes. Mol. Biol. Cell 2003, 14, 4685–4694. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, D.; Aono, R.; Hanada, S.-I.; Okumura, E.; Kishimoto, T. Two New Competing Pathways Establish the Threshold for Cyclin-B-Cdk1 Activation at the Meiotic G2/M Transition. J. Cell Sci. 2016, 129, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Tadenuma, H.; Matsumoto, M.; Takahashi, K.; Katada, T.; Hoshi, M. The Primary Structure of the Alpha Subunit of a Starfish Guanosine-Nucleotide-Binding Regulatory Protein Involved in 1-Methyladenine-Induced Oocyte Maturation. Eur. J. Biochem. 1992, 207, 833–838. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Audic, Y.; Anderson, C.; Bhatty, R.; Hartley, R.S. Zygotic Regulation of Maternal Cyclin A1 and B2 mRNAs. Mol. Cell. Biol. 2001, 21, 1662–1671. [Google Scholar] [CrossRef]
- Tadros, W.; Lipshitz, H.D. The Maternal-to-Zygotic Transition: A Play in Two Acts. Dev. Camb. Engl. 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Wormington, W.M. Developmental Expression and 5S rRNA-Binding Activity of Xenopus Laevis Ribosomal Protein L5. Mol. Cell. Biol. 1989, 9, 5281–5288. [Google Scholar] [CrossRef]
- Baum, E.Z.; Wormington, W.M. Coordinate Expression of Ribosomal Protein Genes during Xenopus Development. Dev. Biol. 1985, 111, 488–498. [Google Scholar] [CrossRef] [PubMed]
- McGrew, L.L.; Dworkin-Rastl, E.; Dworkin, M.B.; Richter, J.D. Poly(A) Elongation during Xenopus Oocyte Maturation Is Required for Translational Recruitment and Is Mediated by a Short Sequence Element. Genes Dev. 1989, 3, 803–815. [Google Scholar] [CrossRef]
- Dai, X.-X.; Jiang, J.-C.; Sha, Q.-Q.; Jiang, Y.; Ou, X.-H.; Fan, H.-Y. A Combinatorial Code for mRNA 3′-UTR-Mediated Translational Control in the Mouse Oocyte. Nucleic Acids Res. 2019, 47, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Ochi, H.; Aoto, S.; Tachibana, K.; Hara, M.; Chiba, K. Block of CDK1-Dependent Polyadenosine Elongation of Cyclin B mRNA in Metaphase-i-Arrested Starfish Oocytes Is Released by Intracellular pH Elevation upon Spawning. Mol. Reprod. Dev. 2016, 83, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.C.; Ryan, K.; Manley, J.L.; Richter, J.D. Symplekin and xGLD-2 Are Required for CPEB-Mediated Cytoplasmic Polyadenylation. Cell 2004, 119, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Warkocki, Z.; Liudkovska, V.; Gewartowska, O.; Mroczek, S.; Dziembowski, A. Terminal Nucleotidyl Transferases (TENTs) in Mammalian RNA Metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20180162. [Google Scholar] [CrossRef]
- Varnum, S.M.; Wormington, W.M. Deadenylation of Maternal mRNAs during Xenopus Oocyte Maturation Does Not Require Specific Cis-Sequences: A Default Mechanism for Translational Control. Genes Dev. 1990, 4, 2278–2286. [Google Scholar] [CrossRef]
- Paynton, B.V.; Bachvarova, R. Polyadenylation and Deadenylation of Maternal mRNAs during Oocyte Growth and Maturation in the Mouse. Mol. Reprod. Dev. 1994, 37, 172–180. [Google Scholar] [CrossRef]
- Lund, E.; Liu, M.; Hartley, R.S.; Sheets, M.D.; Dahlberg, J.E. Deadenylation of Maternal mRNAs Mediated by miR-427 in Xenopus Laevis Embryos. RNA 2009, 15, 2351–2363. [Google Scholar] [CrossRef]
- Audic, Y.; Omilli, F.; Osborne, H.B. Postfertilization Deadenylation of mRNAs in Xenopus Laevis Embryos Is Sufficient to Cause Their Degradation at the Blastula Stage. Mol. Cell. Biol. 1997, 17, 209–218. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, H.; Liu, H.; Lu, F. Poly(A) Inclusive RNA Isoform Sequencing (PAIso-Seq) Reveals Wide-Spread Non-Adenosine Residues within RNA Poly(A) Tails. Nat. Commun. 2019, 10, 5292. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, D.; Lee, Y.-S.; Ha, M.; Lee, M.; Yeo, J.; Chang, H.; Song, J.; Ahn, K.; Kim, V.N. Mixed Tailing by TENT4A and TENT4B Shields mRNA from Rapid Deadenylation. Science 2018, 361, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Gewartowska, O.; Aranaz-Novaliches, G.; Krawczyk, P.S.; Mroczek, S.; Kusio-Kobiałka, M.; Tarkowski, B.; Spoutil, F.; Benada, O.; Kofroňová, O.; Szwedziak, P.; et al. Cytoplasmic Polyadenylation by TENT5A Is Required for Proper Bone Formation. Cell Rep. 2021, 35, 109015. [Google Scholar] [CrossRef]
- Bilska, A.; Kusio-Kobiałka, M.; Krawczyk, P.S.; Gewartowska, O.; Tarkowski, B.; Kobyłecki, K.; Nowis, D.; Golab, J.; Gruchota, J.; Borsuk, E.; et al. Immunoglobulin Expression and the Humoral Immune Response Is Regulated by the Non-Canonical Poly(A) Polymerase TENT5C. Nat. Commun. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Weill, L.; Belloc, E.; Bava, F.-A.; Méndez, R. Translational Control by Changes in Poly(A) Tail Length: Recycling mRNAs. Nat. Struct. Mol. Biol. 2012, 19, 577–585. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, H.; Furuichi, M.; Katagiri, M.; Kajitani, R.; Itoh, T.; Chiba, K. Recycling of Uridylated mRNAs in Starfish Embryos. Biomolecules 2024, 14, 1610. https://doi.org/10.3390/biom14121610
Yamazaki H, Furuichi M, Katagiri M, Kajitani R, Itoh T, Chiba K. Recycling of Uridylated mRNAs in Starfish Embryos. Biomolecules. 2024; 14(12):1610. https://doi.org/10.3390/biom14121610
Chicago/Turabian StyleYamazaki, Haruka, Megumi Furuichi, Mikoto Katagiri, Rei Kajitani, Takehiko Itoh, and Kazuyoshi Chiba. 2024. "Recycling of Uridylated mRNAs in Starfish Embryos" Biomolecules 14, no. 12: 1610. https://doi.org/10.3390/biom14121610
APA StyleYamazaki, H., Furuichi, M., Katagiri, M., Kajitani, R., Itoh, T., & Chiba, K. (2024). Recycling of Uridylated mRNAs in Starfish Embryos. Biomolecules, 14(12), 1610. https://doi.org/10.3390/biom14121610





