Abstract
Autophagy is an important catabolic process to maintain cellular homeostasis and antagonize cellular stresses. The initiation and activation are two of the most important aspects of the autophagic process. This review focuses on mechanisms underlying autophagy initiation and activation and signaling pathways regulating the activation of autophagy found in recent years. These findings include autophagy initiation by liquid–liquid phase separation (LLPS), autophagy initiation in the endoplasmic reticulum (ER) and Golgi apparatus, and the signaling pathways mediated by the ULK1 complex, the mTOR complex, the AMPK complex, and the PI3KC3 complex. Through the review, we attempt to present current research progress in autophagy regulation and forward our understanding of the regulatory mechanisms and signaling pathways of autophagy initiation and activation.
1. Overview of Autophagy
Autophagy is a highly conserved cellular catabolic process [1]. Since Yosuke Ohsumi discovered the key autophagic genes in yeast [2,3], numerous studies have uncovered the molecular events of autophagy from yeast to mammalian cells. Autophagy is divided into three types: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) [4,5]. In microautophagy, cargo proteins are degraded through the direct invagination of lysosomal membranes [6], while in CMA, cargos bind to chaperones that mediate the entry of cargos into lysosomes for degradation [7]. In macroautophagy, cargos are wrapped by double-layer membrane vesicles (autophagosomes) and transported to the lysosome for degradation [8,9] (Figure 1). Autophagy, when mentioned in this article, refers to macroautophagy.
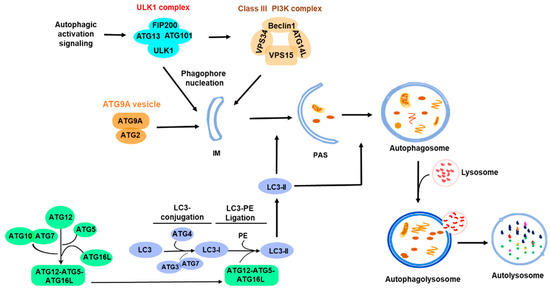
Figure 1.
Overview of autophagy process. The ULK1 complex is activated under various autophagy signals to initiate autophagy, and the nucleation process is completed with the participation of the PI3K complex and the ATG9A system. Furthermore, the extension of the autophagic membrane is achieved through the two ubiquitin-like ATG conjugation and ligation processes: the ATG3-ATG7-mediated LC3 conjugation and the ATG12-ATG5-ATG16L-mediated LC3 ligation with phosphatidylethanolamine (PE). The PAS develops into an autophagosome that eventually fuses with a lysosome for degradation. The shaded area indicates the initiation part of the autophagic processes. IM, isolation membrane; PAS, pre-autophagosomal structure; PE, phosphatidylethanolamine.
Macroautophagy or autophagy is classified into two types, non-selective and selective autophagy, based on the autophagic cargo types. In non-selective autophagy, the cargo, usually a portion of cytoplasm, is randomly wrapped into autophagosomes for lysosomal degradation to provide nutrition for cells. In selective autophagy, the autophagic cargo such as damaged organelles, misfolded proteins, invaded bacteria, or viral particles is recognized by autophagic receptors, recruited to autophagosomes, and transported to lysosomes for degradation [10,11]. As shown in Figure 1, the autophagic process is organized, driven, and regulated by a variety of autophagic signaling pathways and autophagy-related (ATG) proteins [12,13,14], including ULK1 complex (ULK1, FIP200, ATG13, ATG101), PI3KC3 complex (VPS34, VPS15, BECN1, ATG14L), ATG9A vesicle transport system (ATG9A and ATG2A), and two ubiquitin-like conjugation and ligation complexes: the ATG3-ATG7 complex and the ATG12-ATG5-ATG16L complex for LC3 conjugation and ligation with phosphatidylethanolamine (PE) [15,16,17]. The lipidation of ATG8 (or LC3 in mammalian cells) family proteins with PE was thought to be a specific process in autophagy for autophagosomal biogenesis. However, recent studies have found that the lipidation of the ATG8 family proteins occurs not only on autophagosomes, but also on other types of membranes, such as endosomes and lysosomes [18,19,20,21,22], and participates in other cellular processes, such as extracellular vesicle secretion and the repair of lysosomal membrane damage, in addition to autophagy [20,21]. ATG8 family proteins can also be lipidated with phosphatidylserine (PS) to conjugate to single membranes in non-canonical autophagy [23]. Furthermore, it was observed that ATG8 family proteins are conjugated with ATG3 by covalently linking to the lysine 243 residue of ATG3, similar to the reaction in protein ubiquitination [18], suggesting that ATG8 family proteins may function as a ubiquitin-like protein to directly modify other proteins. Thus, it is proposed that ATG8 family proteins, akin to ubiquitin, are a class of general membrane and protein modification molecules. This membrane or protein modification by ATG8 family proteins is designated as ATG8ylation or LC3ylation in mammalian cells [18,19]. ATG8ylation emerges as a new biomolecular modification mode and may play important roles not only in autophagy but also in many other cellular processes.
Autophagy is activated by the ULK1 complex. When the upstream signaling of autophagy, such as inhibition of mTOR or activation of AMPK, is elicited, the ULK1 complex is subsequently assembled, thereby activating the class III PI3K complex. The activated PI3K complex generates phosphatidylinositol 3-phosphate (PI3P) to recruit and assemble a pre-autophagosomal structure (PAS). At the same time, ATG9A provides membrane sources to the PAS for the formation of autophagosomes [24]. Apparently, signaling that activates autophagy is the key factor determining where and when the autophagy occurs. Therefore, the investigation of the mechanisms underlying the initiation and activation of autophagy is crucial for understanding the regulation and biological function of autophagy and targeting autophagy for the therapy of autophagy-associated diseases. This review will go through the recent findings about the initiation and activation of autophagy and hopefully provide insight into the mechanisms underlying the initiation and activation of autophagy.
2. Role of Liquid–Liquid Phase Separation (LLPS) in the Assembly of Pre-Autophagosomal Structure (PAS) During Initiation of Autophagy
Liquid–liquid phase separation (LLPS) refers to the process of forming phase-separated droplets with different components and properties through the interaction of certain biological macromolecules (such as proteins and RNA) in cells [25,26,27,28]. Current studies have found that proteins that drive LLPS usually have one or more intrinsically disordered regions (IDRs) [29], and the promotion of phase separation by IDRs may be related to their lack of hydrophobic amino acids [30]. The abnormality of LLPS is associated with a variety of diseases [31,32,33,34,35].
In recent years, more and more evidence shows that LLPS plays an important role in autophagy [36,37], especially in autophagy initiation and the formation of autophagosome precursors [38,39,40]. The mechanism underlying the LLPS-regulated assembly of pre-autophagosomal structures (PASs) in yeast has been well studied (Figure 2). A PAS is a transient structure formed on yeast vacuoles [41]. An early PAS is an Atg1 complex composed of Atg1, Atg13, Atg17, Atg29, and Atg31, which gradually matures by recruiting downstream ATG proteins and vesicles, forming the initiation site of autophagy [3,40,42]. The PAS-forming protein complex contains a rich IDR domain [3], and the pattern of PAS formation is highly consistent with the molecular aggregates formed by LLPS. Yuko Fujioka’s team showed that a PAS is a molecular condensate, and its formation is regulated by LLPS [40]. It was found that Atg13 binds to different regions of Atg17 through its Atg17 binding region (17BR) and Atg17 linking region (17LR) and forms early PAS droplets through LLPS to initiate autophagy [3]. The droplets are connected to vacuoles through the Vac8 protein [43], which is consistent with the vacuole localization of the PAS in yeast. This process is regulated by the TOR-mediated phosphorylation of Atg13. Under nutrient-rich conditions, Ser428 and Ser429 of Atg13 are phosphorylated by TOR, and phosphorylated Atg13 impairs its interaction with Atg17 and inhibits the LLPS-mediated formation of PASs [44]. In addition, the LLPS of Atg13 and Atg17-Atg29-Atg31 increases Atg1 kinase activity, leading to autophagy activation, and activated Atg1 further inhibits PAS formation by phosphorylating Atg13 [39,40]. This process can be inhibited by the phosphatase Ptc2- and Ptc3-mediated dephosphorylation of Atg1 and Atg13 [45].
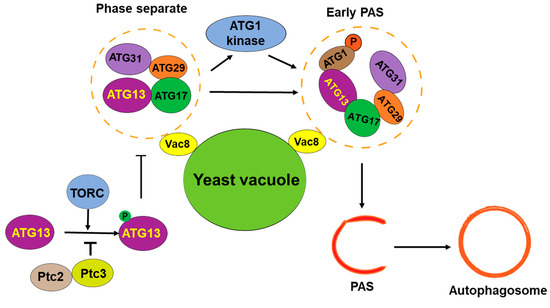
Figure 2.
LLPS in assembly of PAS in yeast cells. The protein complex of autophagy initiation is assembled through phase separation and organized into early PAS. At the same time, TORC, an important TOR protein complex, regulates the phase separation of the autophagy initiation complex.
It has been found that FIP200 also has LLPS in mammalian cells, and when the K276 site of its IDR region is acetylated by the acetyltransferase CREBBP, the protein stability is increased to strengthen its LLPS characteristics further and promotes the formation of autophagosomes [46]. In addition, LLPS affects TOR activity and thus affects the formation of autophagy precursors in yeast. This phenomenon also occurs in mammalian cells and is regulated by the kinase DYRPK3 [47]. In summary, the important role of LLPS in the initiation of yeast autophagy may provide new insight into the mechanism underlying autophagy initiation. However, there may still be some questions about the role of LLPS in autophagy initiation in mammalian cells. For example, does the mammalian ULK1 complex have a similar LLPS phenomenon to the Atg1 complex in yeast? Do other autophagy core proteins in mammalian cells have identical behavior to that of yeast? Is the LLPS of the Atg1 complex regulated by post-translational modifications? These questions need to be answered by further investigation.
3. The Role of Endoplasmic Reticulum (ER) in the Initiation of Autophagy
The formation of autophagosomes is the core event of autophagy initiation, but the membrane source of autophagosomes in multicellular organisms has been debated. According to previous studies, autophagosome has several membrane sources, including the plasma membrane [48], mitochondria [49], endosomes, Golgi, and ER [50,51].
It has been proposed that the ER is the initiation site of autophagosomes (Figure 3) [52,53,54]. Multiple studies observed the formation of isolation membranes (IMs), the membrane structure of pre-autophagosomes, occurring at the ER [54,55,56,57]. The omega-shaped structures associated with the ER, known as omegasomes, are thought to serve as platforms for phagophore assembly by recruiting essential proteins such as DFCP1/ZFYVE1 and facilitating lipid transfer to expand the phagophore [58]. In addition, the COP-II vesicles at the ER function as a membrane source for the formation of autophagosomes [55]. The autophagic initiation complex consisting of FIP200/ATG13/ULK1 directly interacts with the ER protein VAPA/B and forms the initiation site of autophagy in the ER (Figure 3) [59]. The ER-localized transmembrane proteins Atlastin 2 and 3 (ATL2/3) are also involved in this process by helping ULK1 and ATG101 to recruit FIP200 and ATG13 to form the autophagy initiation complex [60]. The phosphatidylinositol 3-kinase catalytic subunit 3 (PI(3)KC3) subsequently produces phosphatidylinositol 3-phosphate (PI(3)P) to recruit autophagic proteins to form omegasomes for autophagosome maturing [58]. Although many studies indicate that the ER plays an important role in autophagy initiation, the signal that determines the initiation of autophagosomes in the ER has not been identified. One report observed that stimulation of autophagy triggers a transient Ca2+ enrichment on the outer surface of the ER membrane, causing FIP200-mediated LLPS to form an autophagy initiation complex [61]. This process is controlled by the ER transmembrane autophagy protein EPG-4/EI24. When EPG-4/EI24 is missing, the autophagosome formation is impaired upon continuous Ca2+ oscillation on the outer surface of the ER [61].
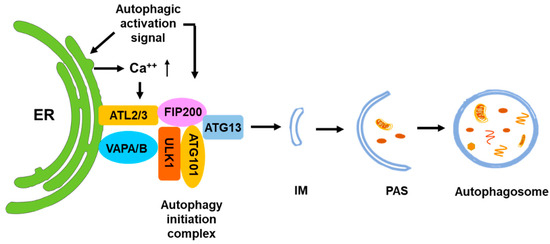
Figure 3.
ER is the major initiation site for autophagy. The ULK1 complex is assembled at ER in response to the autophagic activation signal and binds to the ER membrane through interaction with ATL2/3 and VAPA/B. The ER-bound ULK1 complex recruits ATG9 vesicles and other autophagic initiation complexes for initiation of autophagy. Calcium signaling of ER facilitates the assembly of the ULK1 complex at ER.
4. The Role of ATG9 Vesicles in the Initiation of Autophagy
ATG9 is the only transmembrane ATG protein identified so far. It exists as a trimer in the membrane vesicles [62]. ATG9 has been identified as a phospholipid scramblase that can redistribute phospholipids in both layers of the membranes [63,64]. ATG9 vesicles are initially formed in the ER and transported to the Golgi for maturation [65,66]. The matured ATG9 vesicles are derived from the trans-Golgi network (TGN) and transported to the target sites for autophagy initiation [65,66].
It has been demonstrated that ATG9 vesicles are required for autophagy [67]. The function of ATG9 vesicles in autophagy has been studied extensively. It was proposed that ATG9 vesicles serve as the membrane source for the expansion of phagophores during autophagy initiation [65].
Recent breakthrough studies using the in vitro reconstitution method with yeast autophagic proteins discovered that Atg9 vesicles function as the seeds of the phagophores during the initiation of autophagy [68] (Figure 4). In this model, Atg9 vesicles may interact with the Atg1 (ULK1) complex and other autophagic proteins to obtain phospholipids from the ER membrane or other membrane sources to expand the vesicles. Atg2, a lipid transfer protein, is recruited between the ER membrane and Atg9 vesicles [69,70,71]. At the same time, the PI3KC3 complex (PI3KC3-C1) may be recruited to ATG9 vesicles and subsequently the ATG12-ATG5-ATG16L complex for Atg8 lipidation [24]. The expansion of ATG9 vesicles into the phagophores is processed by transferring the phospholipids from the ER membrane to the Atg9 vesicle through the Atg2 lipid transfer channel [69,70,71]. The phospholipids transferred from the ER membrane are accepted and integrated into the ATG9 vesicle membrane by Atg9 with its phospholipid scramblase activity [63,64]. At the same time, Atg8 may be lipidated with phosphatidylethanolamine (PE) in the membrane of ATG9 vesicles along with the expansion. In this way, ATG9 vesicles function as the seeds of autophagosomes by the formation of the isolation membrane or the phagophore, which is the initiation structure of autophagosomes (Figure 4). Although this in vitro model is established with yeast autophagic proteins, a similar autophagic initiation mechanism is likely to exist in mammalian cells.
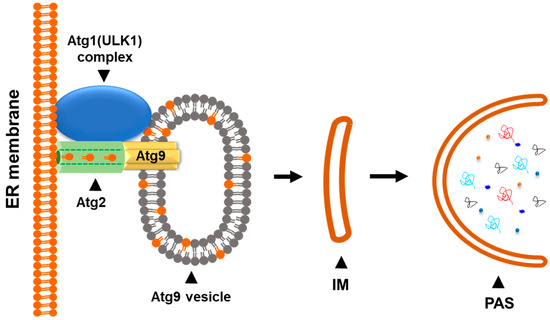
Figure 4.
The Atg9 vesicle functions as a seed for autophagosomal biogenesis. The Atg9 vesicle may interact with the Atg1 (ULK1) complex and other autophagic proteins in response to the autophagic initiation signal. The phospholipid transfer protein Atg2 connects the phospholipid source membrane (ER membrane) and the Atg9 vesicle. Expansion of Atg9 vesicle into the isolation membrane (IM) is processed by the Atg2-mediated phospholipid transferring and the Atg9-mediated phospholipid scrambling.
ATG9 vesicles may also function as the seeds of autophagosomes during the initiation of selective autophagy. ATG9 is capable of interacting with both autophagy receptors and the autophagic initiation complex, such as SQSTM1 and the ULK1 complex [24,72]. Thus, ATG9 vesicles target both the selective autophagic cargos and the source membranes and initiate the expansion of the vesicles to form the PAS [24].
Many questions remain unanswered regarding the role of ATG9 in the initiation of autophagy. For example, how are ATG9 vesicles transported to the initiation site in response to the autophagy activation signaling? How are the autophagy initiation complexes, including the PI3KC3 complex, the LC3 lipidation complex, and the ATG2-ATG9 complex, assembled and organized at the vesicles in response to autophagy activation? Does the ATG9 vesicle also function as a membrane source in the expansion of the PAS in the initiation of autophagy? These interesting questions need further investigation to be answered to further our understanding of the mechanisms underlying the initiation of autophagy.
5. The Cellular Signaling Pathways That Regulate Autophagy
Autophagy functions to mitigate various cellular stresses and protect cells from stress-caused damage. Thus, the signaling pathways involved in the initiation and activation of autophagy are largely related to cellular stresses. The mTOR-related signaling pathway is the first one found to directly regulate the activation of autophagy in response to nutritional stress or other metabolic stress conditions. In recent years multiple non-mTOR signaling pathways have been identified to be involved in the activation of autophagy. The cellular signaling pathways that regulate the initiation and activation of autophagy are summarized in Figure 5.
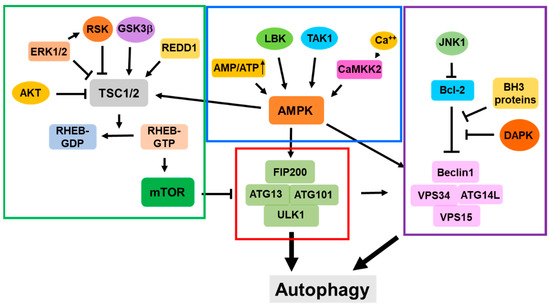
Figure 5.
Cellular signaling pathways for regulation of initiation and activation of autophagy. The red line circled portion is the ULK1 complex; the green line circled portion shows the mTOR complex-related signaling pathways; the blue line circled portion shows the AMPK complex-related signaling pathways; and the purple line circled portion shows the PI3KC3 complex-related signaling pathways.
5.1. The mTOR-Dependent Signaling Pathways in the Regulation of Autophagy
The mTOR participates in multiple metabolic signaling pathways [73]. mTOR signaling is a major negative regulator of autophagy and plays an important role in the regulation of autophagy activation [74].
5.1.1. The AKT-TSC-RHEB—mTOR Signaling Pathway
RHEB is a small GTPase that is capable of directly regulating mTOR kinase activity [75]. The active RHEB directly binds to the kinase domain of mTOR and activates its kinase activity, thus playing an inhibitory role in the regulation of autophagy [76]. The RHEB is inactivated by the TSC1/2 complex, which is the GTPase-activating protein (GAP) of RHEB [75]. However, the RHEB GAP activity of the TSC1/2 complex is negatively regulated by the AKT kinase-mediated phosphorylation of TSC2 [77,78,79,80,81,82]. Thus, the activation effect of RHEB on mTOR kinase activity, which leads to the inhibitory effect on autophagy, is positively regulated by AKT and negatively regulated by the TSC1/2 complex [77,78,79,80,81,82]. Recent studies have shown that RHEB-mediated mTORC1 activation is regulated by ubiquitination [83].
5.1.2. The AMPK-TSC1/2-m TOR Signaling Pathway
AMP-activated protein kinase (AMPK) is a conserved serine/threonine protein kinase and a key metabolic sensor in cells [84]. When the intracellular ATP/AMP ratio decreases, increased AMP activates AMPK activity by binding to the AMPK regulatory subunit γ [85,86,87], which causes allosteric change and the phosphorylation of the Thr172 site [87,88]. Activated AMPK promotes GAP activity of the TSC1/2 complex by directly phosphorylating TSC2 at T1227 and S1345, thus inactivating RHEB and mTOR and activating autophagy [89,90,91]. Intracellular calcium also participates in the AMPK-induced autophagy. An increase in the intracellular Ca2+ concentration activates CaMKK2 kinase [92]. Activated CaMKK2 activates AMPK by the phosphorylation of AMPK at the Thr172 site and triggers autophagy in response to changes in the calcium flux [93,94].
5.1.3. The Ras/Raf-MEK-ERK Signaling Pathway
Recent studies have shown that the Ras/Raf-MEK-ERK signaling pathway regulates autophagy [95]. It has been found that the inhibition of the KRAS-RAF-MEK-ERK signaling induces autophagy, as the inhibition of MEK1/2 leads to the activation of the LKB1-AMPK-ULK1 axis [96]. In addition, the ERK1/2-RSK pathway mediates the phosphorylation of the TSC1/2 complex and inhibits its GAP activity, leading to the activation of RHEB and mTORC1 [97,98]. However, some studies have shown contradictory results, in which the activation of the Ras/Raf-MEK-ERK pathway induces autophagy [99,100], suggesting that the regulation of autophagy by the Ras/Raf-MEK-ERK pathway is complicated and the mechanism needs to be further investigated.
5.1.4. The RAG GTPase Signaling Pathway
Recent studies have found that amino acid deprivation activates autophagy by the inhibition independent of the theTSC1/2 complex; instead, it is dependent on RAG small GTPases [101,102,103,104,105]. There are four RAG GTPases in mammalian cells: RAGA, RAGB, RAGC, and RAGD. Either RAGA or RAGB forms a heterodimer with either RAGC or RAGD. The RAG heterodimers are associated with lysosomal membrane via a lysosomal protein complex composed of p18 (also known as LAMTOR1), p14 (LAMTOR2), MP1 (LAMTOR3), C7orf59 (LAMTOR4), and HBXIP (LAMTOR5) [106]. Amino acid enrichment triggers the active form of RAG heterodimers (RAGA/B-GTP with RAG-C/D-GDP), which recruits mTORC1 to lysosomes [107,108]. At lysosomes, mTORC1 is further activated by RHEB [104,109,110]. Conversely, amino acid deprivation leads to the inactivation of RAG heterodimers (RAGA/B-GDP with RAGC/D-GTP), which dissociates mTORC1 from lysosomes and inactivates mTORC1 (Figure 5).
5.1.5. The GSK3β-TSC Signaling
It has been found that GSK3β, an important negative regulator of the Wnt signaling pathway, phosphorylates and activates the TSC1/2 complex, resulting in the inhibition of mTOR kinase activity and the activation of autophagy [111,112]. Early studies observed that GSK3b was phosphorylated by Akt [113], suggesting that the role of GSK3β in the activation of autophagy may be regulated by Akt-mediated phosphorylation. Indeed, the phosphorylation of GSK3β by Akt inhibits autophagy, while the dephosphorylation of GSK3β enhances autophagy [114,115]. Thus, the Wnt signaling pathway may inhibit autophagy through the phosphorylation and inhibition of GSK3β.
5.2. The mTOR-Independent Signaling Pathways
5.2.1. The mTOR-Independent AMPK Signaling
AMPK phosphorylates multiple sites of ULK1, such as Ser467 and Thr574, to activate ULK1, thereby activating autophagy [116,117]. In addition, AMPK activates autophagy by regulating the PI3KC3 complex. During glucose starvation, AMPK binds to VPS34 and ATG14L, phosphorylates BECN1, and stimulates autophagy activity [118]. Interestingly, AMPK determines the differential function of the PI3KC3 complex in autophagy [119,120]. AMPK phosphorylates the T163/S165 sites of VPS34 to inhibit the non-autophagic function of the PI3KC3 complex and phosphorylates the S91/S94 sites of BECN1 to activate the pro-autophagic function of the PI3KC3 complex and induce autophagy [120]. This illustrates the role of AMPK, which switches between the non-autophagic or pro-autophagic functions of the PI3KC3 complex. Intracellular calcium participates in AMPK-induced autophagy. An increase in the intracellular Ca2+ concentration leads to the activation of CaMKK2 kinase [92]. Activated CaMKK2 activates AMPK by the phosphorylation of AMPK at the Thr172 site and then triggers autophagy in response to changes in the calcium flux [93,94].
5.2.2. The Bcl-2 Signaling in Autophagy
The Bcl-2 protein family plays important roles in the regulation of apoptosis [121]. It has been shown that anti-apoptotic Bcl-2 proteins, such as Bcl-2, Bcl-XL, Bcl-w, and Mcl-1 inhibit autophagy by interacting with the autophagic protein BECN1 [122,123,124,125,126]. The anti-apoptotic Bcl-2 proteins bind to the BH3 domain of BECN1, thus inhibiting the PI3K activity of the PI3KC3 complex [127]. The BH3-only pro-apoptotic proteins, such as BNIP3, Bad, Bik, BimEL, Noxa, and Puma, induce autophagy by competing with the binding of BECN1 to the anti-apoptotic Bcl-2 proteins and releasing the inhibition of BCEN1by the anti-apoptotic Bcl-2 proteins [126,128,129,130,131]. In addition, it has been proved that activated c-jun terminal protein kinase 1 (JNK1) induces autophagy by phosphorylating Bcl-2 and dissociating Bcl-2 from the BECN1 complex [132].
5.3. The Death-Associated Protein Kinase (DAPK) Signaling Pathway
Recently, it has been found that death-associated protein kinase 3 (DAPK3) directly phosphorylates ULK1 at Ser556, and promotes the formation of the ULK1 autophagy initiation complex, thus activating autophagy [133]. In addition, DAPK phosphorylates BECN1 at Thr119 in the BH3 domain, leading to the dissociation of BECN1 from Bcl-XL and the activation of autophagy [134].
5.4. The O-GlcNAcylation Pathway in Autophagy Initiation
O-GlcNAc is a protein post-translational modification that uses uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as a donor molecule to modify the hydroxyl group of serine or threonine of the target proteins [135]. Multiple studies have shown that O-GlcNAcylation activates autophagy [136,137]. O-GlcNAcylation of the autophagy regulatory proteins regulates their activity, stability, and subcellular localization, thus regulating autophagy [138]. Studies have shown that the GlcNAc modification of AKT Thr305 and Ser312 sites antagonizes Ser308 phosphorylation [139], and mouse experiments have shown that OGT (O-GlcNAc transferase) content is negatively correlated with Akt expression [140]. This indicates that O-GlcNAc modification is likely to affect mTOR activation by regulating Akt activity and expression, thereby regulating autophagy initiation. In addition, AMPK, another key protein in the autophagy activation pathway, is also modified by O-GlcNAcylation, which inhibits its kinase activity [141]. Furthermore, the ULK1/2 O-GlcNAcylation level is positively correlated with autophagic activity [76]. These studies indicate that O-GlcNAcylation is a novel regulatory means for autophagy.
6. Conclusions
The initiation and activation of autophagy are the most important steps for autophagic processes. LLPS is the newly identified biophysical process for the assembly of a pre-autophagic complex, and the ER is a major cellular location for the initiation of autophagy and the formation of autophagosomes. Atg9 vesicles have been identified as the seeds for autophagosomal biogenesis and are the crucial component for the initiation of autophagy. The activation of autophagy is regulated by multiple signaling pathways that are in response to cellular stress conditions. As shown in Figure 5, these signaling pathways are centralized by the ULK1 complex, the mTOR complex, the AMPK complex, and the PI3KC3 complex. Interactions with these functional complexes constitute the regulatory networks for the initiation and activation of autophagy.
Currently, many questions remain unanswered regarding the regulation of the initiation and activation of autophagy. For example, how does the autophagy initiation complex assemble temporally and spatially in response to the autophagic activation signal? How does the autophagy initiation complex determine or recognize the initiation site? How do the multiple autophagy regulatory signaling pathways coordinate in response to nutritional or oxidative stress in cells? Furthermore, as the function of Atg8/LC3 is expanded from findings of ATG8ylation, other cellular processes such as endosomal trafficking and vesicle secretion may participate in the regulation of or coordination with autophagy initiation and activation. Further investigation of these questions will greatly improve our understanding of molecular mechanisms underlying autophagy initiation and activation.
Author Contributions
Conceptualization, Q.L. and Z.W.; writing—review and editing, Z.W.; illustration, X.H., L.Z., M.Z. and Y.W.; supervision, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82172942 and No. 81871888 to Q. L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009, 10, 458–467. [Google Scholar] [CrossRef]
- Yamamoto, H.; Fujioka, Y.; Suzuki, S.W.; Noshiro, D.; Suzuki, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Ando, T.; Noda, N.N.; et al. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Dev. Cell 2016, 38, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Zhu, L.; Yang, Q. The Classification and Basic Processes of Autophagy. Adv. Exp. Med. Biol. 2021, 1208, 3–16. [Google Scholar] [PubMed]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Shen, J. Chaperone-mediated autophagy: Molecular mechanisms, biological functions, and diseases. MedComm 2023, 4, e347. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Cregg, J.M.; Dunn, W.A., Jr.; Emr, S.D.; Sakai, Y.; Sandoval, I.V.; Sibirny, A.; Subramani, S.; Thumm, M.; Veenhuis, M.; et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 2003, 5, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Noda, T.; Yoshimori, T.; Tanaka, Y.; Ishii, T.; George, M.D.; Klionsky, D.J.; Ohsumi, M.; Ohsumi, Y. A protein conjugation system essential for autophagy. Nature 1998, 395, 395–398. [Google Scholar] [CrossRef]
- Ohsumi, Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001, 2, 211–216. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Mizushima, N. Two ubiquitin-like conjugation systems essential for autophagy. Semin. Cell Dev. Biol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Agrotis, A.; von Chamier, L.; Oliver, H.; Kiso, K.; Singh, T.; Ketteler, R. Human ATG4 autophagy proteases counteract attachment of ubiquitin-like LC3/GABARAP proteins to other cellular proteins. J. Biol. Chem. 2019, 294, 12610–12621. [Google Scholar] [CrossRef]
- Kumar, S.; Jia, J.; Deretic, V. Atg8ylation as a general membrane stress and remodeling response. Cell Stress 2021, 5, 128–142. [Google Scholar] [CrossRef]
- Deretic, V.; Klionsky, D.J. An expanding repertoire of E3 ligases in membrane Atg8ylation. Nat. Cell Biol. 2024, 26, 307–308. [Google Scholar] [CrossRef]
- Deretic, V.; Duque, T.; Trosdal, E.; Paddar, M.; Javed, R.; Akepati, P. Membrane atg8ylation in Canonical and Noncanonical Autophagy. J. Mol. Biol. 2024, 436, 168532. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Lazarou, M. A guide to membrane atg8ylation and autophagy with reflections on immunity. J. Cell Biol. 2022, 221, e202203083. [Google Scholar] [CrossRef] [PubMed]
- Durgan, J.; Lystad, A.H.; Sloan, K.; Carlsson, S.R.; Wilson, M.I.; Marcassa, E.; Ulferts, R.; Webster, J.; Lopez-Clavijo, A.F.; Wakelam, M.J.; et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 2021, 81, 2031–2040.e8. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Jensen, L.E.; Hurley, J.H. Autophagosome biogenesis comes out of the black box. Nat. Cell Biol. 2021, 23, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e16. [Google Scholar] [CrossRef]
- Li, P.; Banjade, S.; Cheng, H.C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Science China. Life Sci. 2020, 63, 953–985. [Google Scholar] [CrossRef]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, X.; Ma, L.; Li, P.; Yu, H. Protein phase separation and its role in chromatin organization and diseases. Biomed. Pharmacother. 2021, 138, 111520. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Dai, T.; Qin, Z.; Lu, H.; Zhang, L.; Zhou, F. Liquid-liquid phase separation in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Pérez-Berlanga, M.; De Rossi, P.; Polymenidou, M. Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev. Cell 2020, 55, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Boyko, S.; Surewicz, W.K. Tau liquid-liquid phase separation in neurodegenerative diseases. Trends Cell Biol. 2022, 32, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Zhang, J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, R.; Negro, R.; Cheng, J.; Vora, S.M.; Fu, T.M.; Wang, A.; He, K.; Andreeva, L.; Gao, P.; et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 2021, 184, 5759–5774.e20. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Du, Z.; Zhang, H. mTOR Regulates Phase Separation of PGL Granules to Modulate Their Autophagic Degradation. Cell 2018, 174, 1492–1506.e22. [Google Scholar] [CrossRef]
- Sun, D.; Wu, R.; Zheng, J.; Li, P.; Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018, 28, 405–415. [Google Scholar] [CrossRef]
- Noda, N.N.; Wang, Z.; Zhang, H. Liquid-liquid phase separation in autophagy. J. Cell Biol. 2020, 219, e202004062. [Google Scholar] [CrossRef]
- Jin, S.; Cui, J. Igniting autophagy through the regulation of phase separation. Signal Transduct. Target. Ther. 2020, 5, 49. [Google Scholar] [CrossRef]
- Fujioka, Y.; Alam, J.M.; Noshiro, D.; Mouri, K.; Ando, T.; Okada, Y.; May, A.I.; Knorr, R.L.; Suzuki, K.; Ohsumi, Y.; et al. Phase separation organizes the site of autophagosome formation. Nature 2020, 578, 301–305. [Google Scholar] [CrossRef]
- Suzuki, K.; Kirisako, T.; Kamada, Y.; Mizushima, N.; Noda, T.; Ohsumi, Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001, 20, 5971–5981. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; Kotani, T.; Noda, N.N.; Kimura, Y.; Nakatogawa, H. The Atg1 complex, Atg9, and Vac8 recruit PI3K complex I to the pre-autophagosomal structure. J. Cell Biol. 2023, 222, e202210017. [Google Scholar] [CrossRef] [PubMed]
- Gatica, D.; Wen, X.; Cheong, H.; Klionsky, D.J. Vac8 determines phagophore assembly site vacuolar localization during nitrogen starvation-induced autophagy. Autophagy 2021, 17, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Suzuki, S.W.; Yamamoto, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Akada, R.; Inagaki, F.; Ohsumi, Y.; Noda, N.N. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 2014, 21, 513–521. [Google Scholar] [CrossRef]
- Memisoglu, G.; Eapen, V.V.; Yang, Y.; Klionsky, D.J.; Haber, J.E. PP2C phosphatases promote autophagy by dephosphorylation of the Atg1 complex. Proc. Natl. Acad. Sci. USA 2019, 116, 1613–1620. [Google Scholar] [CrossRef]
- Yi, F.; Cai, C.; Ruan, B.; Hao, M.; Yeo, S.K.; Haas, M.; Yang, F.; Zhang, X.; Guan, J.L. Regulation of RB1CC1/FIP200 stability and autophagy function by CREBBP-mediated acetylation in an intrinsically disordered region. Autophagy 2023, 19, 1662–1677. [Google Scholar] [CrossRef]
- Wippich, F.; Bodenmiller, B.; Trajkovska, M.G.; Wanka, S.; Aebersold, R.; Pelkmans, L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 2013, 152, 791–805. [Google Scholar] [CrossRef]
- Ravikumar, B.; Moreau, K.; Jahreiss, L.; Puri, C.; Rubinsztein, D.C. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 2010, 12, 747–757. [Google Scholar] [CrossRef]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef]
- Puri, C.; Renna, M.; Bento, C.F.; Moreau, K.; Rubinsztein, D.C. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 2013, 154, 1285–1299. [Google Scholar] [CrossRef]
- Ge, L.; Melville, D.; Zhang, M.; Schekman, R. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife 2013, 2, e00947. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Graef, M.; Friedman, J.R.; Graham, C.; Babu, M.; Nunnari, J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell 2013, 24, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- Hayashi-Nishino, M.; Fujita, N.; Noda, T.; Yamaguchi, A.; Yoshimori, T.; Yamamoto, A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009, 11, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Kirisako, H.; Nakatogawa, H. COPII vesicles contribute to autophagosomal membranes. J. Cell Biol. 2019, 218, 1503–1510. [Google Scholar] [CrossRef]
- Karanasios, E.; Walker, S.A.; Okkenhaug, H.; Manifava, M.; Hummel, E.; Zimmermann, H.; Ahmed, Q.; Domart, M.C.; Collinson, L.; Ktistakis, N.T. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat. Commun. 2016, 7, 12420. [Google Scholar] [CrossRef]
- Karanasios, E.; Stapleton, E.; Manifava, M.; Kaizuka, T.; Mizushima, N.; Walker, S.A.; Ktistakis, N.T. Dynamic association of the ULK1 complex with omegasomes during autophagy induction. J. Cell Sci. 2013, 126, 5224–5238. [Google Scholar] [CrossRef]
- Nähse, V.; Stenmark, H.; Schink, K.O. Omegasomes control formation, expansion, and closure of autophagosomes. BioEssays: News Rev. Mol. Cell. Dev. Biol. 2024, 46, e2400038. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, N.; Miao, G.; Chen, Y.; Zhao, H.; Zhang, H. The ER Contact Proteins VAPA/B Interact with Multiple Autophagy Proteins to Modulate Autophagosome Biogenesis. Curr. Biol. 2018, 28, 1234–1245.e4. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, H.; Zhao, Y.G.; Hu, J.; Zhang, H. Atlastin 2/3 regulate ER targeting of the ULK1 complex to initiate autophagy. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, Y.; Chen, D.; Zhao, H.; Feng, Y.; Meng, Q.; Zhao, Y.; Zhang, H. Calcium transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites. Cell 2022, 185, 4082–4098.e22. [Google Scholar] [CrossRef] [PubMed]
- Guardia, C.M.; Tan, X.F.; Lian, T.; Rana, M.S.; Zhou, W.; Christenson, E.T.; Lowry, A.J.; Faraldo-Gómez, J.D.; Bonifacino, J.S.; Jiang, J.; et al. Structure of Human ATG9A, the Only Transmembrane Protein of the Core Autophagy Machinery. Cell Rep. 2020, 31, 107837. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Yamamoto, H.; Kinch, L.N.; Garza, C.M.; Takahashi, S.; Otomo, C.; Grishin, N.V.; Forli, S.; Mizushima, N.; Otomo, T. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat. Struct. Mol. Biol. 2020, 27, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Kotani, T.; Tsutsumi, A.; Tsuji, T.; Mori, T.; Noshiro, D.; Sugita, Y.; Nomura, N.; Iwata, S.; Ohsumi, Y.; et al. Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat. Struct. Mol. Biol. 2020, 27, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kakuta, S.; Watanabe, T.M.; Kitamura, A.; Sekito, T.; Kondo-Kakuta, C.; Ichikawa, R.; Kinjo, M.; Ohsumi, Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 2012, 198, 219–233. [Google Scholar] [CrossRef]
- Holzer, E.; Martens, S.; Tulli, S. The Role of ATG9 Vesicles in Autophagosome Biogenesis. J. Mol. Biol. 2024, 436, 168489. [Google Scholar] [CrossRef]
- Mari, M.; Griffith, J.; Rieter, E.; Krishnappa, L.; Klionsky, D.J.; Reggiori, F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 2010, 190, 1005–1022. [Google Scholar] [CrossRef]
- Sawa-Makarska, J.; Baumann, V.; Coudevylle, N.; von Bülow, S.; Nogellova, V.; Abert, C.; Schuschnig, M.; Graef, M.; Hummer, G.; Martens, S. Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science 2020, 369, eaaz7714. [Google Scholar] [CrossRef]
- Maeda, S.; Otomo, C.; Otomo, T. The autophagic membrane tether ATG2A transfers lipids between membranes. eLife 2019, 8, e45777. [Google Scholar] [CrossRef]
- Osawa, T.; Kotani, T.; Kawaoka, T.; Hirata, E.; Suzuki, K.; Nakatogawa, H.; Ohsumi, Y.; Noda, N.N. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat. Struct. Mol. Biol. 2019, 26, 281–288. [Google Scholar] [CrossRef]
- Valverde, D.P.; Yu, S.; Boggavarapu, V.; Kumar, N.; Lees, J.A.; Walz, T.; Reinisch, K.M.; Melia, T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019, 218, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, X.; Mi, N. A lipid membrane-centric role of the SQSTM1/p62 body during autophagosome formation. Autophagy 2024, 20, 1192–1193. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Lin, Y.; Ortiz-Vega, S.; Yonezawa, K.; Avruch, J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 2005, 15, 702–713. [Google Scholar] [CrossRef]
- Ruan, H.B.; Ma, Y.; Torres, S.; Zhang, B.; Feriod, C.; Heck, R.M.; Qian, K.; Fu, M.; Li, X.; Nathanson, M.H.; et al. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017, 31, 1655–1665. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Potter, C.J.; Pedraza, L.G.; Xu, T. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 2002, 4, 658–665. [Google Scholar] [CrossRef]
- Li, Y.; Inoki, K.; Guan, K.L. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol. Cell. Biol. 2004, 24, 7965–7975. [Google Scholar] [CrossRef]
- Mazhab-Jafari, M.T.; Marshall, C.B.; Ishiyama, N.; Ho, J.; Di Palma, V.; Stambolic, V.; Ikura, M. An autoinhibited noncanonical mechanism of GTP hydrolysis by Rheb maintains mTORC1 homeostasis. Structure 2012, 20, 1528–1539. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, C.; Doumpas, N.; Teleman, A.A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014, 156, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chen, L.; Zhao, L.; Xu, Y.; Peng, X.; Wang, X.; Ding, L.; Jin, J.; Teng, H.; Wang, Y.; et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019, 29, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Heath, R.; Saiu, P.; Leiper, F.C.; Leone, P.; Jing, C.; Walker, P.A.; Haire, L.; Eccleston, J.F.; Davis, C.T.; et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 2007, 449, 496–500. [Google Scholar] [CrossRef]
- Hardie, D.G.; Carling, D.; Gamblin, S.J. AMP-activated protein kinase: Also regulated by ADP? Trends Biochem. Sci. 2011, 36, 470–477. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Mäkelä, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, K.L.; Means, A.R.; York, B. The Ca2+/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol. Metab.: TEM 2016, 27, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Huang, Y.; Zhen, Y.; Chen, Y.; Sui, S.; Zhang, L. Unraveling the interplay between RAS/RAF/MEK/ERK signaling pathway and autophagy in cancer: From molecular mechanisms to targeted therapy. Biochem. Pharmacol. 2023, 217, 115842. [Google Scholar] [CrossRef]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef]
- Roux, P.P.; Ballif, B.A.; Anjum, R.; Gygi, S.P.; Blenis, J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 13489–13494. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Erdjument-Bromage, H.; Tempst, P.; Pandolfi, P.P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 2005, 121, 179–193. [Google Scholar] [CrossRef]
- Chen, K.L.; Chang, W.S.; Cheung, C.H.; Lin, C.C.; Huang, C.C.; Yang, Y.N.; Kuo, C.P.; Kuo, C.C.; Chang, Y.H.; Liu, K.J.; et al. Targeting cathepsin S induces tumor cell autophagy via the EGFR-ERK signaling pathway. Cancer Lett. 2012, 317, 89–98. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, S.K.; Wu, P.K.; Richards, A.L.; Jackson, W.T.; Park, J.I. Raf/MEK/ERK can regulate cellular levels of LC3B and SQSTM1/p62 at expression levels. Exp. Cell Res. 2014, 327, 340–352. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Finn, S.G.; Tee, A.R.; Browne, G.J.; Proud, C.G. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J. Biol. Chem. 2005, 280, 18717–18727. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Goraksha-Hicks, P.; Li, L.; Neufeld, T.P.; Guan, K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008, 10, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, K.H.; Kim, Y.M.; Kim, D.H.; Oh, B.H.; Kim, Y.G. Crystal structure of the Gtr1p(GTP)-Gtr2p(GDP) protein complex reveals large structural rearrangements triggered by GTP-to-GDP conversion. J. Biol. Chem. 2012, 287, 29648–29653. [Google Scholar] [CrossRef]
- Shen, K.; Choe, A.; Sabatini, D.M. Intersubunit Crosstalk in the Rag GTPase Heterodimer Enables mTORC1 to Respond Rapidly to Amino Acid Availability. Mol. Cell 2017, 68, 552–565.e8. [Google Scholar] [CrossRef]
- Gong, R.; Li, L.; Liu, Y.; Wang, P.; Yang, H.; Wang, L.; Cheng, J.; Guan, K.L.; Xu, Y. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev. 2011, 25, 1668–1673. [Google Scholar] [CrossRef]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, S.; Kohan, L.; Ghavami, S.; Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Et Biophys. Acta. Mol. Cell Res. 2021, 1868, 118926. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Buttrick, G.J.; Wakefield, J.G. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle 2008, 7, 2621–2625. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, X.; Zhu, Q.; Song, D.; Ding, X.; Li, L.; Jiang, X.; Wang, X.; Tian, R.; Su, H.; et al. Pacer Is a Mediator of mTORC1 and GSK3-TIP60 Signaling in Regulation of Autophagosome Maturation and Lipid Metabolism. Mol. Cell 2019, 73, 788–802.e7. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Tamargo-Gómez, I.; Mariño, G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018, 19, 3812. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Sun, X.; Xu, D.; Wang, C.; Zhang, Q.; Wang, H.; Luo, W.; Chen, Y.; Chen, H.; et al. AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy 2016, 12, 1447–1459. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. AMPK connects energy stress to PIK3C3/VPS34 regulation. Autophagy 2013, 9, 1110–1111. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Fang, C.; Russell, R.C.; Kim, J.H.; Fan, W.; Liu, R.; Zhong, Q.; Guan, K.L. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 2013, 152, 290–303. [Google Scholar] [CrossRef]
- Daniel, P.T.; Schulze-Osthoff, K.; Belka, C.; Güner, D. Guardians of cell death: The Bcl-2 family proteins. Essays Biochem. 2003, 39, 73–88. [Google Scholar] [PubMed]
- Levine, B.; Sinha, S.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Criollo, A.; Tasdemir, E.; Vicencio, J.M.; Tajeddine, N.; Hickman, J.A.; Geneste, O.; Kroemer, G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 2007, 3, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Erlich, S.; Mizrachy, L.; Segev, O.; Lindenboim, L.; Zmira, O.; Adi-Harel, S.; Hirsch, J.A.; Stein, R.; Pinkas-Kramarski, R. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 2007, 3, 561–568. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Le Toumelin, G.; Criollo, A.; Rain, J.C.; Gautier, F.; Juin, P.; Tasdemir, E.; Pierron, G.; Troulinaki, K.; Tavernarakis, N.; et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007, 26, 2527–2539. [Google Scholar] [CrossRef]
- Oberstein, A.; Jeffrey, P.D.; Shi, Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007, 282, 13123–13132. [Google Scholar] [CrossRef]
- Daido, S.; Kanzawa, T.; Yamamoto, A.; Takeuchi, H.; Kondo, Y.; Kondo, S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004, 64, 4286–4293. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R.; Logue, S.E.; Sayen, M.R.; Jinno, M.; Kirshenbaum, L.A.; Gottlieb, R.A.; Gustafsson, A.B. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007, 14, 146–157. [Google Scholar] [CrossRef]
- Rashmi, R.; Pillai, S.G.; Vijayalingam, S.; Ryerse, J.; Chinnadurai, G. BH3-only protein BIK induces caspase-independent cell death with autophagic features in Bcl-2 null cells. Oncogene 2008, 27, 1366–1375. [Google Scholar] [CrossRef][Green Version]
- Abedin, M.J.; Wang, D.; McDonnell, M.A.; Lehmann, U.; Kelekar, A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007, 14, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Li, L.; Li, M.Q.; Chen, X.; Su, Q.; Deng, Z.J.; Liu, H.B.; Li, B.; Zhang, W.H.; Jia, Y.X.; et al. DAPK3 inhibits gastric cancer progression via activation of ULK1-dependent autophagy. Cell Death Differ. 2021, 28, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef]
- King, D.T.; Males, A.; Davies, G.J.; Vocadlo, D.J. Molecular mechanisms regulating O-linked N-acetylglucosamine (O-GlcNAc)-processing enzymes. Curr. Opin. Chem. Biol. 2019, 53, 131–144. [Google Scholar] [CrossRef]
- Leonel, A.V.; Alisson-Silva, F.; Santos, R.C.M.; Silva-Aguiar, R.P.; Gomes, J.C.; Longo, G.M.C.; Faria, B.M.; Siqueira, M.S.; Pereira, M.G.; Vasconcelos-Dos-Santos, A.; et al. Inhibition of O-GlcNAcylation Reduces Cell Viability and Autophagy and Increases Sensitivity to Chemotherapeutic Temozolomide in Glioblastoma. Cancers 2023, 15, 4740. [Google Scholar] [CrossRef]
- Yu, H.; Wen, L.; Mu, Y. O-GlcNAcylation Is Essential for Autophagy in Cardiomyocytes. Oxidative Med. Cell. Longev. 2020, 2020, 5602396. [Google Scholar] [CrossRef]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Sun, D.; Xin, X.; Pan, Q.; Peng, S.; Liang, Z.; Luo, C.; Yang, Y.; Jiang, H.; et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates Akt signaling. PLoS ONE 2012, 7, e37427. [Google Scholar] [CrossRef]
- Mohan, R.; Jo, S.; Da Sol Chung, E.; Oribamise, E.; Lockridge, A.; Abrahante-Lloréns, J.E.; Ruan, H.B.; Yang, X.Y.; Alejandro, E.U. Pancreatic β-Cell O-GlcNAc Transferase Overexpression Increases Susceptibility to Metabolic Stressors in Female Mice. Cells 2021, 10, 2801. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, F.; Dai, G.; Yao, Q.; Xiang, H.; Wang, L.; Xue, B.; Shan, Y.; Liu, X. Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells. Cell. Mol. Biol. Lett. 2020, 25, 17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).