Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications
Abstract
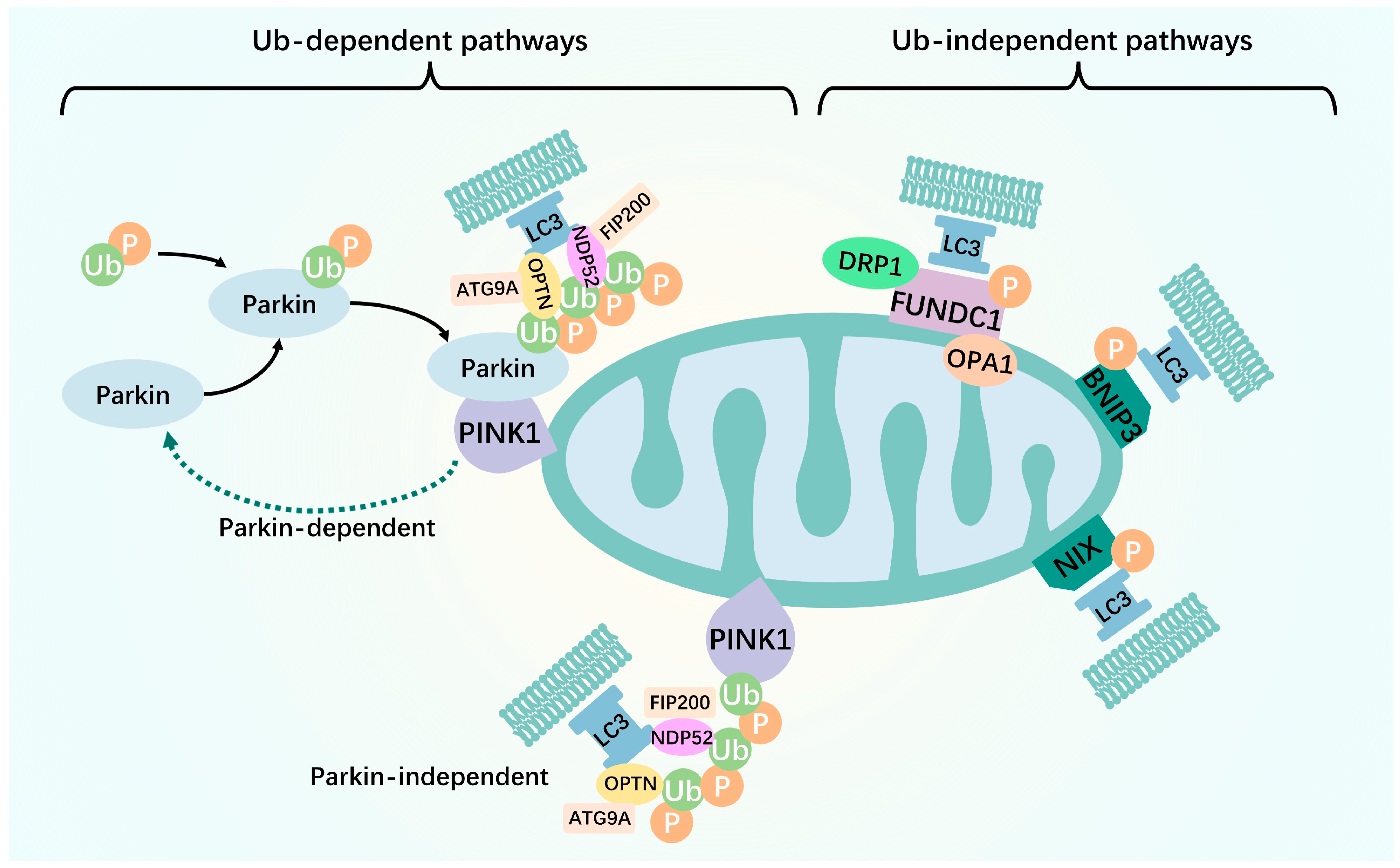
:1. Introduction
2. Mitophagy
2.1. Ubiquitin-Mediated
2.2. Receptor-Mediated
2.2.1. FUNDC1
2.2.2. BNIP3 and NIX
3. Cell Death
3.1. Apoptosis
3.2. Ferroptosis
3.2.1. Iron Metabolism
3.2.2. Lipid Peroxidation
3.2.3. Glutathione (GSH) Metabolism
3.3. Pyroptosis
3.4. NETosis
4. Mitophagy and Cell Death Crosstalk
NETosis
5. Disease-Relevant Cases
6. Targeted Therapy for Mitophagy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular Mitophagy: Mechanism, Roles in Diseases and Small Molecule Pharmacological Regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, R.; Nakahira, K.; Gu, Z. Mitochondrial DNA Mutation, Diseases, and Nutrient-Regulated Mitophagy. Annu. Rev. Nutr. 2019, 39, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and Mitophagy in Acute Kidney Injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in Human Health, Ageing and Disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial Membrane Potential Regulates PINK1 Import and Proteolytic Destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Riley, B.E.; Lougheed, J.C.; Callaway, K.; Velasquez, M.; Brecht, E.; Nguyen, L.; Shaler, T.; Walker, D.; Yang, Y.; Regnstrom, K.; et al. Structure and Function of Parkin E3 Ubiquitin Ligase Reveals Aspects of RING and HECT Ligases. Nat. Commun. 2013, 4, 1982. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.-S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 Stabilized by Mitochondrial Depolarization Recruits Parkin to Damaged Mitochondria and Activates Latent Parkin for Mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Winter, D.; Ashrafi, G.; Schlehe, J.; Wong, Y.L.; Selkoe, D.; Rice, S.; Steen, J.; LaVoie, M.J.; Schwarz, T.L. PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell 2011, 147, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, J.; Li, H.; Yang, B.; Wang, J.; He, Q.; Weng, Q. Emerging Views of OPTN (Optineurin) Function in the Autophagic Process Associated with Disease. Autophagy 2022, 18, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Holzbaur, E.L.F. Optineurin Is an Autophagy Receptor for Damaged Mitochondria in Parkin-Mediated Mitophagy That Is Disrupted by an ALS-Linked Mutation. Proc. Natl. Acad. Sci. USA 2014, 111, E4439–E4448. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Wang, C.; Bunker, E.; Hao, L.; Maric, D.; Schiavo, G.; Randow, F.; Youle, R.J. Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy. Mol. Cell 2019, 74, 347–362.e6. [Google Scholar] [CrossRef]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The Ubiquitin Kinase PINK1 Recruits Autophagy Receptors to Induce Mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Poole, L.P.; Macleod, K.F. Mitophagy in Tumorigenesis and Metastasis. Cell. Mol. Life Sci. 2021, 78, 3817–3851. [Google Scholar] [CrossRef]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial Outer-Membrane Protein FUNDC1 Mediates Hypoxia-Induced Mitophagy in Mammalian Cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef]
- Zhang, W.; Siraj, S.; Zhang, R.; Chen, Q. Mitophagy Receptor FUNDC1 Regulates Mitochondrial Homeostasis and Protects the Heart from I/R Injury. Autophagy 2017, 13, 1080–1081. [Google Scholar] [CrossRef]
- Chen, G.; Han, Z.; Feng, D.; Chen, Y.; Chen, L.; Wu, H.; Huang, L.; Zhou, C.; Cai, X.; Fu, C.; et al. A Regulatory Signaling Loop Comprising the PGAM5 Phosphatase and CK2 Controls Receptor-Mediated Mitophagy. Mol. Cell 2014, 54, 362–377. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Cheng, Q.; Li, Y.; Wu, H.; Zhang, W.; Wang, Y.; Sehgal, S.A.; Siraj, S.; Wang, X.; et al. Mitochondrial E3 Ligase MARCH5 Regulates FUNDC1 to Fine-Tune Hypoxic Mitophagy. EMBO Rep. 2017, 18, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Yuan, F.; Ju, C.; Shang, M.; Ning, J.; Yang, Y.; Ma, J.; Li, G.; Bao, X.; et al. The Role of FUNDC1 in Mitophagy, Mitochondrial Dynamics and Human Diseases. Biochem. Pharmacol. 2022, 197, 114891. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, C.; Wu, K.; Jiang, L.; Wang, X.; Li, W.; Zhuang, H.; Zhang, X.; Chen, H.; Li, S.; et al. FUNDC1 Regulates Mitochondrial Dynamics at the ER-Mitochondrial Contact Site under Hypoxic Conditions. EMBO J. 2016, 35, 1368–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, H.; Xu, C.; Zhu, C.; Wu, H.; Liu, D.; Wang, J.; Liu, L.; Li, W.; Ma, Q.; et al. Hypoxic Mitophagy Regulates Mitochondrial Quality and Platelet Activation and Determines Severity of I/R Heart Injury. eLife 2016, 5, e21407. [Google Scholar] [CrossRef]
- Wang, Y.; Reis, C.; Applegate, R.; Stier, G.; Martin, R.; Zhang, J.H. Ischemic Conditioning-Induced Endogenous Brain Protection: Applications Pre-, Per- or Post-Stroke. Exp. Neurol. 2015, 272, 26–40. [Google Scholar] [CrossRef]
- Pasupathy, S.; Homer-Vanniasinkam, S. Ischaemic Preconditioning Protects against Ischaemia/Reperfusion Injury: Emerging Concepts. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 106–115. [Google Scholar] [CrossRef]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.-H. Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase A2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Li, W.; Chen, H.; Du, L.; Liu, D.; Wang, X.; Xu, T.; Liu, L.; Chen, Q. Deficiency of Mitophagy Receptor FUNDC1 Impairs Mitochondrial Quality and Aggravates Dietary-Induced Obesity and Metabolic Syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef]
- Zhang, J.; Ney, P.A. Mechanisms and Biology of B-Cell Leukemia/Lymphoma 2/Adenovirus E1B Interacting Protein 3 and Nip-like Protein X. Antioxid. Redox Signal. 2011, 14, 1959–1969. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Zhu, S.-H.; Li, V.; Gibson, S.B.; Xu, X.-S.; Kong, J.-M. BNIP3 Interacting with LC3 Triggers Excessive Mitophagy in Delayed Neuronal Death in Stroke. CNS Neurosci. Ther. 2014, 20, 1045–1055. [Google Scholar] [CrossRef]
- He, Y.-L.; Li, J.; Gong, S.-H.; Cheng, X.; Zhao, M.; Cao, Y.; Zhao, T.; Zhao, Y.-Q.; Fan, M.; Wu, H.-T.; et al. BNIP3 Phosphorylation by JNK1/2 Promotes Mitophagy via Enhancing Its Stability under Hypoxia. Cell Death Dis. 2022, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.P.; Bock-Hughes, A.; Berardi, D.E.; Macleod, K.F. ULK1 Promotes Mitophagy via Phosphorylation and Stabilization of BNIP3. Sci. Rep. 2021, 11, 20526. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, W.; Lu, Y.; Zheng, Y.; Pan, L.; Wu, X.; Yuan, Y.; Shen, Z.; Ma, S.; Zhang, X.; et al. BNIP3L/NIX-Mediated Mitophagy: Molecular Mechanisms and Implications for Human Disease. Cell Death Dis. 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zheng, Y.; Zhang, X.; Chen, Y.; Wu, X.; Wu, J.; Shen, Z.; Jiang, L.; Wang, L.; Yang, W.; et al. BNIP3L/NIX-Mediated Mitophagy Protects against Ischemic Brain Injury Independent of PARK2. Autophagy 2017, 13, 1754–1766. [Google Scholar] [CrossRef]
- Chen, M.; Sandoval, H.; Wang, J. Selective Mitochondrial Autophagy during Erythroid Maturation. Autophagy 2008, 4, 926–928. [Google Scholar] [CrossRef]
- Diwan, A.; Koesters, A.G.; Odley, A.M.; Pushkaran, S.; Baines, C.P.; Spike, B.T.; Daria, D.; Jegga, A.G.; Geiger, H.; Aronow, B.J.; et al. Unrestrained Erythroblast Development in Nix-/- Mice Reveals a Mechanism for Apoptotic Modulation of Erythropoiesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6794–6799. [Google Scholar] [CrossRef]
- Esteban-Martínez, L.; Boya, P. BNIP3L/NIX-Dependent Mitophagy Regulates Cell Differentiation via Metabolic Reprogramming. Autophagy 2018, 14, 915–917. [Google Scholar] [CrossRef]
- Esteban-Martínez, L.; Sierra-Filardi, E.; McGreal, R.S.; Salazar-Roa, M.; Mariño, G.; Seco, E.; Durand, S.; Enot, D.; Graña, O.; Malumbres, M.; et al. Programmed Mitophagy Is Essential for the Glycolytic Switch during Cell Differentiation. EMBO J. 2017, 36, 1688–1706. [Google Scholar] [CrossRef]
- Koentjoro, B.; Park, J.-S.; Ha, A.D.; Sue, C.M. Phenotypic Variability of Parkin Mutations in Single Kindred. Mov. Disord. 2012, 27, 1299–1303. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.-W.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Crane, J.D.; Devries, M.C.; Safdar, A.; Hamadeh, M.J.; Tarnopolsky, M.A. The Effect of Aging on Human Skeletal Muscle Mitochondrial and Intramyocellular Lipid Ultrastructure. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Function in Rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.H.C.; Lamb, D.A.; Parry, H.A.; Moore, J.H.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Ruple, B.A.; Huggins, K.W.; et al. Acute and Chronic Effects of Resistance Training on Skeletal Muscle Markers of Mitochondrial Remodeling in Older Adults. Physiol. Rep. 2020, 8, e14526. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Lopus, M. Cell Death Mechanisms in Eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Evan, G.I.; Brown, L.; Whyte, M.; Harrington, E. Apoptosis and the Cell Cycle. Curr. Opin. Cell Biol. 1995, 7, 825–834. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of Aldehydes Derived from Lipid Peroxidation and Membrane Proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Elkin, E.R.; Harris, S.M.; Loch-Caruso, R. Trichloroethylene Metabolite S-(1,2-Dichlorovinyl)-l-Cysteine Induces Lipid Peroxidation-Associated Apoptosis via the Intrinsic and Extrinsic Apoptosis Pathways in a First-Trimester Placental Cell Line. Toxicol. Appl. Pharmacol. 2018, 338, 30–42. [Google Scholar] [CrossRef]
- Cheng, E.H.; Wei, M.C.; Weiler, S.; Flavell, R.A.; Mak, T.W.; Lindsten, T.; Korsmeyer, S.J. BCL-2, BCL-X(L) Sequester BH3 Domain-Only Molecules Preventing BAX- and BAK-Mediated Mitochondrial Apoptosis. Mol. Cell 2001, 8, 705–711. [Google Scholar] [CrossRef]
- Bleicken, S.; Jeschke, G.; Stegmueller, C.; Salvador-Gallego, R.; García-Sáez, A.J.; Bordignon, E. Structural Model of Active Bax at the Membrane. Mol. Cell 2014, 56, 496–505. [Google Scholar] [CrossRef]
- Zha, H.; Aimé-Sempé, C.; Sato, T.; Reed, J.C. Proapoptotic Protein Bax Heterodimerizes with Bcl-2 and Homodimerizes with Bax via a Novel Domain (BH3) Distinct from BH1 and BH2. J. Biol. Chem. 1996, 271, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Walker, G.; Srinivasula, S.M.; Sun, X.M.; Butterworth, M.; Alnemri, E.S.; Cohen, G.M. Recruitment, Activation and Retention of Caspases-9 and -3 by Apaf-1 Apoptosome and Associated XIAP Complexes. EMBO J. 2001, 20, 998–1009. [Google Scholar] [CrossRef]
- Cain, K.; Bratton, S.B.; Langlais, C.; Walker, G.; Brown, D.G.; Sun, X.M.; Cohen, G.M. Apaf-1 Oligomerizes into Biologically Active Approximately 700-kDa and Inactive Approximately 1.4-MDa Apoptosome Complexes. J. Biol. Chem. 2000, 275, 6067–6070. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer Proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome C-Initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9-Dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Eby, M.; Jasmin, A.; Bookwalter, A.; Murray, J.; Hood, L. Death Receptor 5, a New Member of the TNFR Family, and DR4 Induce FADD-Dependent Apoptosis and Activate the NF-kappaB Pathway. Immunity 1997, 7, 821–830. [Google Scholar] [CrossRef]
- Van Antwerp, D.J.; Martin, S.J.; Verma, I.M.; Green, D.R. Inhibition of TNF-Induced Apoptosis by NF-Kappa B. Trends Cell Biol. 1998, 8, 107–111. [Google Scholar] [CrossRef]
- Medema, J.P.; Scaffidi, C.; Kischkel, F.C.; Shevchenko, A.; Mann, M.; Krammer, P.H.; Peter, M.E. FLICE Is Activated by Association with the CD95 Death-Inducing Signaling Complex (DISC). EMBO J. 1997, 16, 2794–2804. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; van Loo, G.; Saelens, X.; Van Gurp, M.; Brouckaert, G.; Kalai, M.; Declercq, W.; Vandenabeele, P. Differential Signaling to Apoptotic and Necrotic Cell Death by Fas-Associated Death Domain Protein FADD. J. Biol. Chem. 2004, 279, 7925–7933. [Google Scholar] [CrossRef]
- Li, J.; Sharma, R.; Patrick, B.; Sharma, A.; Jeyabal, P.V.S.; Reddy, P.M.R.V.; Saini, M.K.; Dwivedi, S.; Dhanani, S.; Ansari, N.H.; et al. Regulation of CD95 (Fas) Expression and Fas-Mediated Apoptotic Signaling in HLE B-3 Cells by 4-Hydroxynonenal. Biochemistry 2006, 45, 12253–12264. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.A.; Legarda-Addison, D.; Skountzos, P.; Yeh, W.C.; Ting, A.T. Ubiquitination of RIP1 Regulates an NF-kappaB-Independent Cell-Death Switch in TNF Signaling. Curr. Biol. 2007, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, I.; Matsuo, K.; Matsushita, Y.; Haruna, Y.; Niwa, M.; Kataoka, T. The C-Terminal Domain of the Long Form of Cellular FLICE-Inhibitory Protein (c-FLIPL) Inhibits the Interaction of the Caspase 8 Prodomain with the Receptor-Interacting Protein 1 (RIP1) Death Domain and Regulates Caspase 8-Dependent Nuclear Factor κB (NF-κB) Activation. J. Biol. Chem. 2014, 289, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.B.; Mehrotra, P.; Arandjelovic, S.; Perry, J.S.A.; Guo, Y.; Morioka, S.; Barron, B.; Walk, S.F.; Ghesquière, B.; Krupnick, A.S.; et al. Metabolites Released from Apoptotic Cells Act as Tissue Messengers. Nature 2020, 580, 130–135. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Find-Me and Eat-Me Signals in Apoptotic Cell Clearance: Progress and Conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef]
- El Hout, M.; Dos Santos, L.; Hamaï, A.; Mehrpour, M. A Promising New Approach to Cancer Therapy: Targeting Iron Metabolism in Cancer Stem Cells. Semin. Cancer Biol. 2018, 53, 125–138. [Google Scholar] [CrossRef]
- Ryu, M.-S.; Zhang, D.; Protchenko, O.; Shakoury-Elizeh, M.; Philpott, C.C. PCBP1 and NCOA4 Regulate Erythroid Iron Storage and Heme Biosynthesis. J. Clin. Investig. 2017, 127, 1786–1797. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative Proteomics Identifies NCOA4 as the Cargo Receptor Mediating Ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia Aggravates Ferroptosis in RPE Cells by Promoting the Fenton Reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Reyhani, A.; McKenzie, T.G.; Fu, Q.; Qiao, G.G. Fenton-Chemistry-Mediated Radical Polymerization. Macromol. Rapid Commun. 2019, 40, e1900220. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated Fatty Acids, Part 1: Occurrence, Biological Activities and Applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Porter, N.A.; Wolf, R.A.; Yarbro, E.M.; Weenen, H. The Autoxidation of Arachidonic Acid: Formation of the Proposed SRS-A Intermediate. Biochem. Biophys. Res. Commun. 1979, 89, 1058–1064. [Google Scholar] [CrossRef]
- Yan, H.-F.; Zou, T.; Tuo, Q.-Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Hishikawa, D.; Shindou, H.; Kobayashi, S.; Nakanishi, H.; Taguchi, R.; Shimizu, T. Discovery of a Lysophospholipid Acyltransferase Family Essential for Membrane Asymmetry and Diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 2830–2835. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Krümmel, B.; Plötz, T.; Jörns, A.; Lenzen, S.; Mehmeti, I. The Central Role of Glutathione Peroxidase 4 in the Regulation of Ferroptosis and Its Implications for Pro-Inflammatory Cytokine-Mediated Beta-Cell Death. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166114. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel Biomarker of Oxidative Stress Is Associated with Risk of Death in Patients with Coronary Artery Disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef]
- Chen, M.; Rong, R.; Xia, X. Spotlight on Pyroptosis: Role in Pathogenesis and Therapeutic Potential of Ocular Diseases. J. Neuroinflamm. 2022, 19, 183. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.-D. The Cell Biology of Inflammasomes: Mechanisms of Inflammasome Activation and Regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular Mechanisms and Functions of Pyroptosis, Inflammatory Caspases and Inflammasomes in Infectious Diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.-D. Inflammasome Activation and Assembly at a Glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A New Frontier in Cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Gong, F.; Chen, Y.; Chen, E. The Role and Mechanism of Pyroptosis and Potential Therapeutic Targets in Sepsis: A Review. Front. Immunol. 2021, 12, 711939. [Google Scholar] [CrossRef]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 Cleaves Gasdermin D for Non-Canonical Inflammasome Signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-Canonical Inflammasome Activation Targets Caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging Connectivity of Programmed Cell Death Pathways and Its Physiological Implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed Interplay between Autophagy and Inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil Migration in Infection and Wound Repair: Going Forward in Reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-Like Disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Naik, P.P.; Panigrahi, D.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Sethi, G.; Bhutia, S.K. Intricate Role of Mitochondrial Lipid in Mitophagy and Mitochondrial Apoptosis: Its Implication in Cancer Therapeutics. Cell. Mol. Life Sci. 2019, 76, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Green, D.R. MOMP, Cell Suicide as a BCL-2 Family Business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sinha, S.; Levine, B. Dual Role of JNK1-Mediated Phosphorylation of Bcl-2 in Autophagy and Apoptosis Regulation. Autophagy 2008, 4, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Hollville, E.; Carroll, R.G.; Cullen, S.P.; Martin, S.J. Bcl-2 Family Proteins Participate in Mitochondrial Quality Control by Regulating Parkin/PINK1-Dependent Mitophagy. Mol. Cell 2014, 55, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-Eating and Self-Killing: Crosstalk between Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Mazure, N.M.; Pouysségur, J. Atypical BH3-Domains of BNIP3 and BNIP3L Lead to Autophagy in Hypoxia. Autophagy 2009, 5, 868–869. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 Antiapoptotic Proteins Inhibit Beclin 1-Dependent Autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Fernández, Á.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.-C.; et al. Disruption of the Beclin 1-BCL2 Autophagy Regulatory Complex Promotes Longevity in Mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef]
- Cheng, M.; Lin, N.; Dong, D.; Ma, J.; Su, J.; Sun, L. PGAM5: A Crucial Role in Mitochondrial Dynamics and Programmed Cell Death. Eur. J. Cell Biol. 2021, 100, 151144. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Z.; Chang, R.; Cheng, H.; Mu, C.; Zhao, T.; Chen, L.; Zhang, C.; Luo, Q.; Lin, J.; et al. Dynamic PGAM5 Multimers Dephosphorylate BCL-xL or FUNDC1 to Regulate Mitochondrial and Cellular Fate. Cell Death Differ. 2020, 27, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xue, D.; Chen, G.; Han, Z.; Huang, L.; Zhu, C.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y.; et al. The BCL2L1 and PGAM5 Axis Defines Hypoxia-Induced Receptor-Mediated Mitophagy. Autophagy 2014, 10, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-J.; Wang, Z.-Y.; Xu, L.; Chen, X.-H.; Li, X.-X.; Liao, W.-T.; Ma, H.-K.; Jiang, M.-D.; Xu, T.-T.; Xu, J.; et al. HIF-1α-BNIP3-Mediated Mitophagy in Tubular Cells Protects against Renal Ischemia/Reperfusion Injury. Redox Biol. 2020, 36, 101671. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1α Alleviates Osteoarthritis via Enhancing Mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 Inflammasome Attenuates Apoptosis in Contrast-Induced Acute Kidney Injury through the Upregulation of HIF1A and BNIP3-Mediated Mitophagy. Autophagy 2021, 17, 2975–2990. [Google Scholar] [CrossRef]
- Shen, J.; Wang, L.; Jiang, N.; Mou, S.; Zhang, M.; Gu, L.; Shao, X.; Wang, Q.; Qi, C.; Li, S.; et al. NLRP3 Inflammasome Mediates Contrast Media-Induced Acute Kidney Injury by Regulating Cell Apoptosis. Sci. Rep. 2016, 6, 34682. [Google Scholar] [CrossRef]
- Yu, S.; Du, M.; Yin, A.; Mai, Z.; Wang, Y.; Zhao, M.; Wang, X.; Chen, T. Bcl-xL Inhibits PINK1/Parkin-Dependent Mitophagy by Preventing Mitochondrial Parkin Accumulation. Int. J. Biochem. Cell Biol. 2020, 122, 105720. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, S.E.; Koh, H.C. PGAM5 Regulates PINK1/Parkin-Mediated Mitophagy via DRP1 in CCCP-Induced Mitochondrial Dysfunction. Toxicol. Lett. 2018, 284, 120–128. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.-N.; Wang, K.; Zhang, L.; Huang, Z.; Liu, J.; Zhang, Z.; Luo, M.; Lei, Y.; Peng, Y.; et al. Ketoconazole Exacerbates Mitophagy to Induce Apoptosis by Downregulating Cyclooxygenase-2 in Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 66–77. [Google Scholar] [CrossRef]
- Li, W.; Jiang, W.-S.; Su, Y.-R.; Tu, K.-W.; Zou, L.; Liao, C.-R.; Wu, Q.; Wang, Z.-H.; Zhong, Z.-M.; Chen, J.-T.; et al. PINK1/Parkin-Mediated Mitophagy Inhibits Osteoblast Apoptosis Induced by Advanced Oxidation Protein Products. Cell Death Dis. 2023, 14, 88. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Qin, J.; Lu, Y.; Shen, H.-M.; Chen, H.-B. Targeting Mitophagy to Promote Apoptosis Is a Potential Therapeutic Strategy for Cancer. Autophagy 2023, 19, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wang, L.; Zhang, C.; Song, R.; Zou, H.; Gu, J.; Liu, X.; Bian, J.; Liu, Z.; Yuan, Y. PINK1/Parkin-Mediated Mitophagy Modulates Cadmium-Induced Apoptosis in Rat Cerebral Cortical Neurons. Ecotoxicol. Environ. Saf. 2022, 244, 114052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-Glucose Induces Retinal Pigment Epithelium Mitochondrial Pathways of Apoptosis and Inhibits Mitophagy by Regulating ROS/PINK1/Parkin Signal Pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Gao, M.; Jiang, X. To Eat or Not to Eat-the Metabolic Flavor of Ferroptosis. Curr. Opin. Cell Biol. 2018, 51, 58–64. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Liesa, M.; Palacín, M.; Zorzano, A. Mitochondrial Dynamics in Mammalian Health and Disease. Physiol. Rev. 2009, 89, 799–845. [Google Scholar] [CrossRef]
- Richardson, D.R.; Lane, D.J.R.; Becker, E.M.; Huang, M.L.-H.; Whitnall, M.; Suryo Rahmanto, Y.; Sheftel, A.D.; Ponka, P. Mitochondrial Iron Trafficking and the Integration of Iron Metabolism between the Mitochondrion and Cytosol. Proc. Natl. Acad. Sci. USA 2010, 107, 10775–10782. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Hu, F.; Feng, H.; Linkermann, A.; Min, W.; Stockwell, B.R. Determination of the Subcellular Localization and Mechanism of Action of Ferrostatins in Suppressing Ferroptosis. ACS Chem. Biol. 2018, 13, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Oberst, A.; Quarato, G.; Milasta, S.; Haller, M.; Wang, R.; Karvela, M.; Ichim, G.; Yatim, N.; Albert, M.L.; et al. Widespread Mitochondrial Depletion via Mitophagy Does Not Compromise Necroptosis. Cell Rep. 2013, 5, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Q.; Liu, H.; Liu, J.; Yang, S.; Luo, X.; Liu, W.; Zheng, H.; Liu, Q.; Cui, Y.; et al. Dynamic O-GlcNAcylation Coordinates Ferritinophagy and Mitophagy to Activate Ferroptosis. Cell Discov. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Gan, B. Mitochondrial Regulation of Ferroptosis. J. Cell Biol. 2021, 220, e202105043. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis Is a Type of Autophagy-Dependent Cell Death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Singh, L.P.; Yumnamcha, T.; Devi, T.S. Mitophagy, Ferritinophagy and Ferroptosis in Retinal Pigment Epithelial Cells under High Glucose Conditions: Implications for Diabetic Retinopathy and Age-Related Retinal Diseases. JOJ Ophthalmol. 2021, 8, 77–85. [Google Scholar]
- Peng, H.; Fu, S.; Wang, S.; Xu, H.; Dhanasekaran, M.; Chen, H.; Shao, C.; Chen, Y.; Ren, J. Ablation of FUNDC1-Dependent Mitophagy Renders Myocardium Resistant to Paraquat-Induced Ferroptosis and Contractile Dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166448. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, Y.; Liu, S.; Jin, W.; Luo, Y.; Sun, M.; Duan, Y.; Ajoolabady, A.; Sowers, J.R.; Fang, Y.; et al. FUNDC1 Insufficiency Sensitizes High Fat Diet Intake-Induced Cardiac Remodeling and Contractile Anomaly through ACSL4-Mediated Ferroptosis. Metabolism 2021, 122, 154840. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-Mediated Mitophagy Is Dependent on VDAC1 and P62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Moras, M.; Hattab, C.; Gonzalez-Menendez, P.; Martino, S.; Larghero, J.; Le Van Kim, C.; Kinet, S.; Taylor, N.; Lefevre, S.D.; Ostuni, M.A. Downregulation of Mitochondrial TSPO Inhibits Mitophagy and Reduces Enucleation during Human Terminal Erythropoiesis. Int. J. Mol. Sci. 2020, 21, 9066. [Google Scholar] [CrossRef]
- Sun, Y.; Vashisht, A.A.; Tchieu, J.; Wohlschlegel, J.A.; Dreier, L. Voltage-Dependent Anion Channels (VDACs) Recruit Parkin to Defective Mitochondria to Promote Mitochondrial Autophagy. J. Biol. Chem. 2012, 287, 40652–40660. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Leeper, T.C.; Carroll, R.T. mitoNEET as a Novel Drug Target for Mitochondrial Dysfunction. Drug Discov. Today 2014, 19, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Lipper, C.H.; Stofleth, J.T.; Bai, F.; Sohn, Y.-S.; Roy, S.; Mittler, R.; Nechushtai, R.; Onuchic, J.N.; Jennings, P.A. Redox-Dependent Gating of VDAC by mitoNEET. Proc. Natl. Acad. Sci. USA 2019, 116, 19924–19929. [Google Scholar] [CrossRef]
- Basit, F.; van Oppen, L.M.; Schöckel, L.; Bossenbroek, H.M.; van Emst-de Vries, S.E.; Hermeling, J.C.; Grefte, S.; Kopitz, C.; Heroult, M.; Hgm Willems, P.; et al. Mitochondrial Complex I Inhibition Triggers a Mitophagy-Dependent ROS Increase Leading to Necroptosis and Ferroptosis in Melanoma Cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef]
- McElroy, G.S.; Reczek, C.R.; Reyfman, P.A.; Mithal, D.S.; Horbinski, C.M.; Chandel, N.S. NAD+ Regeneration Rescues Lifespan, but Not Ataxia, in a Mouse Model of Brain Mitochondrial Complex I Dysfunction. Cell Metab. 2020, 32, 301–308.e6. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Huang, Z.; Zhou, Y.; Xia, J.; Hu, W.; Wang, X.; Du, J.; Tong, X.; Wang, Y. CISD3 Inhibition Drives Cystine-Deprivation Induced Ferroptosis. Cell Death Dis. 2021, 12, 839. [Google Scholar] [CrossRef]
- Fan, P.; Xie, X.-H.; Chen, C.-H.; Peng, X.; Zhang, P.; Yang, C.; Wang, Y.-T. Molecular Regulation Mechanisms and Interactions between Reactive Oxygen Species and Mitophagy. DNA Cell Biol. 2019, 38, 10–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy Contributes to the Pathogenesis of Inflammatory Diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R. Mitophagy Programs: Mechanisms and Physiological Implications of Mitochondrial Targeting by Autophagy. Cell. Mol. Life Sci. 2016, 73, 775–795. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Qiu, X.; Wang, G.; Hu, Z.; Chen, S.; Wu, Z.; Yuan, N.; Gao, H.; Wang, J.; et al. Listeria Hijacks Host Mitophagy through a Novel Mitophagy Receptor to Evade Killing. Nat. Immunol. 2019, 20, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.-M.; Silwal, P.; Jo, E.-K. Inflammasome and Mitophagy Connection in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4714. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting Inflammation-the Negative Regulation of NF-κB and the NLRP3 Inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Yoon, J.-H.; Ryu, J.-H. Mitophagy: A Balance Regulator of NLRP3 Inflammasome Activation. BMB Rep. 2016, 49, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Nagasu, H.; Murakami, T.; Hoang, H.; Broderick, L.; Hoffman, H.M.; Horng, T. Inflammasome Activation Leads to Caspase-1-Dependent Mitochondrial Damage and Block of Mitophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 15514–15519. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.-C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, R.; Cen, S.; Zhou, J. STING Modulators: Predictive Significance in Drug Discovery. Eur. J. Med. Chem. 2019, 182, 111591. [Google Scholar] [CrossRef]
- Hopfner, K.-P.; Hornung, V. Molecular Mechanisms and Cellular Functions of cGAS-STING Signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.-J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New Mitochondrial DNA Synthesis Enables NLRP3 Inflammasome Activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Liu, Y.; Rundberg Nilsson, A.; Gatchalian, R.; Crother, T.R.; Tourtellotte, W.G.; Zhang, Y.; Aleman-Muench, G.R.; Lewis, G.; Chen, W.; et al. Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity 2021, 54, 1463–1477.e11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Wang, X.; Bu, Q.; Wang, Q.; Su, W.; Li, L.; Zhou, H.; Lu, L. XBP1 Deficiency Promotes Hepatocyte Pyroptosis by Impairing Mitophagy to Activate mtDNA-cGAS-STING Signaling in Macrophages during Acute Liver Injury. Redox Biol. 2022, 52, 102305. [Google Scholar] [CrossRef] [PubMed]
- de Torre-Minguela, C.; Gómez, A.I.; Couillin, I.; Pelegrín, P. Gasdermins Mediate Cellular Release of Mitochondrial DNA during Pyroptosis and Apoptosis. FASEB J. 2021, 35, e21757. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Siraj, S.; Jin, H.; Fan, Y.; Yang, X.; Huang, X.; Wang, X.; Wang, J.; Liu, L.; et al. FUN14 Domain-Containing 1-Mediated Mitophagy Suppresses Hepatocarcinogenesis by Inhibition of Inflammasome Activation in Mice. Hepatology 2019, 69, 604–621. [Google Scholar] [CrossRef]
- Bendorius, M.; Neeli, I.; Wang, F.; Bonam, S.R.; Dombi, E.; Buron, N.; Borgne-Sanchez, A.; Poulton, J.; Radic, M.; Muller, S. The Mitochondrion-Lysosome Axis in Adaptive and Innate Immunity: Effect of Lupus Regulator Peptide P140 on Mitochondria Autophagy and NETosis. Front. Immunol. 2018, 9, 2158. [Google Scholar] [CrossRef]
- Chu, C.; Wang, X.; Yang, C.; Chen, F.; Shi, L.; Xu, W.; Wang, K.; Liu, B.; Wang, C.; Sun, D.; et al. Neutrophil Extracellular Traps Drive Intestinal Microvascular Endothelial Ferroptosis by Impairing Fundc1-Dependent Mitophagy. Redox Biol. 2023, 67, 102906. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil Extracellular Traps in Systemic Autoimmune and Autoinflammatory Diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Roy, E.; Comerci, A.J.; van der Windt, D.J.; Zhang, H.; Huang, H.; Loughran, P.; Shiva, S.; Geller, D.A.; Bartlett, D.L.; et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019, 79, 5626–5639. [Google Scholar] [CrossRef]
- Fang, G.; Wen, X.; Jiang, Z.; Du, X.; Liu, R.; Zhang, C.; Huang, G.; Liao, W.; Zhang, Z. FUNDC1/PFKP-Mediated Mitophagy Induced by KD025 Ameliorates Cartilage Degeneration in Osteoarthritis. Mol. Ther. 2023, 31, 3594–3612. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, L.; Chen, G.; Lin, T.; Lin, J.; Zhao, X.; Li, W.; Guo, S.; Wu, R.; Wang, Z.; et al. FUNDC1-Induced Mitophagy Protects Spinal Cord Neurons against Ischemic Injury. Cell Death Discov. 2024, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, C.; Li, Y.; Wang, H.; Wang, H.; Wang, Y.; Wu, T.; Wang, H.; Cheng, G.; Man, J.; et al. Mitophagy Mediated by HIF-1α/FUNDC1 Signaling in Tubular Cells Protects against Renal Ischemia/Reperfusion Injury. Ren. Fail. 2024, 46, 2332492. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, J.; Li, S.; Wang, S.; Zhang, J.; Wang, Y.-P.; Yan, Y.-S.; Hu, H.-Y.; Xiong, M.-F.; Bai, C.-B.; et al. NTRK1 Knockdown Induces Mouse Cognitive Impairment and Hippocampal Neuronal Damage through Mitophagy Suppression via Inactivating the AMPK/ULK1/FUNDC1 Pathway. Cell Death Discov. 2023, 9, 404. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Gu, J.; Ke, P.; Liu, J.; Meng, Y.; Zheng, W.; Que, W.; Fan, R.; Luo, J.; et al. FUDNC1-Dependent Mitophagy Ameliorate Motor Neuron Death in an Amyotrophic Lateral Sclerosis Mouse Model. Neurobiol. Dis. 2024, 197, 106534. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Wang, J.; Zhu, H.; Ren, J.; Chen, Y. Pathogenesis of Cardiac Ischemia Reperfusion Injury Is Associated with CK2α-Disturbed Mitochondrial Homeostasis via Suppression of FUNDC1-Related Mitophagy. Cell Death Differ. 2018, 25, 1080–1093. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, H.-Y.; Chen, Y.; Chen, X.; Wu, Z.-L.; Hu, Q.-Y.; Sun, H. Src Activation Aggravates Podocyte Injury in Diabetic Nephropathy via Suppression of FUNDC1-Mediated Mitophagy. Front. Pharmacol. 2022, 13, 897046. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Ouyang, S.-X.; Zhang, G.-Y.; Cao, Q.; Xin, R.; Yin, H.; Wu, J.-W.; Zhang, Y.; Zhang, Z.; Liu, Y.; et al. GSDME Promotes MASLD by Regulating Pyroptosis, Drp1 Citrullination-Dependent Mitochondrial Dynamic, and Energy Balance in Intestine and Liver. Cell Death Differ. 2024, 60, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jian, L.; Guo, Y.; Tang, C.; Huang, Z.; Gao, J. Liver Cell Mitophagy in Metabolic Dysfunction-Associated Steatotic Liver Disease and Liver Fibrosis. Antioxidants 2024, 13, 729. [Google Scholar] [CrossRef]
- Yao, S.; Pang, M.; Wang, Y.; Wang, X.; Lin, Y.; Lv, Y.; Xie, Z.; Hou, J.; Du, C.; Qiu, Y.; et al. Mesenchymal Stem Cell Attenuates Spinal Cord Injury by Inhibiting Mitochondrial Quality Control-Associated Neuronal Ferroptosis. Redox Biol. 2023, 67, 102871. [Google Scholar] [CrossRef]
- Shen, J.; Xie, P.; Wang, J.; Yang, F.; Li, S.; Jiang, H.; Wu, X.; Zhou, F.; Li, J. Nlrp6 Protects from Corticosterone-Induced NSPC Ferroptosis by Modulating RIG-1/MAVS-Mediated Mitophagy. Redox Biol. 2024, 73, 103196. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; Bevilacqua, A.; Wu, R.-M.; Kao, K.-C.; Lin, C.-P.; Rousseau, L.; Peng, F.-T.; Chuang, Y.-M.; Peng, J.-J.; Park, J.; et al. Regulatory Circuits of Mitophagy Restrict Distinct Modes of Cell Death during Memory CD8+ T Cell Formation. Sci. Immunol. 2023, 8, eadf7579. [Google Scholar] [CrossRef] [PubMed]
- Todkar, K.; Ilamathi, H.S.; Germain, M. Mitochondria and Lysosomes: Discovering Bonds. Front. Cell Dev. Biol. 2017, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Soares, F.; Tattoli, I.; Girardin, S.E. Mitochondria in Innate Immunity. EMBO Rep. 2011, 12, 901–910. [Google Scholar] [CrossRef]
- Lezi, E.; Swerdlow, R.H. Mitochondria in Neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286. [Google Scholar] [CrossRef]
- Yang, M.; Wei, X.; Yi, X.; Jiang, D.-S. Mitophagy-Related Regulated Cell Death: Molecular Mechanisms and Disease Implications. Cell Death Dis. 2024, 15, 505. [Google Scholar] [CrossRef]
- Liu, D.; Qin, H.; Gao, Y.; Sun, M.; Wang, M. Cardiovascular Disease: Mitochondrial Dynamics and Mitophagy Crosstalk Mechanisms with Novel Programmed Cell Death and Macrophage Polarisation. Pharmacol. Res. 2024, 206, 107258. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, E.; Yao, X.; Zhang, X.; Wang, Q.; Wang, Y.; Liu, J.; Fan, W.; Yi, K.; Kang, C.; et al. FUNDC1-Dependent Mitophagy Induced by tPA Protects Neurons against Cerebral Ischemia-Reperfusion Injury. Redox Biol. 2021, 38, 101792. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, Y.; Liu, M.; Li, Y.; Ma, S.; Tang, W.; Yan, W.; Cao, M.; Zheng, W.; Jiang, L.; et al. BNIP3L/NIX Degradation Leads to Mitophagy Deficiency in Ischemic Brains. Autophagy 2021, 17, 1934–1946. [Google Scholar] [CrossRef]
- Cai, C.; Guo, Z.; Chang, X.; Li, Z.; Wu, F.; He, J.; Cao, T.; Wang, K.; Shi, N.; Zhou, H.; et al. Empagliflozin Attenuates Cardiac Microvascular Ischemia/Reperfusion through Activating the AMPKα1/ULK1/FUNDC1/Mitophagy Pathway. Redox Biol. 2022, 52, 102288. [Google Scholar] [CrossRef]
- Yu, W.; Wang, L.; Ren, W.-Y.; Xu, H.-X.; Wu, N.N.; Yu, D.-H.; Reiter, R.J.; Zha, W.-L.; Guo, Q.-D.; Ren, J. SGLT2 Inhibitor Empagliflozin Alleviates Cardiac Remodeling and Contractile Anomalies in a FUNDC1-Dependent Manner in Experimental Parkinson’s Disease. Acta Pharmacol. Sin. 2024, 45, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, J.; Li, Y.; Zhang, Y.; Du, Q.; Hao, P.; Li, J.; Cao, X.; Li, L. Berberine Protects against Simulated Ischemia/Reperfusion Injury-Induced H9C2 Cardiomyocytes Apoptosis In Vitro and Myocardial Ischemia/Reperfusion-Induced Apoptosis In Vivo by Regulating the Mitophagy-Mediated HIF-1α/BNIP3 Pathway. Front. Pharmacol. 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, Y.; Wu, J.; Ma, Q.; Bai, S.; Xia, Z.; Wan, X.; Liang, J. Resveratrol Improves Bnip3-Related Mitophagy and Attenuates High-Fat-Induced Endothelial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, D.; Wei, X.; Yu, L.; Jia, L. Focused Low-Intensity Pulsed Ultrasound Alleviates Osteoarthritis via Restoring Impaired FUNDC1-Mediated Mitophagy. iScience 2023, 26, 107772. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, W.; Liu, Y.; Yu, L.; Mao, X.; Guo, X.; Jiang, F.; Guo, Q.; Lin, N.; Zhang, Y. Artesunate Sensitizes Human Hepatocellular Carcinoma to Sorafenib via Exacerbating AFAP1L2-SRC-FUNDC1 Axis-Dependent Mitophagy. Autophagy 2024, 20, 541–556. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Huang, X.-Q.; Wu, R.-C.; Xiao, Y.; Zhang, S.-H. Antitumor Studies Evaluation of Triphenylphosphine Ruthenium Complexes with 5,7-Dihalo-Substituted-8-Quinolinoline Targeting Mitophagy Pathways. J. Inorg. Biochem. 2023, 248, 112361. [Google Scholar] [CrossRef]
- He, C.; Lu, S.; Wang, X.-Z.; Wang, C.-C.; Wang, L.; Liang, S.-P.; Luo, T.-F.; Wang, Z.-C.; Piao, M.-H.; Chi, G.-F.; et al. FOXO3a Protects Glioma Cells against Temozolomide-Induced DNA Double Strand Breaks via Promotion of BNIP3-Mediated Mitophagy. Acta Pharmacol. Sin. 2021, 42, 1324–1337. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Lu, L.; Yu, K.; Zhao, J.; Chen, M.; Liu, L.; Sun, Q.; Lin, Z.; Zheng, J.; et al. STOML2 Potentiates Metastasis of Hepatocellular Carcinoma by Promoting PINK1-Mediated Mitophagy and Regulates Sensitivity to Lenvatinib. J. Hematol. Oncol. 2021, 14, 16. [Google Scholar] [CrossRef]
- Luo, T.; Jia, X.; Feng, W.; Wang, J.-Y.; Xie, F.; Kong, L.-D.; Wang, X.-J.; Lian, R.; Liu, X.; Chu, Y.-J.; et al. Bergapten Inhibits NLRP3 Inflammasome Activation and Pyroptosis via Promoting Mitophagy. Acta Pharmacol. Sin. 2023, 44, 1867–1878. [Google Scholar] [CrossRef]
- Wei, Y.; You, Y.; Zhang, J.; Ban, J.; Min, H.; Li, C.; Chen, J. Crystalline Silica-Induced Macrophage Pyroptosis Interacting with Mitophagy Contributes to Pulmonary Fibrosis via Modulating Mitochondria Homeostasis. J. Hazard. Mater. 2023, 454, 131562. [Google Scholar] [CrossRef]
- Gong, L.-J.; Wang, X.-Y.; Gu, W.-Y.; Wu, X. Pinocembrin Ameliorates Intermittent Hypoxia-Induced Neuroinflammation through BNIP3-Dependent Mitophagy in a Murine Model of Sleep Apnea. J. Neuroinflamm. 2020, 17, 337. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yang, L.; Ren, F.; Dong, K.; Zhao, Z.; Duan, W.; Wei, W.; Guo, R. Ginsenoside Rb1 Protects Hippocampal Neurons in Depressed Rats Based on Mitophagy-Regulated Astrocytic Pyroptosis. Phytomedicine 2023, 121, 155083. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin Hinders Microglial Activation to Alleviate Neurotoxicity via the Interplay between NLRP3 Inflammasome and Mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic Acid Inhibits Pyroptosis in Spinal Cord Injury by Augmenting Autophagy via the AMPK-mTOR-TFEB Signaling Pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef]
- Xia, J.; Chu, C.; Li, W.; Chen, H.; Xie, W.; Cheng, R.; Hu, K.; Li, X. Mitochondrial Protein UCP1 Inhibits the Malignant Behaviors of Triple-Negative Breast Cancer through Activation of Mitophagy and Pyroptosis. Int. J. Biol. Sci. 2022, 18, 2949–2961. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Jiang, P.; Chen, L.; Xing, L.; Shen, X.; Li, L.; Huang, Y. Biomimetic Nanoparticle Synchronizing Pyroptosis Induction and Mitophagy Inhibition for Anti-Tumor Therapy. Biomaterials 2023, 301, 122293. [Google Scholar] [CrossRef]
- Ye, Y.; Ren, K.; Dong, Y.; Yang, L.; Zhang, D.; Yuan, Z.; Ma, N.; Song, Y.; Huang, X.; Qiao, H. Mitochondria-Targeting Pyroptosis Amplifier of Lonidamine-Modified Black Phosphorus Nanosheets for Glioblastoma Treatments. ACS Appl. Mater. Interfaces 2023, 15, 26285–26297. [Google Scholar] [CrossRef]
- Granata, S.; Votrico, V.; Spadaccino, F.; Catalano, V.; Netti, G.S.; Ranieri, E.; Stallone, G.; Zaza, G. Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxidants 2022, 11, 769. [Google Scholar] [CrossRef]
- Rademaker, G.; Boumahd, Y.; Peiffer, R.; Anania, S.; Wissocq, T.; Liégeois, M.; Luis, G.; Sounni, N.E.; Agirman, F.; Maloujahmoum, N.; et al. Myoferlin Targeting Triggers Mitophagy and Primes Ferroptosis in Pancreatic Cancer Cells. Redox Biol. 2022, 53, 102324. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, S.; Jiang, B.; Cao, S.; Dong, Y.; Cao, L.; Guo, S. Adipose-Derived Stem Cells Regulate Metabolic Homeostasis and Delay Aging by Promoting Mitophagy. FASEB J. 2021, 35, e21709. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Luo, Y.; Gu, F.; Zhu, Y.; Liu, X.; Yan, C.; Hu, W.; Wang, S.; Chao, X.; et al. Natural Compounds Modulating Mitophagy: Implications for Cancer Therapy. Cancer Lett. 2024, 582, 216590. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, T.; Qu, X.; Sun, G.; Fu, Q.; Han, G. Stress/Cell Death Pathways, Neuroinflammation, and Neuropathic Pain. Immunol. Rev. 2024, 321, 33–51. [Google Scholar] [CrossRef]



| Ub-Dependent Pathways | Ub-Independent Pathways | |
|---|---|---|
| Mechanism of labeling | Recognition of ubiquitination markers and specific receptors with LIR, such as OPTN, NDP52, etc. | Directly through specific receptors (such as FUNDC1, NIX and BNIP3) interact with autophagosome. |
| Mainly involved in protein | PINK1 and Parkin. | DRP1, OPA1, etc. |
| The activation conditions | Usually activated in response to mitochondrial damage or abnormal function (e.g., loss of membrane potential). | Usually activated under specific physiological or pathological conditions (e.g., erythrocyte maturation, hypoxia). |
| Functional goals | Remove damaged or unwanted mitochondria to maintain cell health and function. | |
| Core process | Formation of autophagosomes, fusion of autophagosomes with lysosomes, and eventual degradation of mitochondria. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Chen, X.; Du, Y.; Li, J.; Guo, T.; Luo, S. Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications. Biomolecules 2024, 14, 1270. https://doi.org/10.3390/biom14101270
Lin J, Chen X, Du Y, Li J, Guo T, Luo S. Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications. Biomolecules. 2024; 14(10):1270. https://doi.org/10.3390/biom14101270
Chicago/Turabian StyleLin, Jiani, Xinyao Chen, Yuyang Du, Jiapeng Li, Tingting Guo, and Sai Luo. 2024. "Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications" Biomolecules 14, no. 10: 1270. https://doi.org/10.3390/biom14101270
APA StyleLin, J., Chen, X., Du, Y., Li, J., Guo, T., & Luo, S. (2024). Mitophagy in Cell Death Regulation: Insights into Mechanisms and Disease Implications. Biomolecules, 14(10), 1270. https://doi.org/10.3390/biom14101270








