Is Hemopexin a Nephrotoxin or a Marker of Kidney Injury in Renal Ischemia-Reperfusion?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Kidney Function and Histopathological Studies
2.3. Immunohistochemistry
2.4. Western Blot Analysis
2.5. Enzyme Linked Immunosorbent Assay
2.6. Statistical Analysis
3. Results
3.1. Kidney Function and Renal Injury Scores
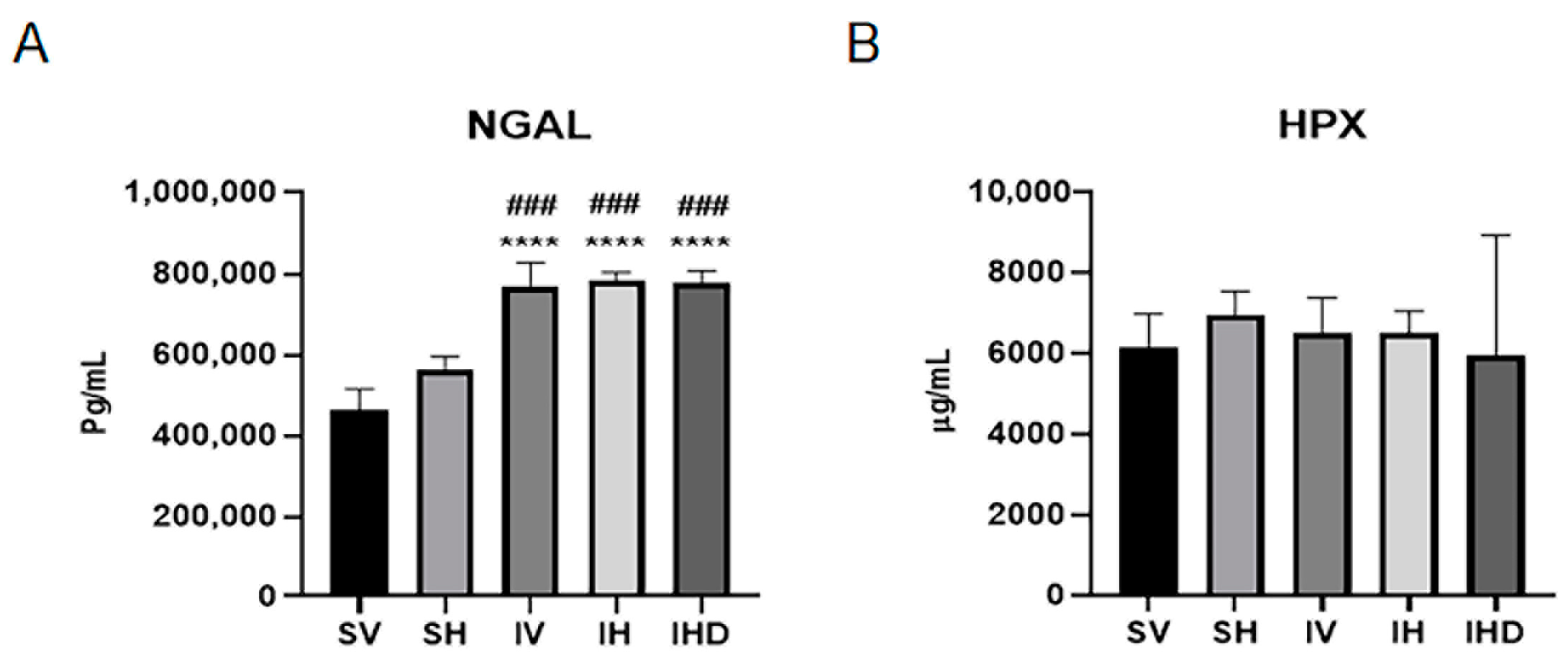
3.2. Increased NGAL Expression in IRI Groups Independent of Hpx Infusion
3.3. Distinct Serum Profiles of NGAL and Hpx in Response to IRI
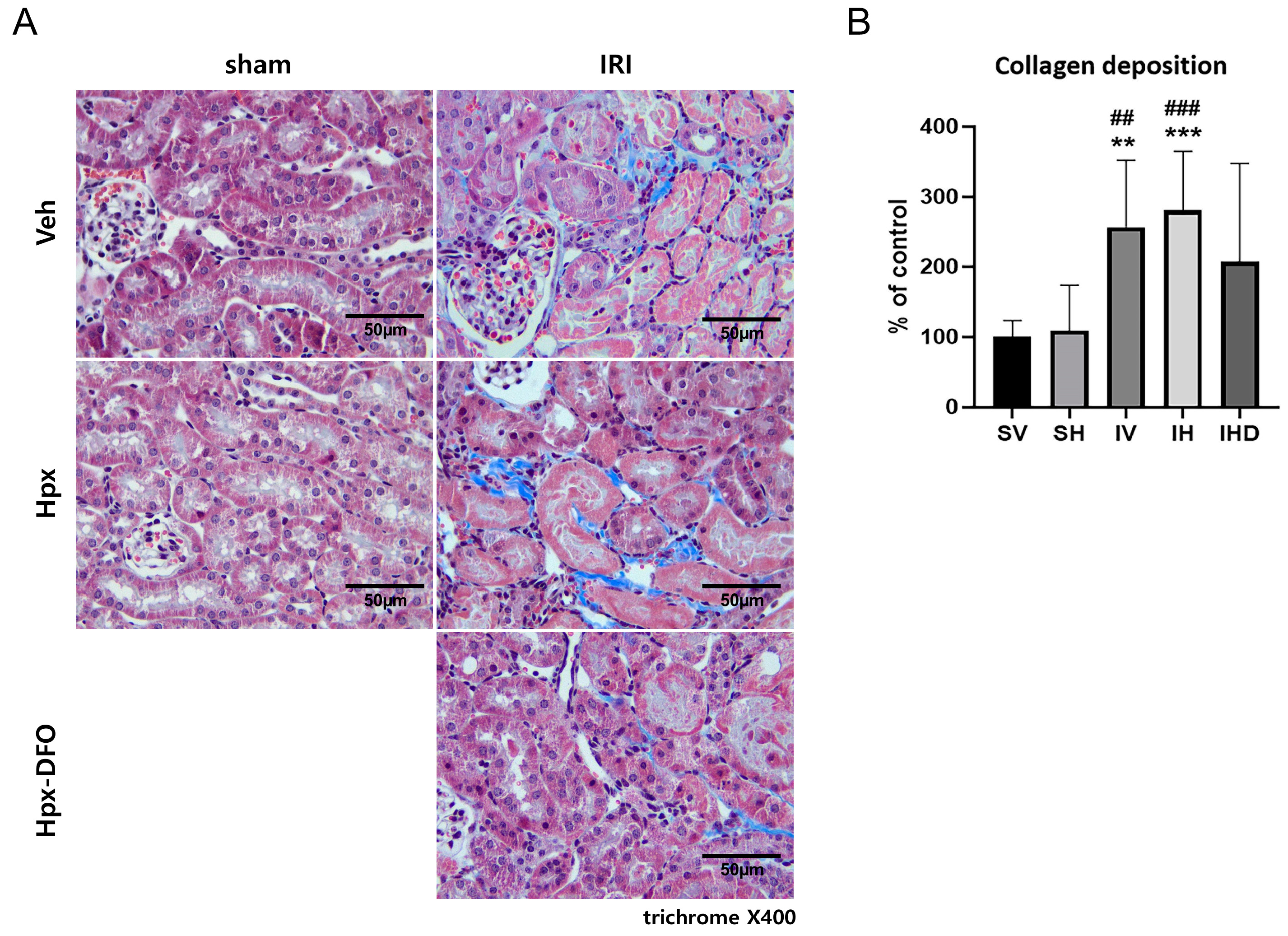
3.4. Collagen Deposition in the IRI Groups
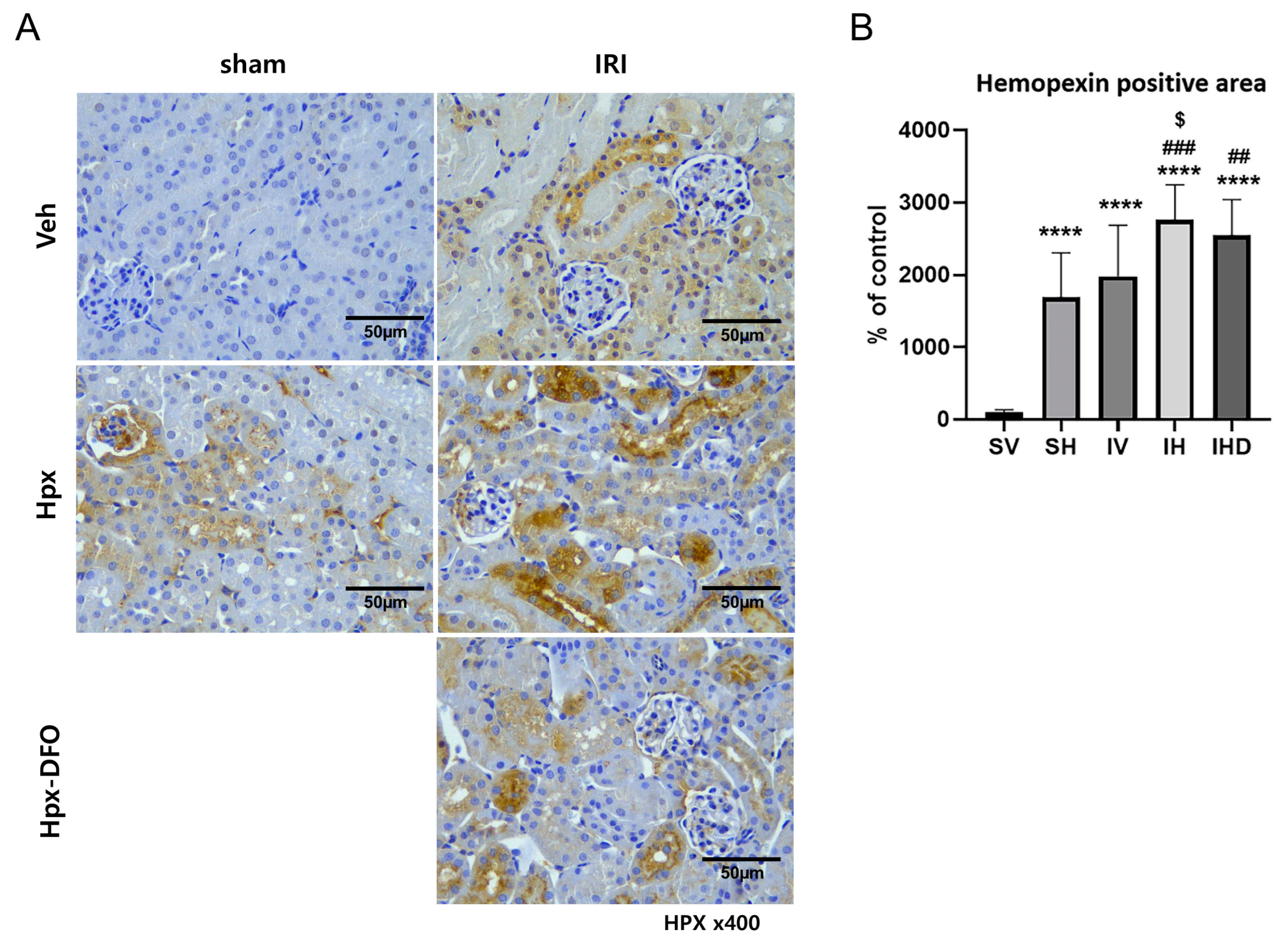
3.5. Hpx Deposition Induced by IRI and Hpx Infusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Goldstein, S.L.; Vijayan, A.; Parikh, C.R.; Kashani, K.; Okusa, M.D.; Agarwal, A.; Cerda, J.; AKI!Now Initiative of the American Society of Nephrology. AKI!Now Initiative: Recommendations for Awareness, Recognition, and Management of AKI. Clin. J. Am. Soc. Nephrol. 2020, 15, 1838–1847. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Schetz, M.; Schortgen, F. Ten shortcomings of the current definition of AKI. Intensive Care Med. 2017, 43, 911–913. [Google Scholar] [CrossRef]
- Albert, C.; Zapf, A.; Haase, M.; Röver, C.; Pickering, J.W.; Albert, A.; Bellomo, R.; Breidthardt, T.; Camou, F.; Chen, Z.; et al. Neutrophil Gelatinase-Associated Lipocalin Measured on Clinical Laboratory Platforms for the Prediction of Acute Kidney Injury and the Associated Need for Dialysis Therapy: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 826–841.e1. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Qin, Z.; Su, B. The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: A systematic review and Bayesian meta-analysis. J. Transl. Med. 2021, 19, 105. [Google Scholar] [CrossRef]
- Pickkers, P.; Darmon, M.; Hoste, E.; Joannidis, M.; Legrand, M.; Ostermann, M.; Prowle, J.R.; Schneider, A.; Schetz, M. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensive Care Med. 2021, 47, 835–850. [Google Scholar] [CrossRef]
- Gaut, J.P.; Liapis, H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2021, 14, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Van Avondt, K.; Nur, E.; Zeerleder, S. Mechanisms of haemolysis-induced kidney injury. Nat. Rev. Nephrol. 2019, 15, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.D.T.G.; Volk, H.-D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different Faces of the Heme-Heme Oxygenase System in Inflammation. Pharmacol. Rev. 2003, 55, 551. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Singh, R.D.; Croatt, A.J.; Adams, C.M. Heme Proteins and Kidney Injury: Beyond Rhabdomyolysis. Kidney360 2022, 3, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D.; Mayer, R.D.; Ewing, J.F.; McCoubrey, W.K., Jr. Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: Possible role of heme as both promotor of tissue damage and regulator of HSP32. J. Pharmacol. Exp. Ther. 1993, 264, 457–462. [Google Scholar]
- Agarwal, A.; Balla, J.; Alam, J.; Croatt, A.J.; Nath, K.A. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995, 48, 1298–1307. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Nath, K.A.; Grande, J.P.; Farrugia, G.; Croatt, A.J.; Belcher, J.D.; Hebbel, R.P.; Vercellotti, G.M.; Katusic, Z.S. Age sensitizes the kidney to heme protein-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2013, 304, F317–F325. [Google Scholar] [CrossRef]
- Nath, K.A.; Balla, G.; Vercellotti, G.M.; Balla, J.; Jacob, H.S.; Levitt, M.D.; Rosenberg, M.E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 1992, 90, 267–270. [Google Scholar] [CrossRef]
- Everse, J.; Hsia, N. The toxicities of native and modified hemoglobins. Free Radic. Biol. Med. 1997, 22, 1075–1099. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Leaf, D.E.; Rajapurkar, M.; Lele, S.S.; Mukhopadhyay, B.; Boerger, E.A.S.; Mc Causland, F.R.; Eisenga, M.F.; Singh, K.; Babitt, J.L.; Kellum, J.A.; et al. Iron, Hepcidin, and Death in Human AKI. J. Am. Soc. Nephrol. 2019, 30, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.V.; Walker, P.D. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am. J. Physiol. 1988, 255, F438–F443. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.S. Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am. J. Physiol. 1988, 255, F539–F544. [Google Scholar] [CrossRef]
- Tolosano, E.; Altruda, F. Hemopexin: Structure, function, and regulation. DNA Cell Biol. 2002, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Acquah, S.F.; Hazra, R.; Orikogbo, O.O.; Crosby, D.; Flage, B.; Ackah, E.B.; Lenhart, D.; Tan, R.J.; Vitturi, D.A.; Paintsil, V.; et al. Hemopexin deficiency promotes acute kidney injury in sickle cell disease. Blood 2020, 135, 1044–1048. [Google Scholar] [CrossRef]
- Bakker, W.W.; Borghuis, T.; Harmsen, M.C.; van den Berg, A.; Kema, I.P.; Niezen, K.E.; Kapojos, J.J. Protease activity of plasma hemopexin. Kidney Int. 2005, 68, 603–610. [Google Scholar] [CrossRef]
- Cheung, P.K.; Klok, P.A.; Baller, J.F.; Bakker, W.W. Induction of experimental proteinuria in vivo following infusion of human plasma hemopexin. Kidney Int. 2000, 57, 1512–1520. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Liu, L.C.; Zhang, S.; Pelger, C.B.; Lughmani, H.Y.; Haller, S.T.; Gunning, W.T., 3rd; Cooper, C.J.; Gong, R.; et al. Hemopexin accumulates in kidneys and worsens acute kidney injury by causing hemoglobin deposition and exacerbation of iron toxicity in proximal tubules. Kidney Int. 2022, 102, 1320–1330. [Google Scholar] [CrossRef]
- Poillerat, V.; Gentinetta, T.; Leon, J.; Wassmer, A.; Edler, M.; Torset, C.; Luo, D.; Tuffin, G.; Roumenina, L.T. Hemopexin as an Inhibitor of Hemolysis-Induced Complement Activation. Front. Immunol. 2020, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Ikeda, M.; Miyamoto, H.D.; Furusawa, S.; Abe, K.; Watanabe, M.; Kanamura, T.; Fujita, S.; Nishimura, R.; Toyohara, T.; et al. Deferasirox Targeting Ferroptosis Synergistically Ameliorates Myocardial Ischemia Reperfusion Injury in Conjunction With Cyclosporine A. J. Am. Heart Assoc. 2024, 13, e031219. [Google Scholar] [CrossRef]
- Oishi, S.; Tsukiji, N.; Otake, S.; Oishi, N.; Sasaki, T.; Shirai, T.; Yoshikawa, Y.; Takano, K.; Shinmori, H.; Inukai, T.; et al. Heme activates platelets and exacerbates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRγ. Blood Adv. 2021, 5, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Boudhabhay, I.; Poillerat, V.; Grunenwald, A.; Torset, C.; Leon, J.; Daugan, M.V.; Lucibello, F.; El Karoui, K.; Ydee, A.; Chauvet, S.; et al. Complement activation is a crucial driver of acute kidney injury in rhabdomyolysis. Kidney Int. 2021, 99, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; McCulloh, R.J. Hemopexin and haptoglobin: Allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.J.; Alam, J.; Nath, K.A. Physiology and pathophysiology of heme: Implications for kidney disease. J. Am. Soc. Nephrol. 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Belcher, J.D.; Nath, K.A.; Vercellotti, G.M. Vasculotoxic and Proinflammatory Effects of Plasma Heme: Cell Signaling and Cytoprotective Responses. ISRN Oxidative Med. 2013, 2013, 831596. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Haggard, J.J.; Croatt, A.J.; Grande, J.P.; Poss, K.D.; Alam, J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am. J. Pathol. 2000, 156, 1527–1535. [Google Scholar] [CrossRef]
- Vinchi, F.; Costa da Silva, M.; Ingoglia, G.; Petrillo, S.; Brinkman, N.; Zuercher, A.; Cerwenka, A.; Tolosano, E.; Muckenthaler, M.U. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 2016, 127, 473–486. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Abdulla, F.; Zhang, P.; Nguyen, H.; Nguyen, P.; Killeen, T.; Miescher, S.M.; Brinkman, N.; et al. Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: Role of heme oxygenase-1 induction. PLoS ONE 2018, 13, e0196455. [Google Scholar] [CrossRef]
- Ashouri, R.; Fangman, M.; Burris, A.; Ezenwa, M.O.; Wilkie, D.J.; Doré, S. Critical Role of Hemopexin Mediated Cytoprotection in the Pathophysiology of Sickle Cell Disease. Int. J. Mol. Sci. 2021, 22, 6408. [Google Scholar] [CrossRef]
- Lennon, R.; Singh, A.; Welsh, G.I.; Coward, R.J.; Satchell, S.; Ni, L.; Mathieson, P.W.; Bakker, W.W.; Saleem, M.A. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J. Am. Soc. Nephrol. 2008, 19, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Bakker, W.W.; van Dael, C.M.; Pierik, L.J.; van Wijk, J.A.; Nauta, J.; Borghuis, T.; Kapojos, J.J. Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr. Nephrol. 2005, 20, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.; Becker, K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am. J. Physiol. Renal Physiol. 2012, 303, F1460–F1472. [Google Scholar] [CrossRef] [PubMed]
- Cowled, P.; Fitridge, R. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; Chapter 18; pp. 331–350. [Google Scholar]
- Kalapotharakos, G.; Murtoniemi, K.; Åkerström, B.; Hämäläinen, E.; Kajantie, E.; Räikkönen, K.; Villa, P.; Laivuori, H.; Hansson, S.R. Plasma Heme Scavengers Alpha-1-Microglobulin and Hemopexin as Biomarkers in High-Risk Pregnancies. Front. Physiol. 2019, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.M.; Ward, L.J.; Forssell, C.; Siraj, N.; Li, W. Carotid Atheroma From Men Has Significantly Higher Levels of Inflammation and Iron Metabolism Enabled by Macrophages. Stroke 2018, 49, 419–425. [Google Scholar] [CrossRef]
- Estelius, J.; Lengqvist, J.; Ossipova, E.; Idborg, H.; Le Maître, E.; Andersson, M.L.A.; Brundin, L.; Khademi, M.; Svenungsson, E.; Jakobsson, P.J.; et al. Mass spectrometry-based analysis of cerebrospinal fluid from arthritis patients-immune-related candidate proteins affected by TNF blocking treatment. Arthritis Res. Ther. 2019, 21, 60. [Google Scholar] [CrossRef]
- Brewin, J.; Tewari, S.; Menzel, S.; Kirkham, F.; Inusa, B.; Renney, G.; Ward, M.; Rees, D.C. The effects of hydroxycarbamide on the plasma proteome of children with sickle cell anaemia. Br. J. Haematol. 2019, 186, 879–886. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Chebotareva, N.V.; Bugrova, A.E.; Brzhozovskiy, A.G.; Krasnova, T.N.; Nasibullina, K.Z.; Kononikhin, A.S.; Moiseev, S.V. Study of urinary markers of different podocytopathies by proteomic analysis. Ter. Arkh. 2023, 95, 457–461. [Google Scholar] [CrossRef]
- Zhou, L.; Han, S.; Guo, J.; Qiu, T.; Zhou, J.; Shen, L. Ferroptosis-A New Dawn in the Treatment of Organ Ischemia-Reperfusion Injury. Cells 2022, 11, 3653. [Google Scholar] [CrossRef]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Morel, C.M.; Provance, D.W.; De-Simone, S.G. New Insights into Hemopexin-Binding to Hemin and Hemoglobin. Int. J. Mol. Sci. 2022, 23, 3789. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.H.; Oh, E.-J.; Oh, S.-H.; Lim, J.-H.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Kim, C.-D. Is Hemopexin a Nephrotoxin or a Marker of Kidney Injury in Renal Ischemia-Reperfusion? Biomolecules 2024, 14, 1522. https://doi.org/10.3390/biom14121522
Jeon YH, Oh E-J, Oh S-H, Lim J-H, Jung H-Y, Choi J-Y, Cho J-H, Park S-H, Kim Y-L, Kim C-D. Is Hemopexin a Nephrotoxin or a Marker of Kidney Injury in Renal Ischemia-Reperfusion? Biomolecules. 2024; 14(12):1522. https://doi.org/10.3390/biom14121522
Chicago/Turabian StyleJeon, You Hyun, Eun-Joo Oh, Se-Hyun Oh, Jeong-Hoon Lim, Hee-Yeon Jung, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, Yong-Lim Kim, and Chan-Duck Kim. 2024. "Is Hemopexin a Nephrotoxin or a Marker of Kidney Injury in Renal Ischemia-Reperfusion?" Biomolecules 14, no. 12: 1522. https://doi.org/10.3390/biom14121522
APA StyleJeon, Y. H., Oh, E.-J., Oh, S.-H., Lim, J.-H., Jung, H.-Y., Choi, J.-Y., Cho, J.-H., Park, S.-H., Kim, Y.-L., & Kim, C.-D. (2024). Is Hemopexin a Nephrotoxin or a Marker of Kidney Injury in Renal Ischemia-Reperfusion? Biomolecules, 14(12), 1522. https://doi.org/10.3390/biom14121522










