Oxidative Phosphorylation as a Predictive Biomarker of Oxaliplatin Response in Colorectal Cancer
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Collection of Data
2.3. GO, KEGG Pathway, and Over-Representation Enrichment Analysis
2.4. Oxaliplatin Response Categorization
2.5. OXPHOS Gene Expression in Solid and Colorectal Tumors
2.6. Colon Tumorsphere Generation
2.7. Sphere-Forming Efficiency (SFE) Assay
2.8. RT-qPCR
2.9. Cardiolipin Content and Mitochondrial Mass Analysis
2.10. Western Blot
2.11. Overall Survival and Relapse-Free Analysis
2.12. Statistical Analysis
3. Results
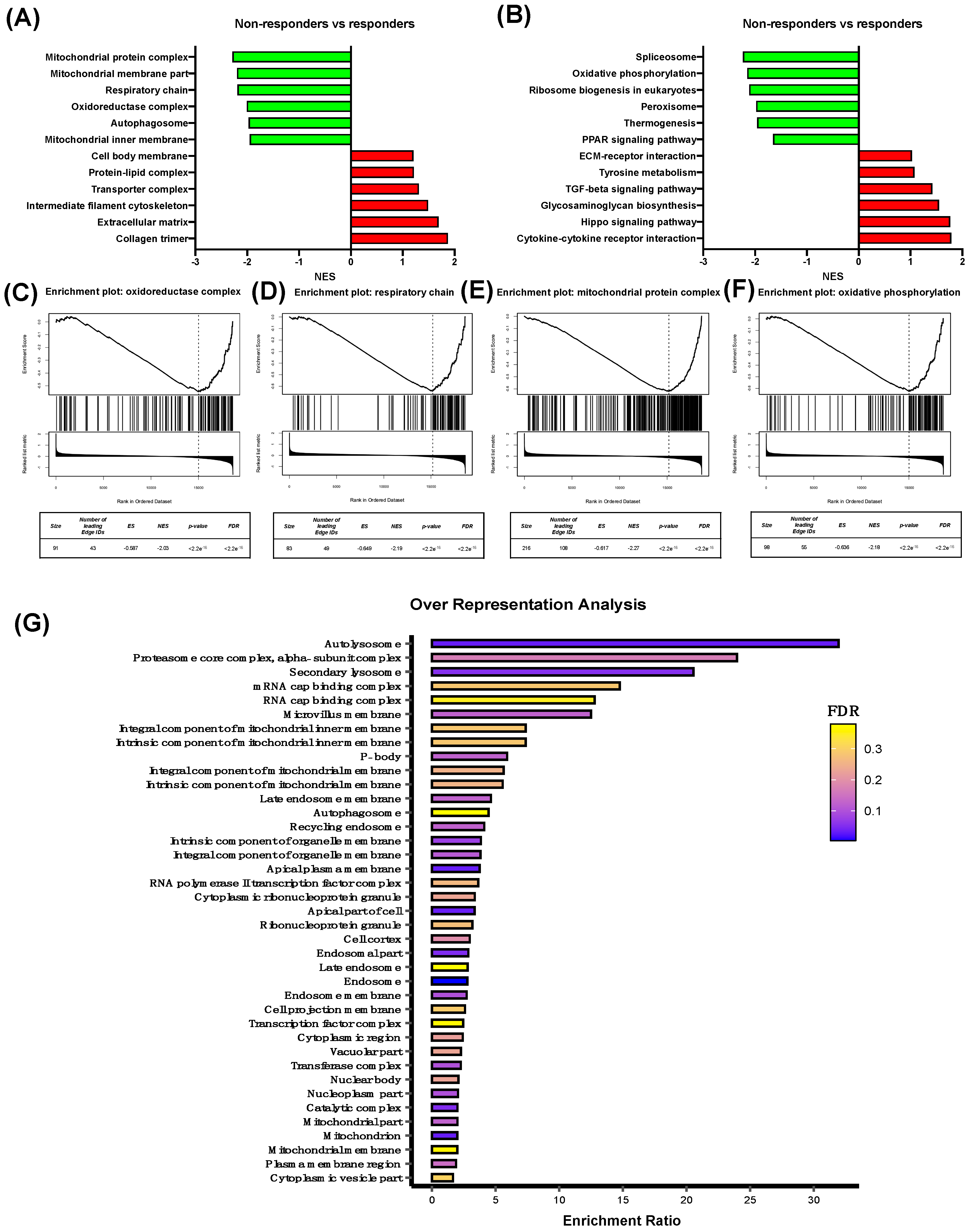
3.1. Characterization of Differentially Expressed Genes Between Oxaliplatin Non-Responder vs Responder Colorectal Cancer Patients
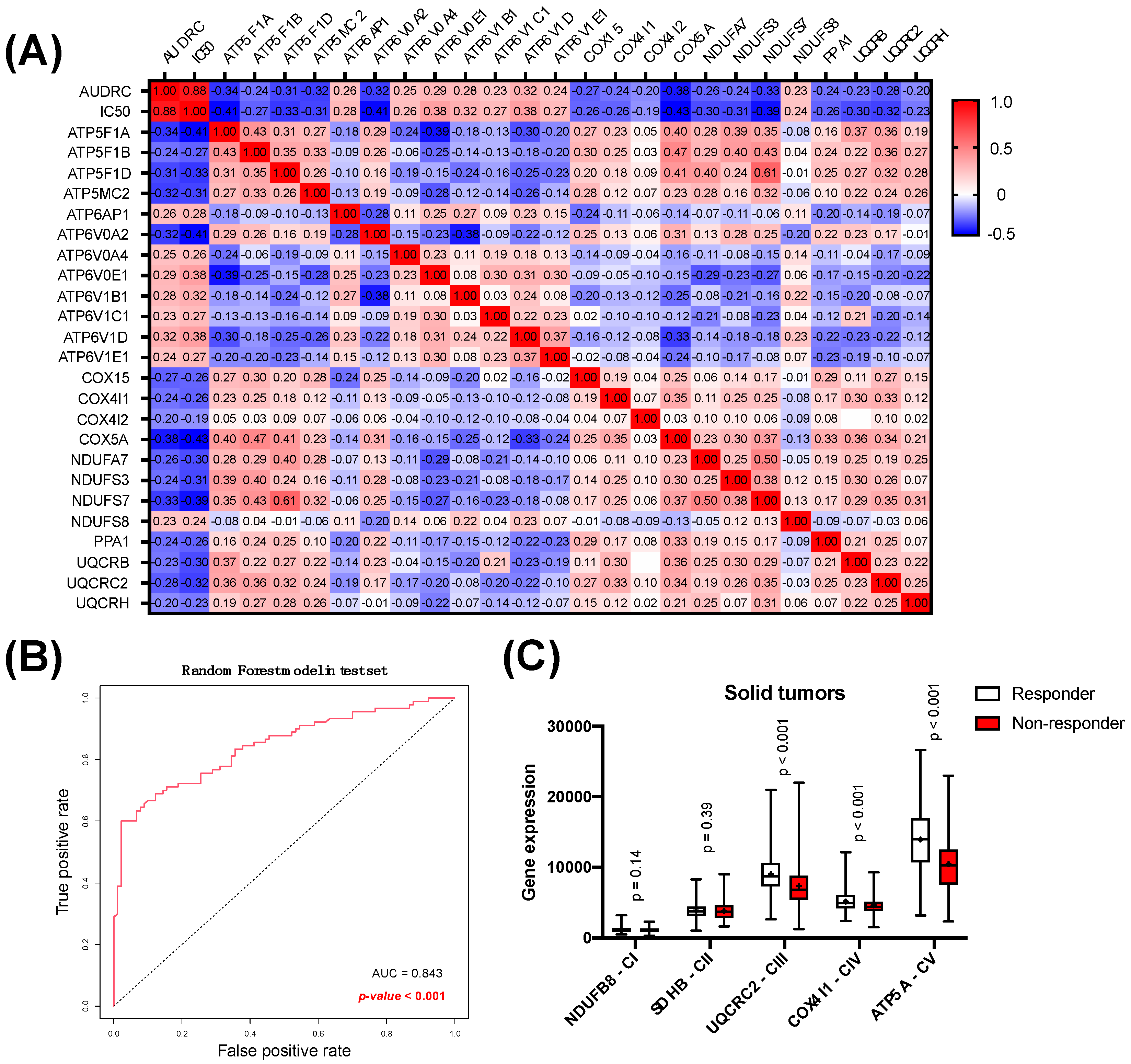
3.2. Analysis of Oxidative Phosphorylation as a Predictor of Response to Oxaliplatin in Solid Tumors
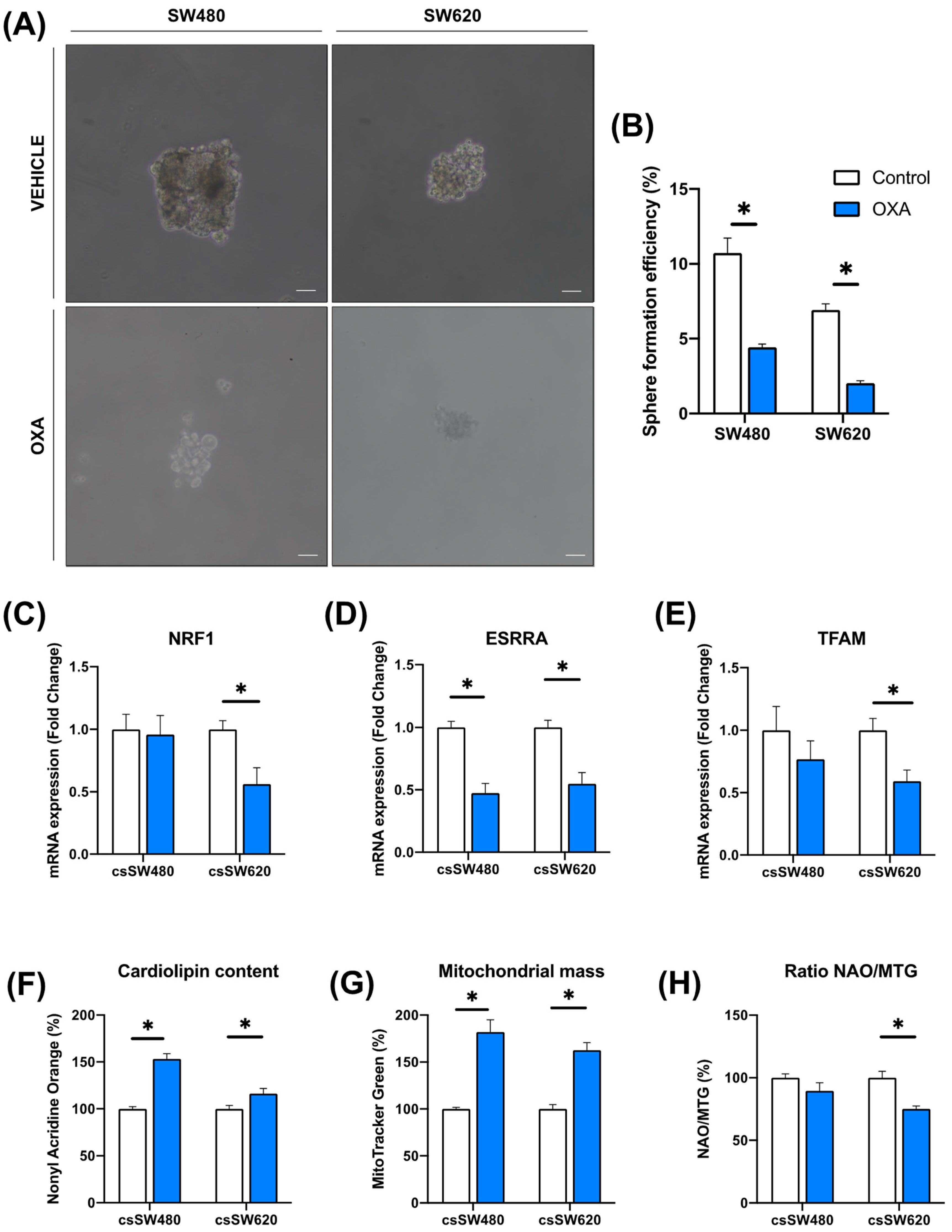
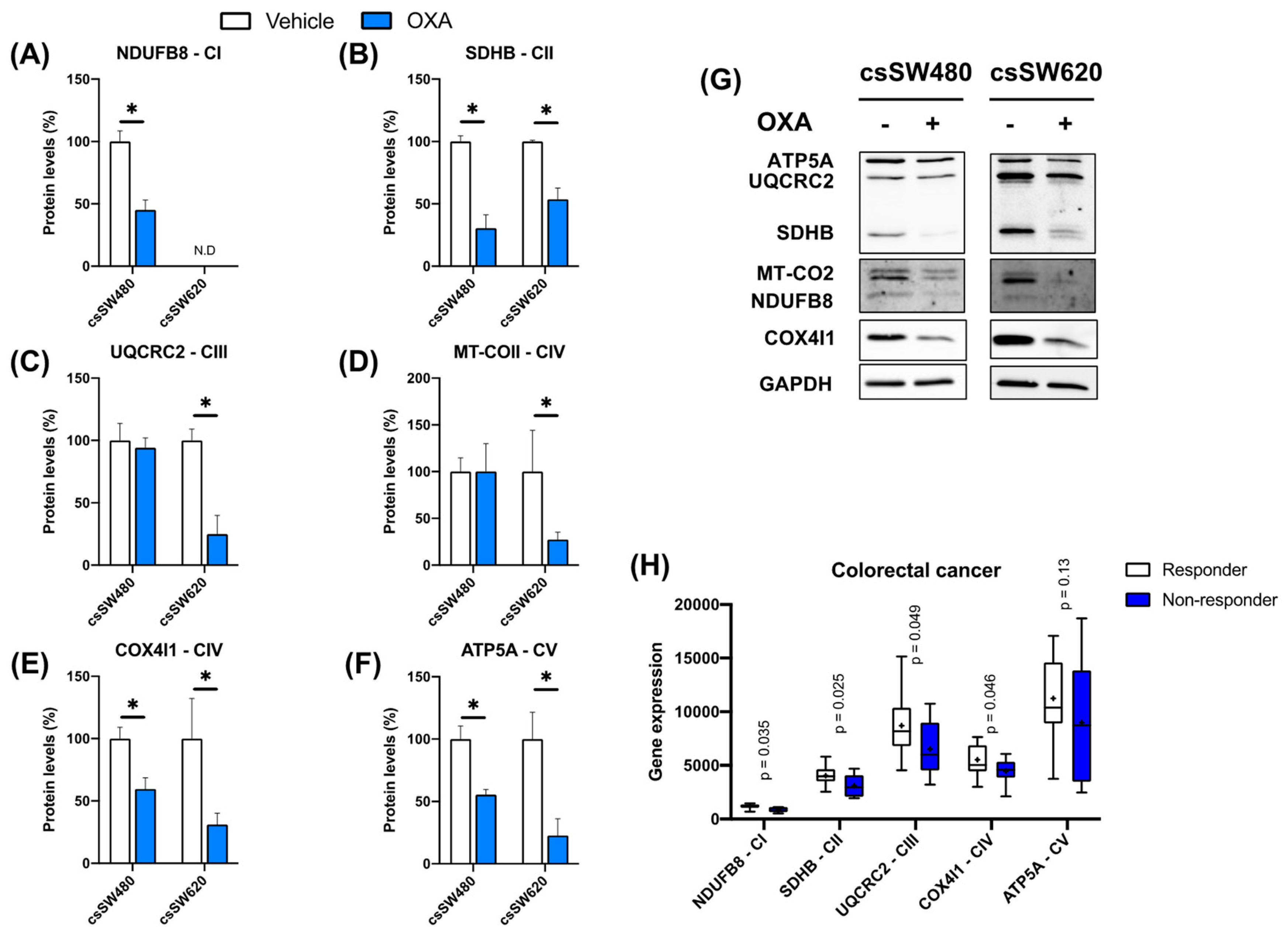
3.3. Validation of Oxidative Phosphorylation as a Predictor of Response to Oxaliplatin in Colorectal Tumorspheres
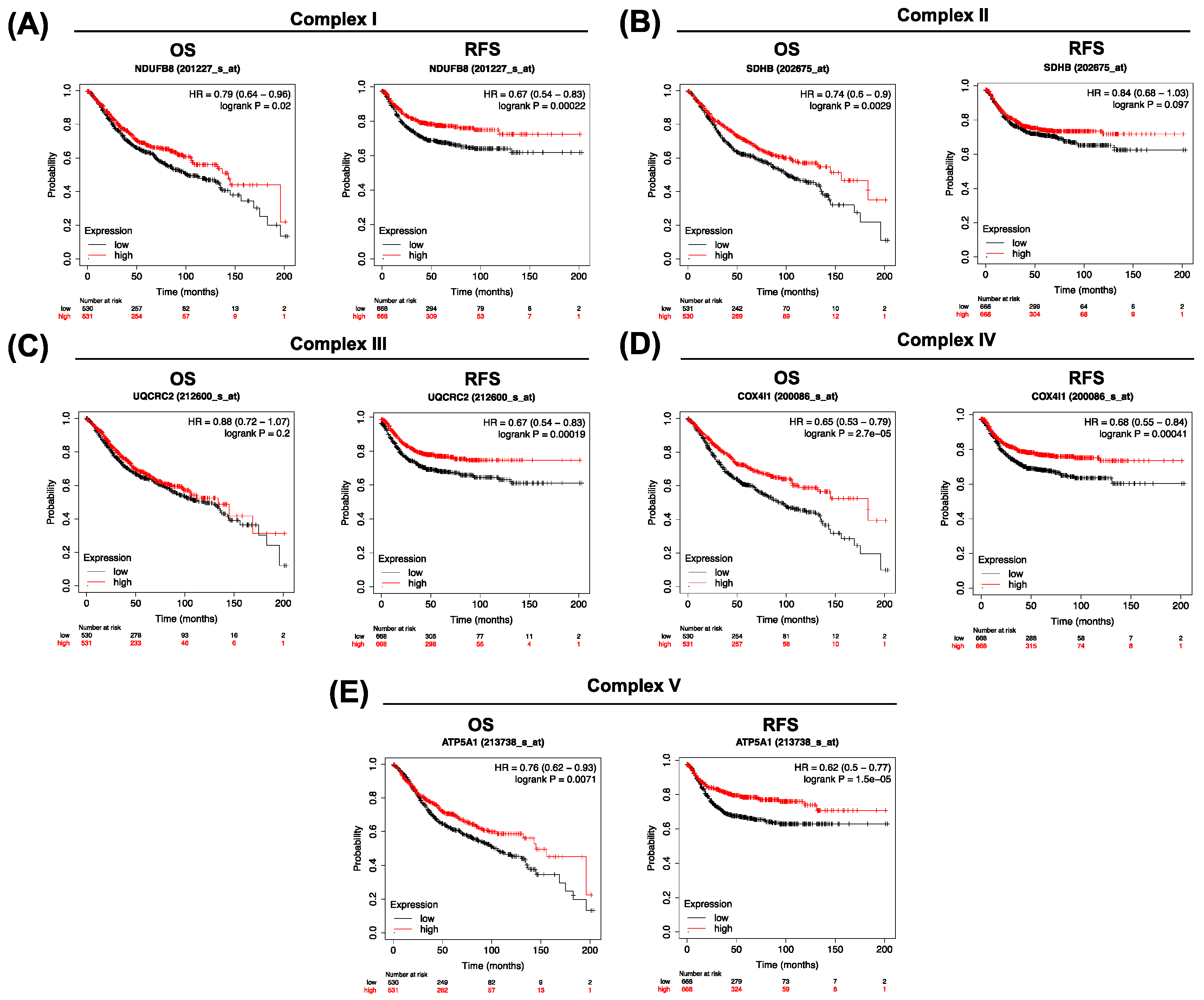
3.4. Clinical Significance of Low OXPHOS Expression in Colorectal Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jansman, F.G.; Sleijfer, D.T.; de Graaf, J.C.; Coenen, J.L.; Brouwers, J.R. Management of chemotherapy-induced adverse effects in the treatment of colorectal cancer. Drug Saf. 2001, 24, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, M.; Mizera, M.; Witowski, J.; Major, P.; Torbicz, G.; Gajewska, N.; Budzyński, A. Primary tumor resection in stage IV unresectable colorectal cancer: What has changed? Med. Oncol. 2017, 34, 188. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical Outcome from Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Marschner, N.; Arnold, D.; Engel, E.; Hutzschenreuter, U.; Rauh, J.; Freier, W.; Hartmann, H.; Frank, M.; Jänicke, M. Oxaliplatin-based first-line chemotherapy is associated with improved overall survival compared to first-line treatment with irinotecan-based chemotherapy in patients with metastatic colorectal cancer—Results from a prospective cohort study. Clin. Epidemiol. 2015, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Rasola, C.; Laurent-Puig, P.; André, T.; Falcoz, A.; Lepage, C.; Aparicio, T.; Bouché, O.; Lievre, A.; Mineur, L.; Bennouna, J.; et al. Time to recurrence and its relation to survival after recurrence in patients resected for stage III colon cancer. Eur. J. Cancer 2023, 194, 113321. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Mauri, G.; Tosi, F.; Bencardino, K.; Bonazzina, E.; Gori, V.; Ruggieri, L.; Arena, S.; Bardelli, A.; Marsoni, S.; et al. Efficacy of Retreatment with Oxaliplatin-Based Regimens in Metastatic Colorectal Cancer Patients: The RETROX-CRC Retrospective Study. Cancers 2022, 14, 1197. [Google Scholar] [CrossRef]
- Gérard, B.; William-Faltaos, S.; Rouillard, D.; Lechat, P.; Bastian, G. Cell Cycle Arrest and Apoptosis Induced by Oxaliplatin (L-OHP) on Four Human Cancer Cell Lines. Anticancer Res. 2006, 26, 2093–2099. [Google Scholar]
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006, 12, 4147–4153. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Liau, J.-Y.; Yu, S.-C.; Ou, D.-L.; Lin, L.-I.; Tseng, L.-H.; Chang, Y.-L.; Yeh, K.-H.; Cheng, A.-L. KRAS Mutation Is a Predictor of Oxaliplatin Sensitivity in Colon Cancer Cells. PLoS ONE 2012, 7, e50701. [Google Scholar] [CrossRef]
- Kassem, A.B.; Salem, S.E.; Abdelrahim, M.E.; Said, A.S.; Salahuddin, A.; Hussein, M.M.; Bahnassy, A.A. ERCC1 and ERCC2 as predictive biomarkers to oxaliplatin-based chemotherapy in colorectal cancer patients from Egypt. Exp. Mol. Pathol. 2017, 102, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; de Gramont, A.; Seitz, J.-F.; et al. Microsatellite Instability in Patients with Stage III Colon Cancer Receiving Fluoropyrimidine with or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J. Clin. Oncol. 2021, 39, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Yu, C.-C.; Wang, B.-Y.; Chang, W.-W. Tumorsphere as an effective in vitro platform for screening anti-cancer stem cell drugs. Oncotarget 2016, 7, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Fotinós, J.; Marks, M.P.; Barberis, L.; Vellón, L. Assessing the distribution of cancer stem cells in tumorspheres. Sci. Rep. 2024, 14, 11013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2018, 9, 33403–33415. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Kaufmann, W.K.; Kaufman, D.G.; Stenstrom, M.; Grisham, J.W. Requirements for adenosine triphosphate in DNA repair in isolated hepatic nuclei. Biochem. Biophys. Res. Commun. 1982, 108, 1040–1047. [Google Scholar] [CrossRef]
- Skulachev, V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996, 29, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Phoon, Y.P.; Karlinsey, K.; Tian, Y.F.; Thapaliya, S.; Thongkum, A.; Qu, L.; Matz, A.J.; Cameron, M.; Cameron, C.; et al. A high OXPHOS CD8 T cell subset is predictive of immunotherapy resistance in melanoma patients. J. Exp. Med. 2022, 219, e20202084. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.H.; Lyskjær, I.; Jersie-Christensen, R.R.; Tarpgaard, L.S.; Primdal-Bengtson, B.; Nielsen, M.M.; Pedersen, J.S.; Hansen, T.P.; Hansen, F.; Olsen, J.V.; et al. miR-625-3p regulates oxaliplatin resistance by targeting MAP2K6-p38 signalling in human colorectal adenocarcinoma cells. Nat. Commun. 2016, 7, 12436. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3104 breast cancer patients. Int. J. Cancer 2019, 145, 3140–3151. [Google Scholar] [CrossRef]
- Morla-Barcelo, P.M.; Laguna-Macarrilla, D.; Cordoba, O.; Matheu, G.; Oliver, J.; Roca, P.; Nadal-Serrano, M.; Sastre-Serra, J. Unraveling malignant phenotype of peritumoral tissue: Transcriptomic insights into early-stage breast cancer. Breast Cancer Res. 2024, 26, 89. [Google Scholar] [CrossRef] [PubMed]
- Fekete, J.T.; Győrffy, B. A unified platform enabling biomarker ranking and validation for 1562 drugs using transcriptomic data of 1250 cancer cell lines. Comput. Struct. Biotechnol. J. 2022, 20, 2885–2894. [Google Scholar] [CrossRef]
- Martinez-Bernabe, T.; Oliver, J.; Sastre-Serra, J.; Pons, D.G. Inflammation-Related Signature Profile Expression as a Poor Prognosis Marker after Oxaliplatin Treatment in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 3821. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Hernández-López, R.; Pons, D.-G.; Roca, P.; Oliver, J.; Sastre-Serra, J. Sirtuin 3 silencing impairs mitochondrial biogenesis and metabolism in colon cancer cells. Am. J. Physiol. Physiol. 2019, 317, C398–C404. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Vilanova-Llompart, J.; Gaya-Bover, A.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 2019, 70, 941–949. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Ahmiane, Y.; Roca, P.; Oliver, J.; Pons, D.G. Xanthohumol, a hop-derived prenylflavonoid present in beer, impairs mitochondrial functionality of SW620 colon cancer cells. Int. J. Food Sci. Nutr. 2019, 70, 396–404. [Google Scholar] [CrossRef]
- Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Roca, P.; Oliver, J. Mitochondrial dynamics is affected by 17β-estradiol in the MCF-7 breast cancer cell line. Effects on fusion and fission related genes. Int. J. Biochem. Cell Biol. 2012, 44, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, A.; Lyssiotis, C.A.; Homicsko, K.; Collisson, E.A.; Gibb, W.J.; Wullschleger, S.; Ostos, L.C.G.; Lannon, W.A.; Grötzinger, C.; Del Rio, M.; et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 2013, 19, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef]
- Yamashita, N.; Withers, H.; Morimoto, Y.; Bhattacharya, A.; Haratake, N.; Daimon, T.; Fushimi, A.; Nakashoji, A.; Thorner, A.R.; Isenhart, E.; et al. MUC1-C integrates aerobic glycolysis with suppression of oxidative phosphorylation in triple-negative breast cancer stem cells. iScience 2023, 26, 108168. [Google Scholar] [CrossRef]
- Uslu, C.; Kapan, E.; Lyakhovich, A. Cancer resistance and metastasis are maintained through oxidative phosphorylation. Cancer Lett. 2024, 587, 216705. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Q.; Chen, J.; Cai, G.; Zhang, Z.; Zou, H.; Zou, J.X.; Liu, Q.; Ji, S.; Shao, G.; et al. Tumor mitochondrial oxidative phosphorylation stimulated by the nuclear receptor RORγ represents an effective therapeutic opportunity in osteosarcoma. Cell Rep. Med. 2024, 5, 101519. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Omar, M.F.M.; Soong, R. The Warburg effect and drug resistance. Br. J. Pharmacol. 2016, 173, 970–979. [Google Scholar] [CrossRef]
- Pendleton, K.E.; Wang, K.; Echeverria, G.V. Rewiring of mitochondrial metabolism in therapy-resistant cancers: Permanent and plastic adaptations. Front. Cell Dev. Biol. 2023, 11, 1254313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Bernabe, T.; Pons, D.G.; Oliver, J.; Sastre-Serra, J. Oxidative Phosphorylation as a Predictive Biomarker of Oxaliplatin Response in Colorectal Cancer. Biomolecules 2024, 14, 1359. https://doi.org/10.3390/biom14111359
Martinez-Bernabe T, Pons DG, Oliver J, Sastre-Serra J. Oxidative Phosphorylation as a Predictive Biomarker of Oxaliplatin Response in Colorectal Cancer. Biomolecules. 2024; 14(11):1359. https://doi.org/10.3390/biom14111359
Chicago/Turabian StyleMartinez-Bernabe, Toni, Daniel G. Pons, Jordi Oliver, and Jorge Sastre-Serra. 2024. "Oxidative Phosphorylation as a Predictive Biomarker of Oxaliplatin Response in Colorectal Cancer" Biomolecules 14, no. 11: 1359. https://doi.org/10.3390/biom14111359
APA StyleMartinez-Bernabe, T., Pons, D. G., Oliver, J., & Sastre-Serra, J. (2024). Oxidative Phosphorylation as a Predictive Biomarker of Oxaliplatin Response in Colorectal Cancer. Biomolecules, 14(11), 1359. https://doi.org/10.3390/biom14111359







