Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Systems Biology Approach
2.2. Generating Male and Female Virtual Patient Populations
2.3. Simulating Recombinant IL12 + Nivolumab Combination Therapy
3. Results
3.1. Sex-Specific Differences in Basal Immune System
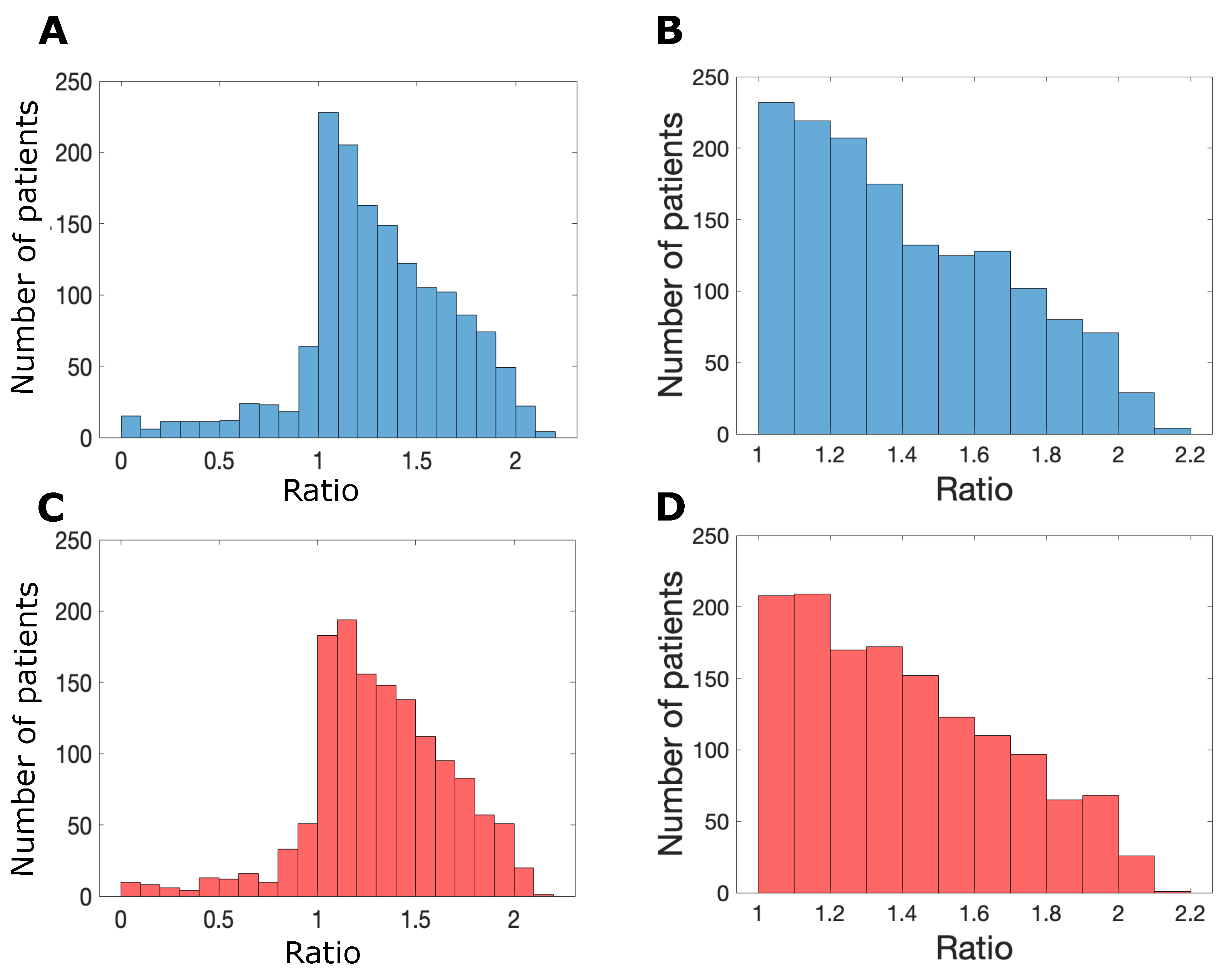
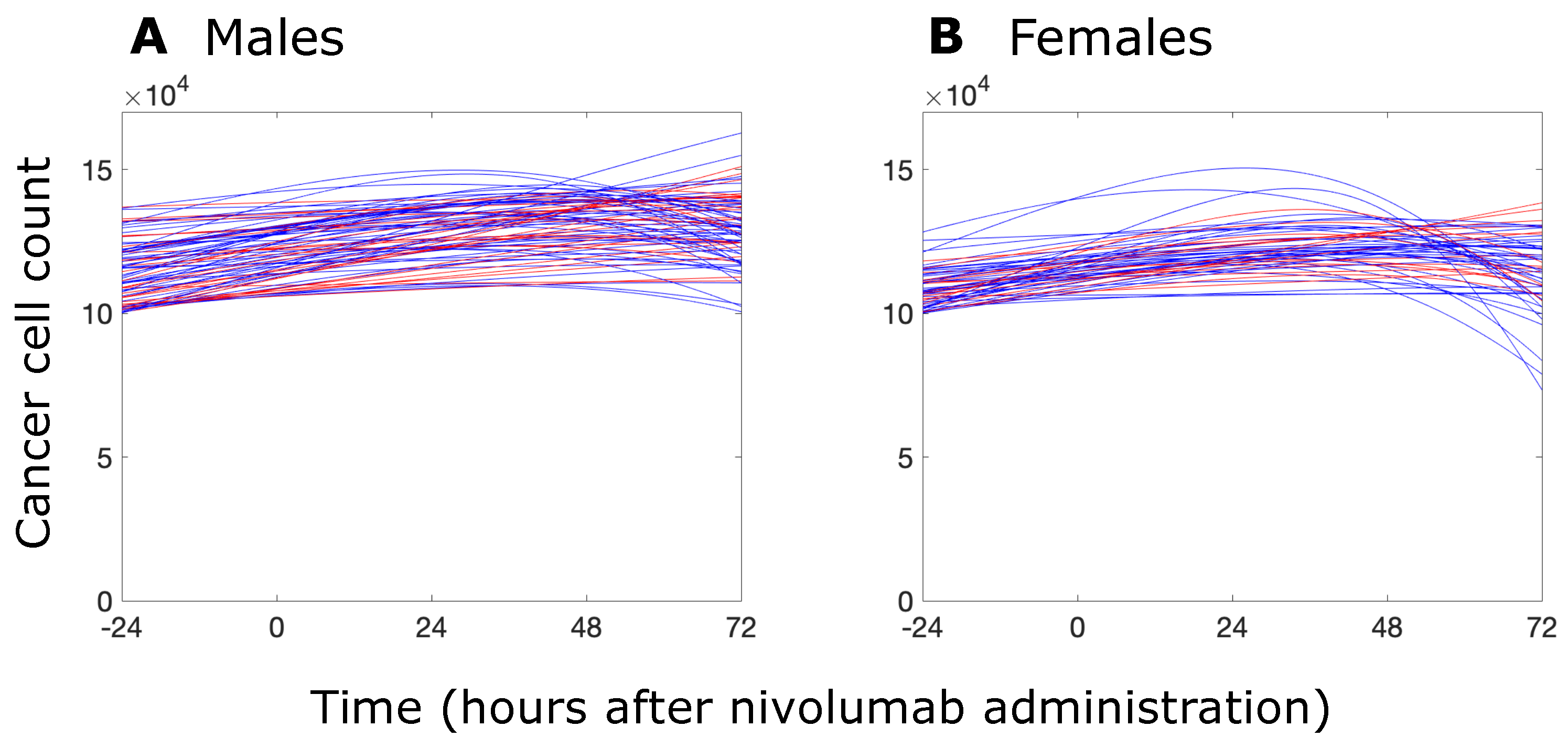
3.2. Sex-Specific Responses to Nivolumab
3.3. Success of Simulated Recombinant IL12–Nivolumab Combination Therapy Depends on Patient Sex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DeLucia, D.C.; Lee, J.K. Development of Cancer Immunotherapies. In Cancer Treatment and Research; Springer: Cham, Switzerland, 2022; Volume 183, pp. 1–48. [Google Scholar] [CrossRef]
- Garon, E.B. Cancer immunotherapy trials not immune from imprecise selection of patients. N. Engl. J. Med. 2017, 376, 2483–2485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Guha, M. The new era of immune checkpoint inhibitors. Acute Pain 2019, 10. [Google Scholar]
- Özdemir, B.C.; Dotto, G.P. Sex hormones and anticancer immunity. Clin. Cancer Res. 2019, 25, 4603–4610. [Google Scholar] [CrossRef]
- Neigh, G.; Mitzelfelt, M. Sex Differences in Physiology; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Medici, N.; Bilancio, A.; Migliaccio, A.; Castoria, G. Breast cancer stem cells: The role of sex steroid receptors. World J. Stem Cells 2019, 11, 594. [Google Scholar] [CrossRef]
- Fu, X.D.; Russo, E.; Zullino, S.; Genazzani, A.R.; Simoncini, T. Sex steroids and breast cancer metastasis. Horm. Mol. Biol. Clin. Investig. 2010, 3, 383–389. [Google Scholar] [CrossRef]
- Christoforou, P.; Christopoulos, P.F.; Koutsilieris, M. The role of estrogen receptor β in prostate cancer. Mol. Med. 2014, 20, 427–434. [Google Scholar] [CrossRef]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 386034. [Google Scholar] [CrossRef]
- Smalley, M.; Przedborski, M.; Thiyagarajan, S.; Pellowe, M.; Verma, A.; Brijwani, N.; Datta, D.; Jain, M.; Shanthappa, B.U.; Kapoor, V.; et al. Integrating Systems Biology and an Ex Vivo Human Tumor Model Elucidates PD-1 Blockade Response Dynamics. Iscience 2020, 23, 101229. [Google Scholar] [CrossRef]
- Przedborski, M.; Smalley, M.; Thiyagarajan, S.; Goldman, A.; Kohandel, M. Systems biology informed neural networks (SBINN) predict response and novel combinations for PD-1 checkpoint blockade. Commun. Biol. 2021, 4, 877. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cowley, L.A.; Liu, X.S. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Dipietro Mager, N.A. Women’s involvement in clinical trials: Historical perspective and future implications. Pharm. Pract. 2016, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Freeman, G.J.; Wherry, E.J.; Ahmed, R.; Sharpe, A.H. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J. Exp. Med. 2006, 203, 2223–2227. [Google Scholar] [CrossRef]
- Okazaki, T.; Honjo, T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006, 27, 195–201. [Google Scholar] [CrossRef]
- Sznol, M.; Chen, L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer—Response. Clin. Cancer Res. 2013, 19, 5542. [Google Scholar] [CrossRef]
- Sakaguchi, S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000, 101, 455–458. [Google Scholar] [CrossRef]
- Ridge, J.P.; Di Rosa, F.; Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998, 393, 474. [Google Scholar] [CrossRef]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. The th1/th2 paradigm. Immunol. Today 1997, 18, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Rincón, M.; Anguita, J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+ T cells. J. Exp. Med. 1997, 185, 461–470. [Google Scholar] [CrossRef]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar] [CrossRef]
- Carreño, V.; Zeuzem, S.; Hopf, U.; Marcellin, P.; Cooksley, W.G.E.; Fevery, J.; Diago, M.; Reddy, R.; Peters, M.; Rittweger, K.; et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J. Hepatol. 2000, 32, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.A.; Perelson, A.S. Th1/Th2 cross regulation. J. Theor. Biol. 1994, 170, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.A.; Perelson, A.S. Th1/Th2 differentiation and cross-regulation. Bull. Math. Biol. 1999, 61, 403–436. [Google Scholar] [CrossRef]
- Morel, B.F.; Kalagnanam, J.; Morel, P.A. Mathematical modeling of Th1-Th2 dynamics. In Theoretical and Experimental Insights into Immunology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 171–190. [Google Scholar]
- Lai, X.; Friedman, A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model. PLoS ONE 2017, 12, e0178479. [Google Scholar] [CrossRef]
- Capone, I.; Ascierto, P.M.P.A.; Malorni, W.; Gabriele, L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef]
- Zhang, M.A.; Rego, D.; Moshkova, M.; Kebir, H.; Chruscinski, A.; Nguyen, H.; Akkermann, R.; Stanczyk, F.Z.; Prat, A.; Steinman, L.; et al. Preoxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. USA 2012, 109, 9505–9510. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tang, Y.Y.; Wa, J.X.; Zou, J.Y.; Lu, C.G.; Zhu, H.S.; Sheng, S.Y.; Wang, Y.F.; Liu, H.C.; Yang, J.; et al. Sex difference in the expression of PD-1 of non-small cell lung cancer. Front. Immunol. 2022, 13, 1026214. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Hussain, T.; Kallies, A.; Vasanthakumar, A. Sex-bias in CD8+ T-cell stemness and exhaustion in cancer. Clin. Transl. Immunol. 2022, 11, e1414. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Ferrer, A.; Padros, G.; Soto, A.L.; Sarro, M.; Pujol, R. Differences according to gender and health status in CD4:CD8 ratio in a sample of community-dwelling oldest old. The OCTABAIX immune study. Aging Clin. Exp. Res. 2011, 23, 268–272. [Google Scholar] [CrossRef]
- The MathWorks Inc. rand. 2024. Available online: https://ww2.mathworks.cn/help/matlab/ref/rand.html (accessed on 20 March 2024).
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161. [Google Scholar] [CrossRef]
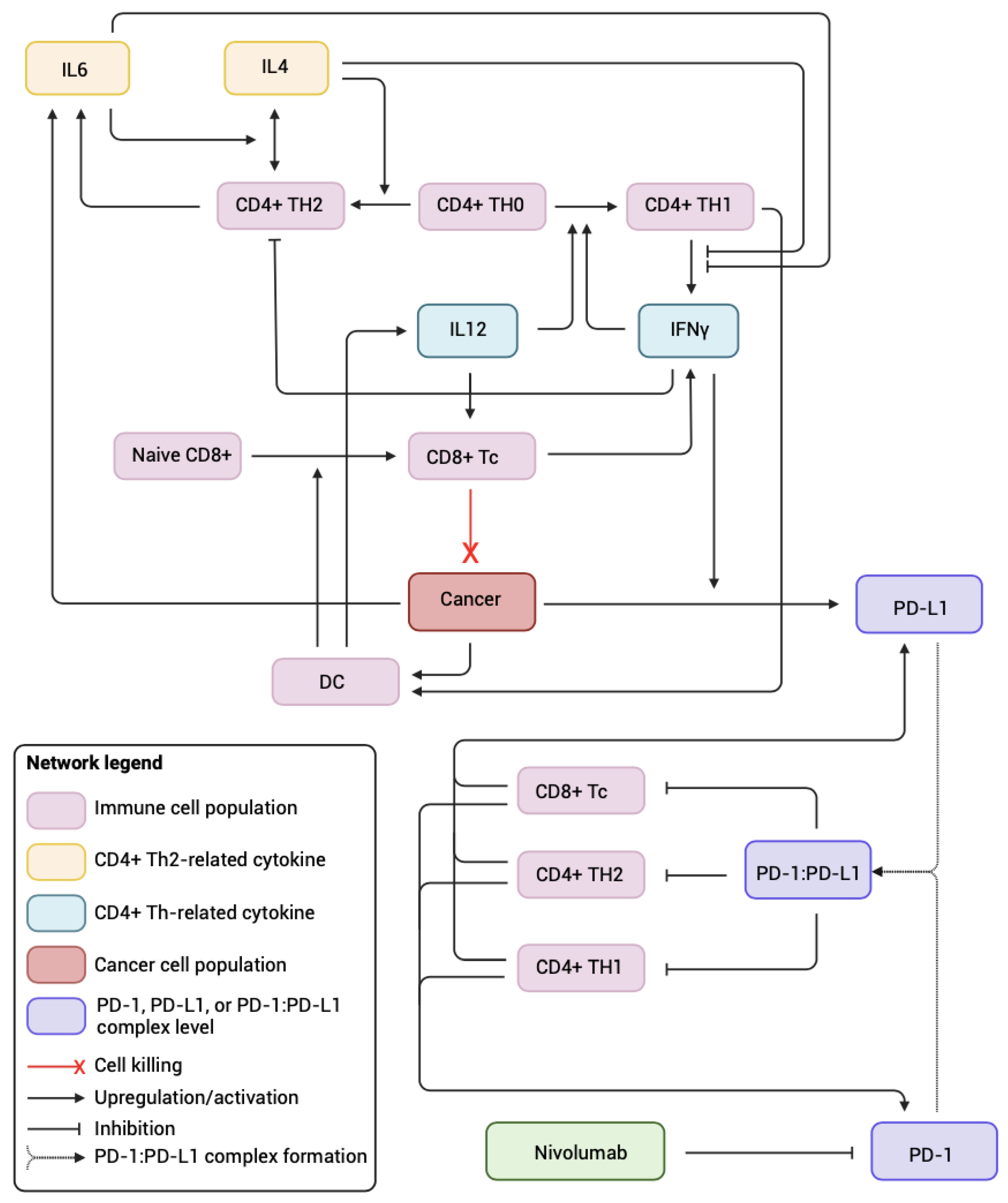
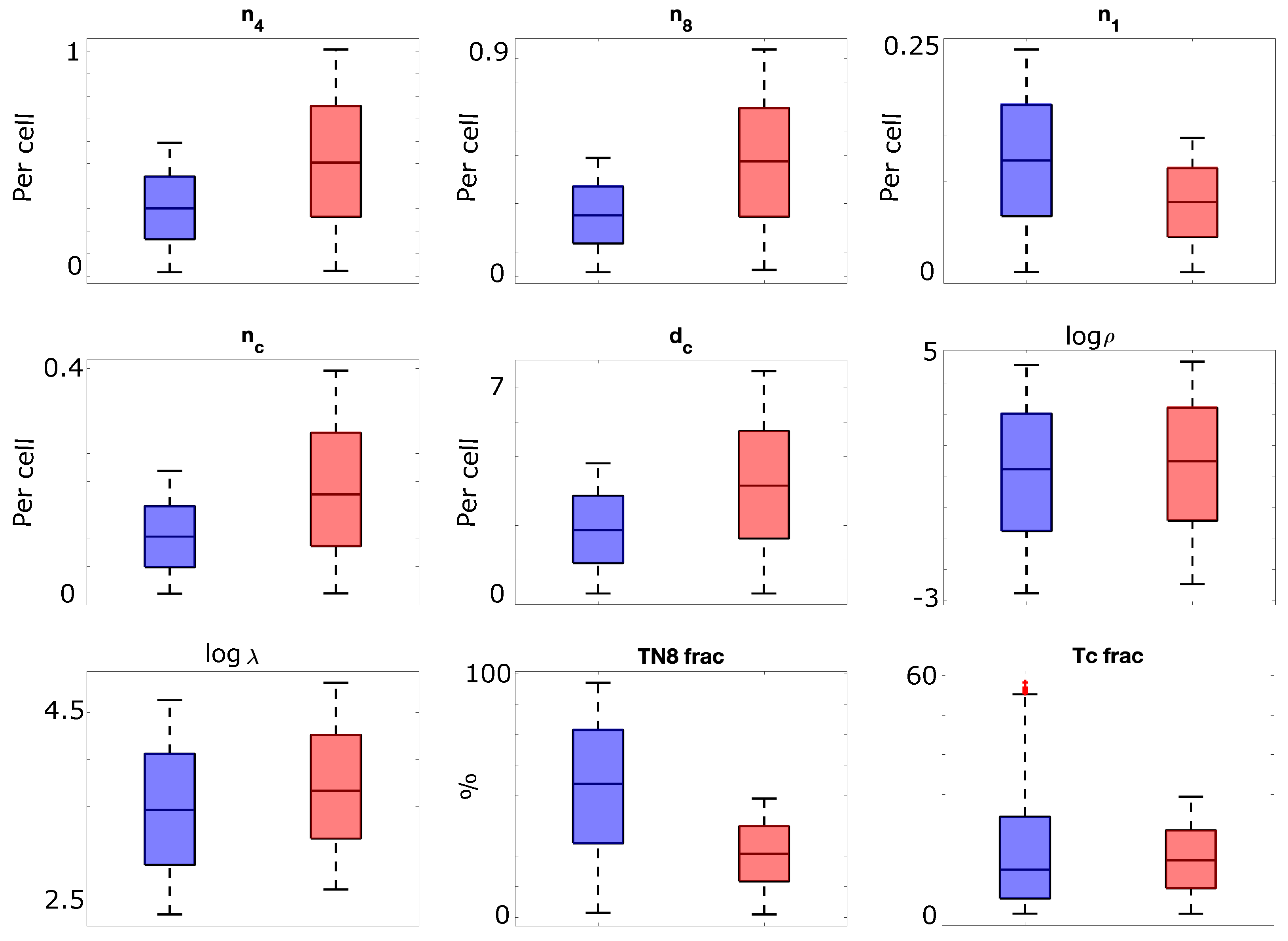


| Sex Difference | Associated Parameter(s) Name (Description) | Reference |
|---|---|---|
| Increased IFN production by CD4+ T-cells in females | (rate of production of IFN by cells) | [34] |
| Higher activated and proliferating CD4+ T-cells in females | (net proliferation rate of ) | [33] |
| Higher activated and proliferating CD8+ T-cells in females | (net proliferation rate of ), (IL12-independent net proliferation rate of cells) | [33] |
| Higher PD-1 expression on CD4+ T-cells in females | (per-cell expression level of PD-1) | [35] |
| Higher PD-1 expression on CD8+ T-cells in males | (per-cell expression level of PD-1) | [37] |
| High PD-L1 expression more likely on female tumors than male | (per-cell expression level of PD-L1), (IFN-dependent PD-L1 expression per cancer cell) | [36] |
| Increased function in females | (rate of IL6-independent production of IL4 by Th2 cells), (rate of IL6 production by Th2 cells) | [33] |
| Increased function in males | (net proliferation rate of cells) | [33] |
| Higher IFN production by CD8+ in females | (rate of IFN production by ) cells | [37] |
| Increased CD8+ cytotoxic activity in females | (rate of differentiation of cells into cells) | [33] |
| Higher CD4:CD8 ratio in females | Tc fraction (fraction of T-cells that are cytotoxic), TN8 fraction (fraction of T-cells that are CD8+ naive) | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotra, S.; Kohandel, M.; Przedborski, M. Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy. Biomolecules 2024, 14, 1513. https://doi.org/10.3390/biom14121513
Cotra S, Kohandel M, Przedborski M. Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy. Biomolecules. 2024; 14(12):1513. https://doi.org/10.3390/biom14121513
Chicago/Turabian StyleCotra, Sonja, Mohammad Kohandel, and Michelle Przedborski. 2024. "Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy" Biomolecules 14, no. 12: 1513. https://doi.org/10.3390/biom14121513
APA StyleCotra, S., Kohandel, M., & Przedborski, M. (2024). Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy. Biomolecules, 14(12), 1513. https://doi.org/10.3390/biom14121513








