Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease
Abstract
1. Introduction
2. The Physiological Role of Lipids in the Brain
2.1. Classification and Functions
2.2. Lipid Rafts
2.3. Lipid Droplets
3. Brain Lipid Alterations in Aging: Implications for Structure and Function
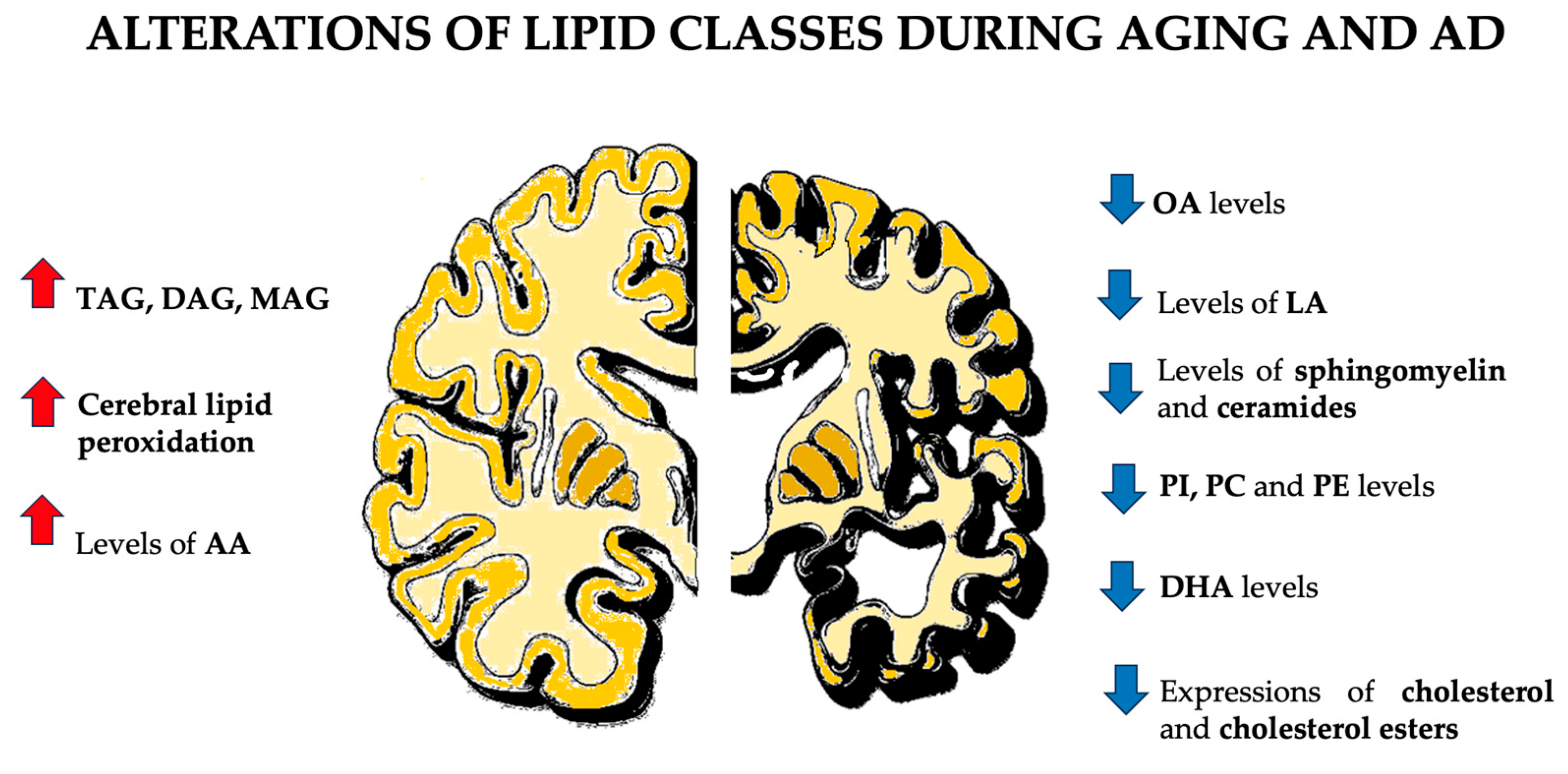
3.1. How Do Lipids Age?
3.2. Lipid Rafts in Aging: The Anti-Inflammatory Role of A–I Binding Protein (AIBP)
3.3. Lipid Droplets in Aging: The Controversial Role of Accumulation in the Microglia
4. Brain Lipid Dysregulation in Alzheimer’s Disease: Mechanisms and Implications
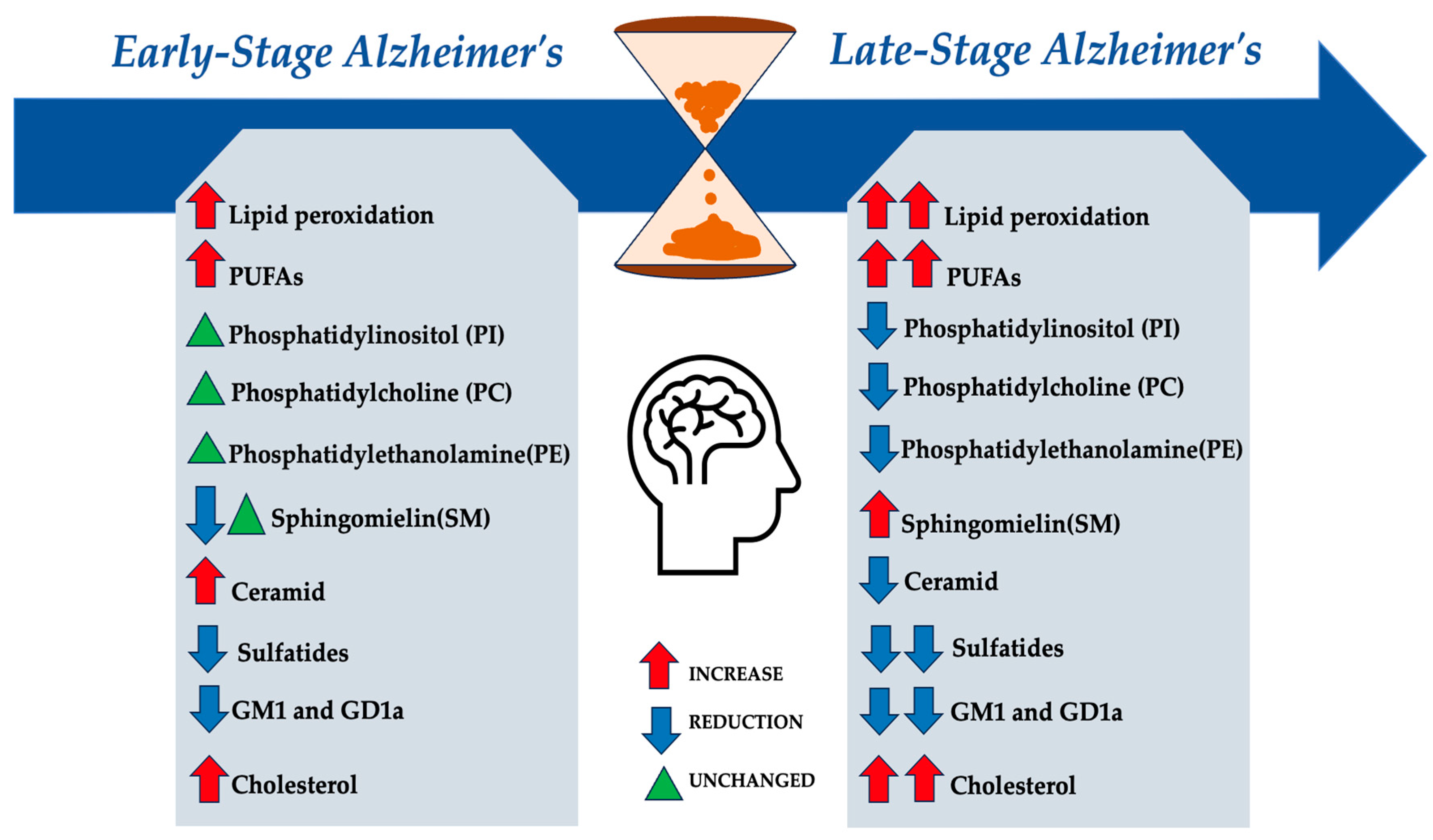
4.1. How Do Lipids Behave in Alzheimer’s Disease?
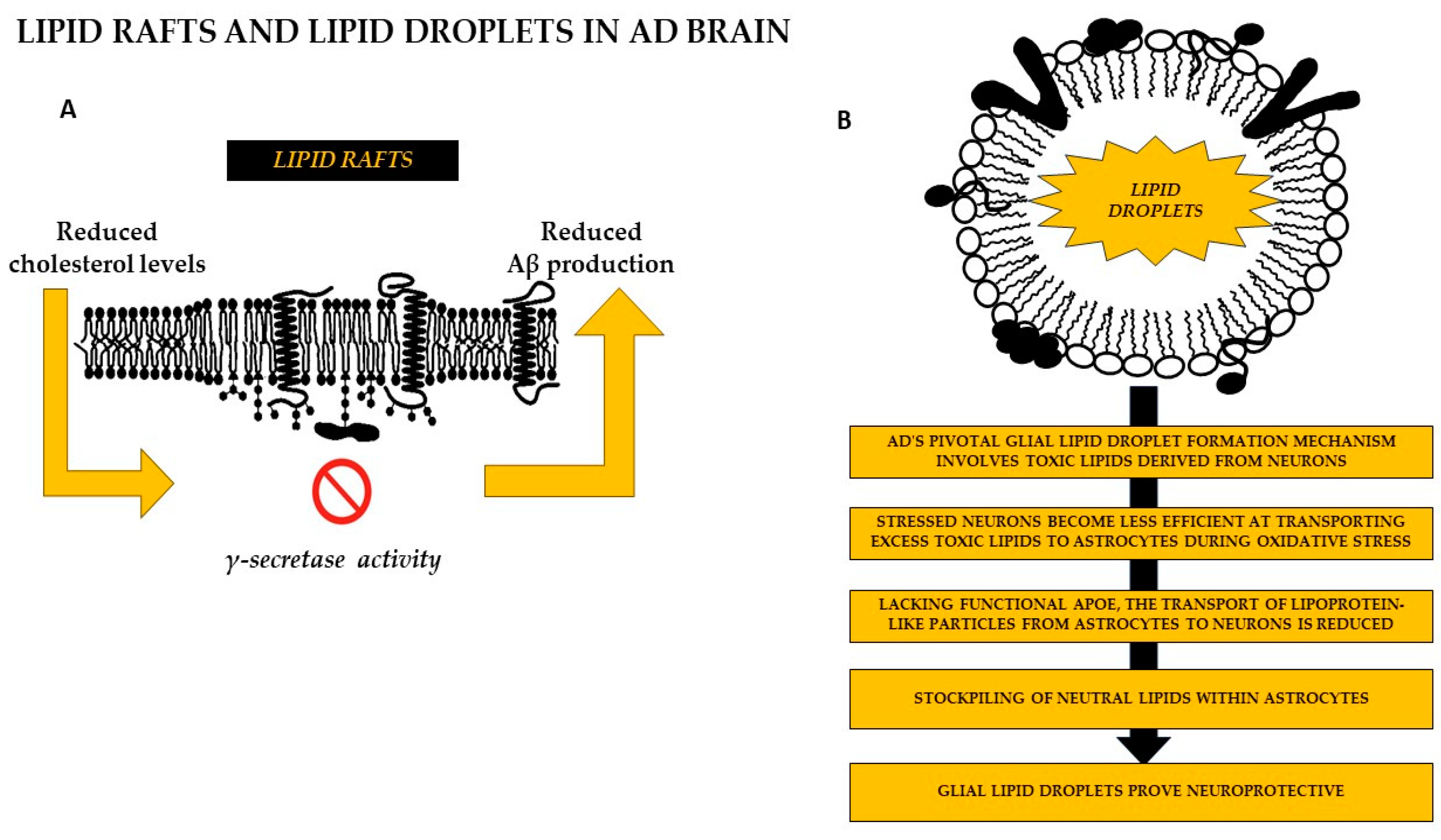
4.2. Lipid Rafts
4.3. Lipid Droplets
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, K.S.; Thinakaran, G. Membrane Rafts in Alzheimer’s Disease Beta-Amyloid Production. Biochim. Et. Biophys. Acta BBA Mol. Cell Biol. Lipids 2010, 1801, 860–867. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, G.; Mitro, N.; Audano, M.; Melcangi, R.C.; Crestani, M.; De Fabiani, E.; Caruso, D. Lipids in the Nervous System: From Biochemistry and Molecular Biology to Patho-Physiology. Biochim. Biophys. Acta 2015, 1851, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A.; Genové, G.; Li, T.; Lütjohann, D.; Olin, M.; Mast, N.; Pikuleva, I.A.; Crick, P.; Wang, Y.; Griffiths, W.; et al. Effects of a Disrupted Blood-Brain Barrier on Cholesterol Homeostasis in the Brain. J. Biol. Chem. 2014, 289, 23712–23722. [Google Scholar] [CrossRef]
- Di Scala, C.; Troadec, J.D.; Lelièvre, C.; Garmy, N.; Fantini, J.; Chahinian, H. Mechanism of Cholesterol-Assisted Oligomeric Channel Formation by a Short Alzheimer β-Amyloid Peptide. J. Neurochem. 2014, 128, 186–195. [Google Scholar] [CrossRef]
- Mota-Martorell, N.; Andrés-Benito, P.; Martín-Gari, M.; Galo-Licona, J.D.; Sol, J.; Fernández-Bernal, A.; Portero-Otín, M.; Ferrer, I.; Jove, M.; Pamplona, R. Selective brain regional changes in lipid profile with human aging. Geroscience 2022, 44, 763–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saher, G. Cholesterol Metabolism in Aging and Age-Related Disorders. Annu. Rev. Neurosci. 2023, 46, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Hatch, G.M. Fatty Acid Transport into the Brain: Of Fatty Acid Fables and Lipid Tails. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 293–302. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty Acid Transport Protein Expression in Human Brain and Potential Role in Fatty Acid Transport Across Human Brain Microvessel Endothelial Cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, E.J.; Sepúlveda, F.J.; Peters, C.; Bascuñán, D.; Riffo-Lepe, N.O.; González-Sanmiguel, J.; Sánchez, S.A.; Peoples, R.W.; Vicente, B.; Aguayo, L.G. Effect of Cholesterol on Membrane Fluidity and Association of Aβ Oligomers and Subsequent Neuronal Damage: A Double-Edged Sword. Front. Aging Neurosci. 2018, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Lütjohann, D.; Mulder, M. Brain Cholesterol in Normal and Pathological Aging. Oléagineux Corps Gras Lipides 2011, 18, 214–217. [Google Scholar] [CrossRef]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid Alterations in Lipid Rafts from Alzheimer’s Disease Human Brain Cortex. J. Alzheimers Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef]
- Colin, J.; Gregory-Pauron, L.; Lanhers, M.C.; Claudepierre, T.; Corbier, C.; Yen, F.T.; Malaplate-Armand, C.; Oster, T. Membrane Raft Domains and Remodeling in Aging Brain. Biochimie 2016, 130, 178–187. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic Acid (DHA), a Fundamental Fatty Acid for the Brain: New Dietary Sources. Prostaglandins Leukot. Essent. Fat. Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Ralhan, I.; Chang, C.L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid Droplets in the Nervous System. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Campos-Peña, V.; Pichardo-Rojas, P.; Sánchez-Barbosa, T.; Ortíz-Islas, E.; Rodríguez-Pérez, C.E.; Montes, P.; Ramos-Palacios, G.; Silva-Adaya, D.; Valencia-Quintana, R.; Cerna-Cortes, J.F.; et al. Amyloid β, Lipid Metabolism, Basal Cholinergic System, and Therapeutics in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12092. [Google Scholar] [CrossRef]
- Hamilton, L.K.; Dufresne, M.; Joppé, S.E.; Petryszyn, S.; Aumont, A.; Calon, F.; Barnabé-Heider, F.; Furtos, A.; Parent, M.; Chaurand, P.; et al. Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 2015, 17, 397–411. [Google Scholar] [CrossRef]
- Ahmed, S.; Shah, P.; Ahmed, O. Biochemistry, Lipids. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, K.G.N.; Ando, H.; Komura, N.; Fujiwara, T.K.; Kiso, M.; Kusumi, A. Development of new ganglioside probes and unraveling of raft domain structure by single-molecule imaging. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islimye, E.; Girard, V.; Gould, A.P. Functions of Stress-Induced Lipid Droplets in the Nervous System. Front. Cell Dev. Biol. 2022, 10, 863907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farmer, B.C.; Walsh, A.E.; Kluemper, J.C.; Johnson, L.A. Lipid Droplets in Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 742. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ishihara, T.; Ogasa, K.; Iwanami, S.; Hori, A.; Takahashi, M.; Yamada, Y.; Satoh-Takayama, N.; Ohno, H.; Minoda, A.; et al. A lipidome landscape of aging in mice. Nat. Aging 2024, 4, 709–726. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; Kim, T.W. Linking Lipids to Alzheimer’s Disease: Cholesterol and Beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef]
- Szrok-Jurga, S.; Turyn, J.; Hebanowska, A.; Swierczynski, J.; Czumaj, A.; Sledzinski, T.; Stelmanska, E. The Role of Acyl-CoA β-Oxidation in Brain Metabolism and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13977. [Google Scholar] [CrossRef]
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2016, 15, 534–542. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Petrovic, S.; Arsic, A. Fatty Acids: Fatty Acids. Encycl. Food Health 2016, 623–631. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. Why Does Brain Metabolism Not Favor Burning of Fatty Acids to Provide Energy-Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J. Cereb. Blood Flow. Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Medicherla, S.; Sheikh, N.; Terry, B.; Phillipps, A.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 537–546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, R.B.; Oliveira, T.G.; Cortes, E.P.; Honig, L.S.; Duff, K.E.; Small, S.A.; Wenk, M.R.; Shui, G.; Di Paolo, G. Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease. J. Biol. Chem. 2012, 287, 2678–2688. [Google Scholar] [CrossRef]
- Jové, M.; Pradas, I.; Dominguez-Gonzalez, M.; Ferrer, I.; Pamplona, R. Lipids and lipoxidation in human brain aging. Mitochondrial ATP-synthase as a key lipoxidation target. Redox Biol. 2019, 23, 101082. [Google Scholar] [CrossRef] [PubMed]
- Shamim, A.; Mahmood, T.; Ahsan, F.; Kumar, A.; Bagga, P. Lipids: An insight into the neurodegenerative disorders. Clin. Nutr. Exp. 2018, 20, 1–19. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Tidhar, R.; Futerman, A.H. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochim. Biophys. Acta 2013, 1833, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane Microdomains in Brain Development, Function and Neurological Diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef]
- Mei, M.; Liu, M.; Mei, Y.; Zhao, J.; Li, Y. Sphingolipid metabolism in brain insulin resistance and neurological diseases. Front. Endocrinol. 2023, 14, 1243132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Segatto, M.; Di Giovanni, A.; Marino, M.; Pallottini, V. Analysis of the Protein Network of Cholesterol Homeostasis in Different Brain Regions: An Age and Sex Dependent Perspective. J. Cell. Physiol. 2013, 228, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Korinek, M.; Gonzalez-Gonzalez, I.M.; Smejkalova, T.; Hajdukovic, D.; Skrenkova, K.; Krusek, J.; Horak, M.; Vyklicky, L. Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci. Rep. 2020, 10, 12651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid Rafts and Neurodegeneration: Structural and Functional Roles in Physiologic Aging and Neurodegenerative Diseases. J. Lipid Res. 2020, 61, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Shabanzadeh, A.P.; Tassew, N.G.; Szydlowska, K.; Tymianski, M.; Banerjee, P.; Vigouroux, R.J.; Eubanks, J.H.; Huang, L.; Geraerts, M.; Koeberle, P.D.; et al. Uncoupling Neogenin association with lipid rafts promotes neuronal survival and functional recovery after stroke. Cell Death Dis. 2015, 6, e1744. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-Droplet-Accumulating Microglia Represent a Dysfunctional and Proinflammatory State in the Aging Brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Cohen, S. Lipid Droplets as Organelles. Int. Rev. Cell Mol. Biol. 2018, 337, 83–110. [Google Scholar] [CrossRef]
- Pol, A.; Gross, S.P.; Parton, R.G. Biogenesis of the Multifunctional Lipid Droplet: Lipids, Proteins, and Sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef]
- Wilfling, F.; Haas, J.T.; Walther, T.C.; Farese Jr., R. V. Lipid Droplet Biogenesis. Curr. Opin. Cell Biol. 2014, 29, 39–45. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Wang, J.; Wang, X.; Chen, W.; Lu, N.; Siniossoglou, S.; Yao, Z.; Liu, K. Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration. Neuron 2020, 105, 276–292.e5. [Google Scholar] [CrossRef]
- Inloes, J.M.; Hsu, K.L.; Dix, M.M.; Viader, A.; Masuda, K.; Takei, T.; Wood, M.R.; Cravatt, B.F. The Hereditary Spastic Paraplegia-Related Enzyme DDHD2 Is a Principal Brain Triglyceride Lipase. Proc. Natl. Acad. Sci. USA 2014, 111, 14924–14929. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Baba, T.; Maemoto, Y.; Hara-Miyauchi, C.; Hasegawa-Ogawa, M.; Okano, H.J.; Enda, Y.; Matsumoto, K.; Arimitsu, N.; Nakao, K.; et al. Loss of DDHD2, whose mutation causes spastic paraplegia, promotes reactive oxygen species generation and apoptosis. Cell Death Dis. 2018, 9, 797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, R.E.; Wang, Y.; Ahmadian, M.; Lu, J.; Sarkadi-Nagy, E.; Sul, H.S. Characterization of desnutrin functional domains: Critical residues for triacylglycerol hydrolysis in cultured cells. J. Lipid Res. 2010, 51, 309–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouimet, M.; Franklin, V.; Mak, E.; Liao, X.; Tabas, I.; Marcel, Y.L. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2017, 13, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, N.; Garcia-Macia, M.; Sahu, S.; Athonvarangkul, D.; Liebling, E.; Merlo, P.; Cecconi, F.; Schwartz, G.J.; Singh, R. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 2016, 23, 113–127. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Ge, Z.Y.; Lv, S.K.; Zhao, B.; Li, C.X. The regulatory role of lipophagy in central nervous system diseases. Cell Death Discov. 2023, 9, 229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulze, R.J.; Sathyanarayan, A.; Mashek, D.G. Breaking Fat: The Regulation and Mechanisms of Lipophagy. Biochim. Et. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 1178–1187. [Google Scholar] [CrossRef]
- Norris, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. Human Prefrontal Cortex Phospholipids Containing Docosahexaenoic Acid Increase during Normal Adult Aging, Whereas Those Containing Arachidonic Acid Decrease. Neurobiol. Aging 2015, 36, 1659–1669. [Google Scholar] [CrossRef]
- De Luca, C.; Papa, M. Looking Inside the Matrix: Perineuronal Nets in Plasticity, Maladaptive Plasticity and Neurological Disorders. Neurochem. Res. 2016, 41, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Colangelo, A.M.; Bianco, M.R.; Cavaliere, C.; Zaccaro, L.; Sarmientos, P.; Alberghina, L.; Papa, M. BB14, a Nerve Growth Factor (NGF)-like peptide shown to be effective in reducing reactive astrogliosis and restoring synaptic homeostasis in a rat model of peripheral nerve injury. Biotechnol. Adv. 2012, 30, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. Decreases in Phospholipids Containing Adrenic and Arachidonic Acids Occur in the Human Hippocampus over the Adult Lifespan. Lipids 2015, 50, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Naudí, A.; Cabré, R.; Jové, M.; Ayala, V.; Gonzalo, H.; Portero-Otín, M.; Ferrer, I.; Pamplona, R. Lipidomics of human brain aging and Alzheimer’s disease pathology. Int. Rev. Neurobiol. 2015, 122, 133–189. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Seidel, K. Chemical Biomorphosis of the Human Brain and Sciatic Nerve; a Survey. Z. Alternsforsch. 1958, 12, 52–79. [Google Scholar]
- Rouser, G.; Yamamoto, A. Curvilinear Regression Course of Human Brain Lipid Composition Changes with Age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef]
- Yu, Q.; He, Z.; Zubkov, D.; Huang, S.; Kurochkin, I.; Yang, X.; Halene, T.; Willmitzer, L.; Giavalisco, P.; Akbarian, S.; et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol. Psychiatry 2020, 25, 2952–2969. [Google Scholar] [CrossRef]
- Hancock, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. The Phospholipid Composition of the Human Entorhinal Cortex Remains Relatively Stable over 80 Years of Adult Aging. Geroscience 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Ica, R.; Zamfir, A.D. Gangliosides as Biomarkers of Human Brain Diseases: Trends in Discovery and Characterization by High-Performance Mass Spectrometry. Int. J. Mol. Sci. 2022, 23, 693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Ryan, J.; Tonkin, A.M.; Zoungas, S.; Lacaze, P.; Wolfe, R.; Orchard, S.G.; Murray, A.M.; McNeil, J.J.; Yu, C.; et al. Association Between Triglycerides and Risk of Dementia in Community-Dwelling Older Adults: A Prospective Cohort Study. Neurology 2023, 101, e2288–e2299. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Frazier, D.T.; Bettcher, B.M.; Jastrzab, L.; Chao, L.; Reed, B.; Mungas, D.; Weiner, M.; DeCarli, C.; Chui, H.; et al. Triglycerides are negatively correlated with cognitive function in nondemented aging adults. Neuropsychology 2017, 31, 682–688. [Google Scholar] [CrossRef]
- Kadyrov, M.; Whiley, L.; Brown, B.; Erickson, K.I.; Holmes, E. Associations of the Lipidome with Ageing, Cognitive Decline and Exercise Behaviours. Metabolites 2022, 12, 822. [Google Scholar] [CrossRef]
- Furukawa, K.; Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Kondo, Y.; Shuting, J.; Hashimoto, N.; Furukawa, K. Gangliosides in Inflammation and Neurodegeneration. Prog. Mol. Biol. Transl. Sci. 2018, 156, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D. Neurosteroids as Regenerative Agents in the Brain: Therapeutic Implications. Nat. Rev. Endocrinol. 2013, 9, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Fabelo, N.; Fernández-Echevarría, C.; Canerina-Amaro, A.; Rodríguez-Barreto, D.; Quinto-Alemany, D.; Mesa-Herrera, F.; Díaz, M. Lipid Raft Alterations in Aged-Associated Neuropathologies. Curr. Alzheimer Res. 2016, 13, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Fabelo, N.; Ferrer, I.; Marín, R. “Lipid Raft Aging” in the Human Frontal Cortex during Nonpathological Aging: Gender Influences and Potential Implications in Alzheimer’s Disease. Neurobiol. Aging 2018, 67, 42–52. [Google Scholar] [CrossRef]
- Cabré, R.; Naudí, A.; Dominguez-Gonzalez, M.; Jové, M.; Ayala, V.; Mota-Martorell, N.; Pradas, I.; Nogueras, L.; Rué, M.; Portero-Otín, M.; et al. Lipid Profile in Human Frontal Cortex Is Sustained Throughout Healthy Adult Life Span to Decay at Advanced Ages. J. Gerontol. Ser. A 2018, 73, 703–710. [Google Scholar] [CrossRef]
- Díaz, M.; Pereda de Pablo, D.; Valdés-Baizabal, C.; Santos, G.; Marin, R. Molecular and biophysical features of hippocampal “lipid rafts aging” are modified by dietary n-3 long-chain polyunsaturated fatty acids. Aging Cell 2023, 22, e13867. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, L.; Miller, Y.I.; Miller, Y.; Opin, C.; Author, L. Regulation of Lipid Rafts, Angiogenesis and Inflammation by AIBP HHS Public Access Author Manuscript. Curr. Opin. Lipidol. 2019, 30, 218–223. [Google Scholar] [CrossRef]
- Woller, S.A.; Choi, S.H.; An, E.J.; Low, H.; Schneider, D.A.; Ramachandran, R.; Kim, J.; Bae, Y.S.; Sviridov, D.; Corr, M.; et al. Inhibition of Neuroinflammation by AIBP: Spinal Effects upon Facilitated Pain States. Cell Rep. 2018, 23, 2667. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Kovacs, M.; Lahnsteiner, A.; Felder, T.K.; Rinnerthaler, M. The Janus-Faced Role of Lipid Droplets in Aging: Insights from the Cellular Perspective. Biomolecules 2023, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Savarese, L.; Colangelo, A.M.; Bianco, M.R.; Cirillo, G.; Alberghina, L.; Papa, M. Astrocytes and Microglia-Mediated Immune Response in Maladaptive Plasticity Is Differently Modulated by NGF in the Ventral Horn of the Spinal Cord Following Peripheral Nerve Injury. Cell. Mol. Neurobiol. 2016, 36, 37–46. [Google Scholar] [CrossRef]
- Beas, A.O.; Gordon, P.B.; Prentiss, C.L.; Olsen, C.P.; Kukurugya, M.A.; Bennett, B.D.; Parkhurst, S.M.; Gottschling, D.E. Independent regulation of age associated fat accumulation and longevity. Nat. Commun. 2020, 11, 2790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef]
- Moulton, M.J.; Barish, S.; Ralhan, I.; Chang, J.; Goodman, L.D.; Harland, J.G.; Marcogliese, P.C.; Johansson, J.O.; Ioannou, M.S.; Bellen, H.J. Neuronal ROS-Induced Glial Lipid Droplet Formation Is Altered by Loss of Alzheimer’s Disease-Associated Genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2112095118. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.; Deng, S.; Zhu, L.; Cao, X.; Bao, X.; Xia, S.; Xu, Y.; Zhang, B. Pharmacological Upregulation of Microglial Lipid Droplet Alleviates Neuroinflammation and Acute Ischemic Brain Injury. Inflammation 2023, 46, 1832–1848. [Google Scholar] [CrossRef]
- Kim, J.; Bai, H. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging. Antioxidants 2022, 11, 192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O.; Van Veldhoven, P.P. Aging, age-related diseases and peroxisomes. Subcell. Biochem. 2013, 69, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Nadeem, A.; Li, Z.; Javed, M.; Liu, Q.; Azhar, J.; Rehman, M.S.; Cui, K.; Rehman, S.U. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. Int. J. Mol. Sci. 2021, 22, 12463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uzor, N.E.; McCullough, L.D.; Tsvetkov, A.S. Peroxisomal Dysfunction in Neurological Diseases and Brain Aging. Front. Cell. Neurosci. 2020, 14, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, F. Lipid Metabolism and Alzheimer’s Disease: Clinical Evidence, Mechanistic Link and Therapeutic Promise. FEBS J. 2023, 290, 1420. [Google Scholar] [CrossRef]
- Cerasuolo, M.; Papa, M.; Colangelo, A.M.; Rizzo, M.R. Alzheimer’s Disease from the Amyloidogenic Theory to the Puzzling Crossroads between Vascular, Metabolic and Energetic Maladaptive Plasticity. Biomedicines 2023, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An Integral Player in the Pathogenesis of Alzheimer’s Disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572. [Google Scholar] [CrossRef]
- Lazar, A.N.; Hanbouch, L.; Boussicaut, L.; Fourmaux, B.; Daira, P.; Millan, M.J.; Bernoud-Hubac, N.; Potier, M.C. Lipid Dys-Homeostasis Contributes to APOE4-Associated AD Pathology. Cells 2022, 11, 3616. [Google Scholar] [CrossRef]
- Giri, M.; Zhang, M.; Lü, Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging 2016, 11, 665–681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Qu, F.; Yu, X.; Yang, S.; Zhao, B.; Chen, Y.; Li, P.; Zhang, Z.; Zhang, J.; Han, X.; et al. Cortical lipid metabolic pathway alteration of early Alzheimer’s disease and candidate drugs screen. Eur. J. Med. Res. 2024, 29, 199. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Lovell, M.A. Biomarkers of lipid peroxidation in Alzheimer disease (AD): An update. Arch. Toxicol. 2015, 89, 1035–1044. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, Z.; Yan, L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar] [PubMed] [PubMed Central]
- Di Domenico, F.; Barone, E.; Perluigi, M.; Butterfield, D.A. The Triangle of Death in Alzheimer’s Disease Brain: The Aberrant Cross-Talk Among Energy Metabolism, Mammalian Target of Rapamycin Signaling, and Protein Homeostasis Revealed by Redox Proteomics. Antioxid. Redox Signal. 2017, 26, 364–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S.; Sanders, A.R.; Duan, J. Brain Lipids and Lipid Droplet Dysregulation in Alzheimer’s Disease and Neuropsychiatric Disorders. Complex. Psychiatry 2023, 9, 154–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milos, T.; Rojo, D.; Nedic Erjavec, G.; Konjevod, M.; Tudor, L.; Vuic, B.; Svob Strac, D.; Uzun, S.; Mimica, N.; Kozumplik, O.; et al. Metabolic profiling of Alzheimer’s disease: Untargeted metabolomics analysis of plasma samples. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 127, 110830. [Google Scholar] [CrossRef] [PubMed]
- Etschmaier, K.; Becker, T.; Eichmann, T.O.; Schweinzer, C.; Scholler, M.; Tam-Amersdorfer, C.; Poeckl, M.; Schuligoi, R.; Kober, A.; Chirackal Manavalan, A.P.; et al. Adipose triglyceride lipase affects triacylglycerol metabolism at brain barriers. J. Neurochem. 2011, 119, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Robb, C.; Tonkin, A.M.; Lacaze, P.; Chong, T.T.; Beilin, L.J.; Yu, C.; Watts, G.F.; Ryan, J.; Ernst, M.E.; et al. Association of plasma high-density lipoprotein cholesterol level with risk of incident dementia: A cohort study of healthy older adults. Lancet Reg. Health West. Pac. 2023, 43, 100963. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2014, 572491. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C. Alleviation of Neuropathology by Inhibition of Monoacylglycerol Lipase in APP Transgenic Mice Lacking CB2 Receptors. Mol. Neurobiol. 2018, 55, 4802–4810. [Google Scholar] [CrossRef]
- Pihlaja, R.; Takkinen, J.; Eskola, O.; Vasara, J.; López-Picón, F.R.; Haaparanta-Solin, M.; Rinne, J.O. Monoacylglycerol Lipase Inhibitor JZL184 Reduces Neuroinflammatory Response in APdE9 Mice and in Adult Mouse Glial Cells. J. Neuroinflamm. 2015, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Ugur, Z.; Yilmaz, A.; Ustun, I.; Gorti, S.K.K.; Oh, K.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Maddens, M.E.; et al. Lipid Profiling of Alzheimer’s Disease Brain Highlights Enrichment in Glycerol(phospho)lipid, and Sphingolipid Metabolism. Cells 2021, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, e0191815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Kebede, M.T.; Kemeh, M.M.; Islam, S.; Lee, B.; Bleck, S.D.; Wurfl, L.A.; Lazo, N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24, 2316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanai, H. Effects of N-3 Polyunsaturated Fatty Acids on Dementia. J. Clin. Med. Res. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Shatshat, A.; Pham, A.T.; Rao, P.P.N. Interactions of Polyunsaturated Fatty Acids with Amyloid Peptides Aβ40 and Aβ42. Arch. Biochem. Biophys. 2019, 663, 34–43. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between Fatty Acid Metabolism in the Brain and Alzheimer Disease Neuropathology and Cognitive Performance: A Nontargeted Metabolomic Study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Emre, C.; Do, K.V.; Jun, B.; Hjorth, E.; Alcalde, S.G.; Kautzmann, M.-A.I.; Gordon, W.C.; Nilsson, P.; Bazan, N.G.; Schultzberg, M. Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 116. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-associated phospholipase A2: The story continues. Med. Res. Rev. 2020, 40, 79–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, Y.; Kasuga, K.; Tokutake, T.; Kitamura, K.; Ikeuchi, T.; Nakamura, K. Alterations in Glycerolipid and Fatty Acid Metabolic Pathways in Alzheimer’s Disease Identified by Urinary Metabolic Profiling: A Pilot Study. Front. Neurol. 2021, 12, 719159. [Google Scholar] [CrossRef] [PubMed]
- Ahsanul Haque, M.; Omori, N.; Md Sheikh, A.; Yano, S.; Osago, H.; Mitaki, S.; Kalam Azad, A.; Sakai, H.; Michikawa, M.; Nagai, A. Analysis of the time-dependent changes of phospholipids in the brain regions of a mouse model of Alzheimer’s disease. Brain Res. 2023, 1800, 148197. [Google Scholar] [CrossRef] [PubMed]
- Kosicek, M.; Hecimovic, S. Phospholipids and Alzheimer’s disease: Alterations, mechanisms and potential biomarkers. Int. J. Mol. Sci. 2013, 14, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- de Wit, N.M.; Mol, K.; Rodríguez-Lorenzo, S.; de Vries, H.E.; Kooij, G. The Role of Sphingolipids and Specialized Pro-Resolving Mediators in Alzheimer’s Disease. Front. Immunol. 2021, 11, 620348. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V.; Bieberich, E. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef]
- Lu, M.H.; Ji, W.L.; Xu, D.E.; Yao, P.P.; Zhao, X.Y.; Wang, Z.T.; Fang, L.P.; Huang, R.; Lan, L.J.; Chen, J.B.; et al. Inhibition of Sphingomyelin Synthase 1 Ameliorates Alzheimer-like Pathology in APP/PS1 Transgenic Mice through Promoting Lysosomal Degradation of BACE1. Exp. Neurol. 2019, 311, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Walter, J.; Saher, G.; Don, A.; Nordström, V.; Herzer, S.; Hagan, C.; Von Gerichten, J.; Dieterle, V.; Munteanu, B.; et al. Deletion of Specific Sphingolipids in Distinct Neurons Improves Spatial Memory in a Mouse Model of Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 206. [Google Scholar] [CrossRef]
- Han, X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 774–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, S.; Palavicini, J.P.; Wang, J.; Gonzalez, N.S.; He, S.; Dustin, E.; Zou, C.; Ding, L.; Bhattacharjee, A.; Van Skike, C.E.; et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer’s disease-like neuroinflammation and cognitive impairment. Mol. Neurodegener. 2021, 16, 64. [Google Scholar] [CrossRef]
- Mielke, M.M.; Haughey, N.J.; Han, D.; An, Y.; Bandaru, V.V.R.; Lyketsos, C.G.; Ferrucci, L.; Resnick, S.M. The Association Between Plasma Ceramides and Sphingomyelins and Risk of Alzheimer’s Disease Differs by Sex and APOE in the Baltimore Longitudinal Study of Aging. J. Alzheimers Dis. 2017, 60, 819–828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukami, Y.; Ariga, T.; Yamada, M.; Yuki, N. Brain Gangliosides in Alzheimer’s Disease: Increased Expression of Cholinergic Neuron-Specific Gangliosides. Curr. Alzheimer Res. 2017, 14, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Büşra Lüleci, H.; Oommen, A.M.; Varma, S.; Blackshear, C.T.; Griswold, M.E.; An, Y.; Roberts, J.A.; O’Brien, R.; Pletnikova, O.; et al. Abnormal brain cholesterol homeostasis in Alzheimer’s disease—A targeted metabolomic and transcriptomic study. NPJ Aging Mech. Dis. 2021, 7, 11. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J. Cholesterol-Dependent Amyloid β Production: Space for Multifarious Interactions between Amyloid Precursor Protein, Secretases, and Cholesterol. Cell Biosci. 2023, 13, 171. [Google Scholar] [CrossRef]
- Kang, S.H.; Yoo, H.; Cheon, B.K.; Park, Y.H.; Kim, S.-J.; Ham, H.; Jang, H.; Kim, H.J.; Oh, K.; Koh, S.-B.; et al. Distinct effects of cholesterol profile components on amyloid and vascular burdens. Alzheimers Res. Ther. 2023, 15, 197. [Google Scholar] [CrossRef]
- Marwarha, G.; Raza, S.; Prasanthi, J.R.P.; Ghribi, O. Gadd153 and NF-KB Crosstalk Regulates 27-Hydroxycholesterol-Induced Increase in BACE1 and β-Amyloid Production in Human Neuroblastoma SH-SY5Y Cells. PLoS ONE 2013, 8, e70773. [Google Scholar] [CrossRef]
- Han, X.; Rozen, S.; Boyle, S.H.; Hellegers, C.; Cheng, H.; Burke, J.R.; Welsh-Bohmer, K.A.; Doraiswamy, P.M.; Kaddurah-Daouk, R. Metabolomics in early Alzheimer’s disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE 2011, 6, e21643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Gaamouch, F.; Jing, P.; Xia, J.; Cai, D. Alzheimer’s Disease Risk Genes and Lipid Regulators. J. Alzheimers Dis. 2016, 53, 15–29. [Google Scholar] [CrossRef]
- Schengrund, C.L. Lipid Rafts: Keys to Neurodegeneration. Brain Res. Bull. 2010, 82, 7–17. [Google Scholar] [CrossRef]
- Díaz, M.; Fabelo, N.; Martín, V.; Ferrer, I.; Gómez, T.; Marín, R. Biophysical Alterations in Lipid Rafts from Human Cerebral Cortex Associate with Increased BACE1/AβPP Interaction in Early Stages of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 43, 1185–1198. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, J.; Fleeman, R.M.; Kuhn, M.K.; Swulius, M.T.; Proctor, E.A.; Dokholyan, N.V. Monosialotetrahexosylganglioside Promotes Early Aβ42 Oligomer Formation and Maintenance. ACS Chem. Neurosci. 2022, 13, 1979–1991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef]
- Deng, C.; Barr, A.; Rojo, L.E.; Gaspar, P.A.; Castillo, R.I.; Henriquez-Henriquez, M.; Silva, H.; Maturana, A.; Villar, M.J.; Fuentes, M. From Molecules to the Clinic: Linking Schizophrenia and Metabolic Syndrome through Sphingolipids Metabolism. Front. Neurosci. 2016, 10, 488. [Google Scholar] [CrossRef]
- Sonnino, S.; Prinetti, A. The Role of Sphingolipids in Neuronal Plasticity of the Brain. J. Neurochem. 2016, 137, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G.M. Neuroimaging in Autism Spectrum Disorder: Brain Structure and Function across the Lifespan. Lancet Neurol. 2015, 14, 1121–1134. [Google Scholar] [CrossRef]
- Hussain, G.; Schmitt, F.; Loeffler, J.P.; de Aguilar, J.L.G. Fatting the Brain: A Brief of Recent Research. Front. Cell. Neurosci. 2013, 7, 56983. [Google Scholar] [CrossRef]
- Liu, L.; MacKenzie, K.R.; Putluri, N.; Maletić-Savatić, M.; Bellen, H.J. The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 2017, 26, 719–737.e6. [Google Scholar] [CrossRef]
- Frieden, C.; Wang, H.; Ho, C.M.W. A Mechanism for Lipid Binding to ApoE and the Role of Intrinsically Disordered Regions Coupled to Domain-Domain Interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Liu, Z.; Lippincott-Schwartz, J. A Neuron-Glia Co-Culture System for Studying Intercellular Lipid Transport. Curr. Protoc. Cell Biol. 2019, 84, e95. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef]
- Victor, M.B.; Leary, N.; Luna, X.; Meharena, H.S.; Scannail, A.N.; Bozzelli, P.L.; Samaan, G.; Murdock, M.H.; von Maydell, D.; Effenberger, A.H.; et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell, 1197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narayan, P.; Sienski, G.; Bonner, J.M.; Lin, Y.T.; Seo, J.; Baru, V.; Haque, A.; Milo, B.; Akay, L.A.; Graziosi, A.; et al. PICALM Rescues Endocytic Defects Caused by the Alzheimer’s Disease Risk Factor APOE4. Cell Rep. 2020, 33, 108224. [Google Scholar] [CrossRef] [PubMed]
- Nuriel, T.; Peng, K.Y.; Ashok, A.; Dillman, A.A.; Figueroa, H.Y.; Apuzzo, J.; Ambat, J.; Levy, E.; Cookson, M.R.; Mathews, P.M.; et al. The Endosomal-Lysosomal Pathway Is Dysregulated by APOE4 Expression In Vivo. Front. Neurosci. 2017, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in brain development and function. Biochim. Biophys. Acta 2016, 1863, 934–955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deb, R.; Joshi, N.; Nagotu, S. Peroxisomes of the Brain: Distribution, Functions, and Associated Diseases. Neurotox. Res. 2021, 39, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Coronel, C.; Waser, M.; Caravias, G.; Ransmayr, G. Differential Diagnosis between Patients with Probable Alzheimer’s Disease, Parkinson’s Disease Dementia, or Dementia with Lewy Bodies and Frontotemporal Dementia, Behavioral Variant, Using Quantitative Electroencephalographic Features. J. Neural Transm. 2017, 124, 569–581. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Salvadó, G.; Shekari, M.; Falcon, C.; Operto, G.D.S.; Milà-Alomà, M.; Sánchez-Benavides, G.; Cacciaglia, R.; Arenaza-Urquijo, E.; Niñerola-Baizán, A.; Perissinotti, A.D.S.; et al. Brain Alterations in the Early Alzheimer’s Continuum with Amyloid-β, Tau, Glial and Neurodegeneration CSF Markers. Brain Commun. 2022, 4, fcac134. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Díaz, M.; Catalá, A. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in Brain Oxysterols at Different Stages of Alzheimer’s Disease: Their Involvement in Neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Ansari, M.A.; Mufson, E.J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 2016, 42, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosén, C.; Mattsson, N.; Johansson, P.M.; Andreasson, U.; Wallin, A.; Hansson, O.; Johansson, J.O.; Lamont, J.; Svensson, J.; Blennow, K.; et al. Discriminatory Analysis of Biochip-Derived Protein Patterns in CSF and Plasma in Neurodegenerative Diseases. Front. Aging Neurosci. 2011, 3, 8473. [Google Scholar] [CrossRef] [PubMed]
- Sepe, F.N.; Chiasserini, D.; Parnetti, L. Role of FABP3 as Biomarker in Alzheimer’s Disease and Synucleinopathies. Future Neurol. 2018, 13, 199–207. [Google Scholar] [CrossRef]
- Chiasserini, D.; Parnetti, L.; Andreasson, U.; Zetterberg, H.; Giannandrea, D.; Calabresi, P.; Blennow, K. CSF Levels of Heart Fatty Acid Binding Protein Are Altered During Early Phases of Alzheimer’s Disease. J. Alzheimers Dis. 2010, 22, 1281–1288. [Google Scholar] [CrossRef]
- Harari, O.; Cruchaga, C.; Kauwe, J.S.K.; Ainscough, B.J.; Bales, K.; Pickering, E.H.; Bertelsen, S.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C.; et al. Phosphorylated Tau-Aβ42 Ratio as a Continuous Trait for Biomarker Discovery for Early-Stage Alzheimer’s Disease in Multiplex Immunoassay Panels of Cerebrospinal Fluid. Biol. Psychiatry 2014, 75, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Medici, V.; Malagoli, D.; Chiariello, A.; Cirrincione, A.; Davin, A.; Chikhladze, M.; Vasuri, F.; Legname, G.; Ferrer, I.; et al. Expression Pattern of Perilipins in Human Brain during Aging and in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12756. [Google Scholar] [CrossRef]
- Strefeler, A.; Jan, M.; Quadroni, M.; Teav, T.; Rosenberg, N.; Chatton, J.-Y.; Guex, N.; Gallart-Ayala, H.; Ivanisevic, J. Molecular insights into sex-specific metabolic alterations in Alzheimer’s mouse brain using multi-omics approach. Alzheimer Res. Ther. 2023, 15, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Thalamuthu, A.; Mather, K.A.; Crawford, J.; Ulanova, M.; Wong, M.W.K.; Pickford, R.; Sachdev, P.S.; Braidy, N. Plasma lipidome is dysregulated in Alzheimer’s disease and is associated with disease risk genes. Transl. Psychiatry 2021, 11, 344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalli, E. Nutritional Lipidomics in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1195, 95–104. [Google Scholar] [CrossRef] [PubMed]

| Lipid Class | Key Findings | Increase/ Decrease |
|---|---|---|
| Glycerolipids and Fatty Acids |
| TAG: Increase DAG: Increase MAG: Increase |
| Glycero phospholipids |
| PC: Decrease PE: Decrease PI: Decrease |
| Sphingolipids |
| SM: Variable Ceramide: Increase Sulfatides: Decrease Gangliosides: Altered |
| Cholesterol |
| Total Cholesterol: Increase Oxysterols: Increase |
| Lipid Rafts |
| PUFAs: Decrease Cholesterol: Variable |
| Lipid Droplets |
| Lipid Droplets: Increase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerasuolo, M.; Di Meo, I.; Auriemma, M.C.; Paolisso, G.; Papa, M.; Rizzo, M.R. Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease. Biomolecules 2024, 14, 1362. https://doi.org/10.3390/biom14111362
Cerasuolo M, Di Meo I, Auriemma MC, Paolisso G, Papa M, Rizzo MR. Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease. Biomolecules. 2024; 14(11):1362. https://doi.org/10.3390/biom14111362
Chicago/Turabian StyleCerasuolo, Michele, Irene Di Meo, Maria Chiara Auriemma, Giuseppe Paolisso, Michele Papa, and Maria Rosaria Rizzo. 2024. "Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease" Biomolecules 14, no. 11: 1362. https://doi.org/10.3390/biom14111362
APA StyleCerasuolo, M., Di Meo, I., Auriemma, M. C., Paolisso, G., Papa, M., & Rizzo, M. R. (2024). Exploring the Dynamic Changes of Brain Lipids, Lipid Rafts, and Lipid Droplets in Aging and Alzheimer’s Disease. Biomolecules, 14(11), 1362. https://doi.org/10.3390/biom14111362