MGACL: Prediction Drug–Protein Interaction Based on Meta-Graph Association-Aware Contrastive Learning
Abstract
1. Introduction
- MGACL prevents negative transfer through the DTI association-aware contrastive learning strategy and safely incorporates heterogeneous auxiliary information into the model under the graph contrastive learning paradigm.
- MGACL combines meta-knowledge transfer networks with novel contrastive learning strategies to transmit personalized signals between different relationship views.
- Extensive experiments on five datasets show that MGACL can significantly improve performance over other strong baselines. Moreover, we validated the effectiveness of MGACL prediction results using molecular docking, providing an effective strategy for identifying drug–target interactions.
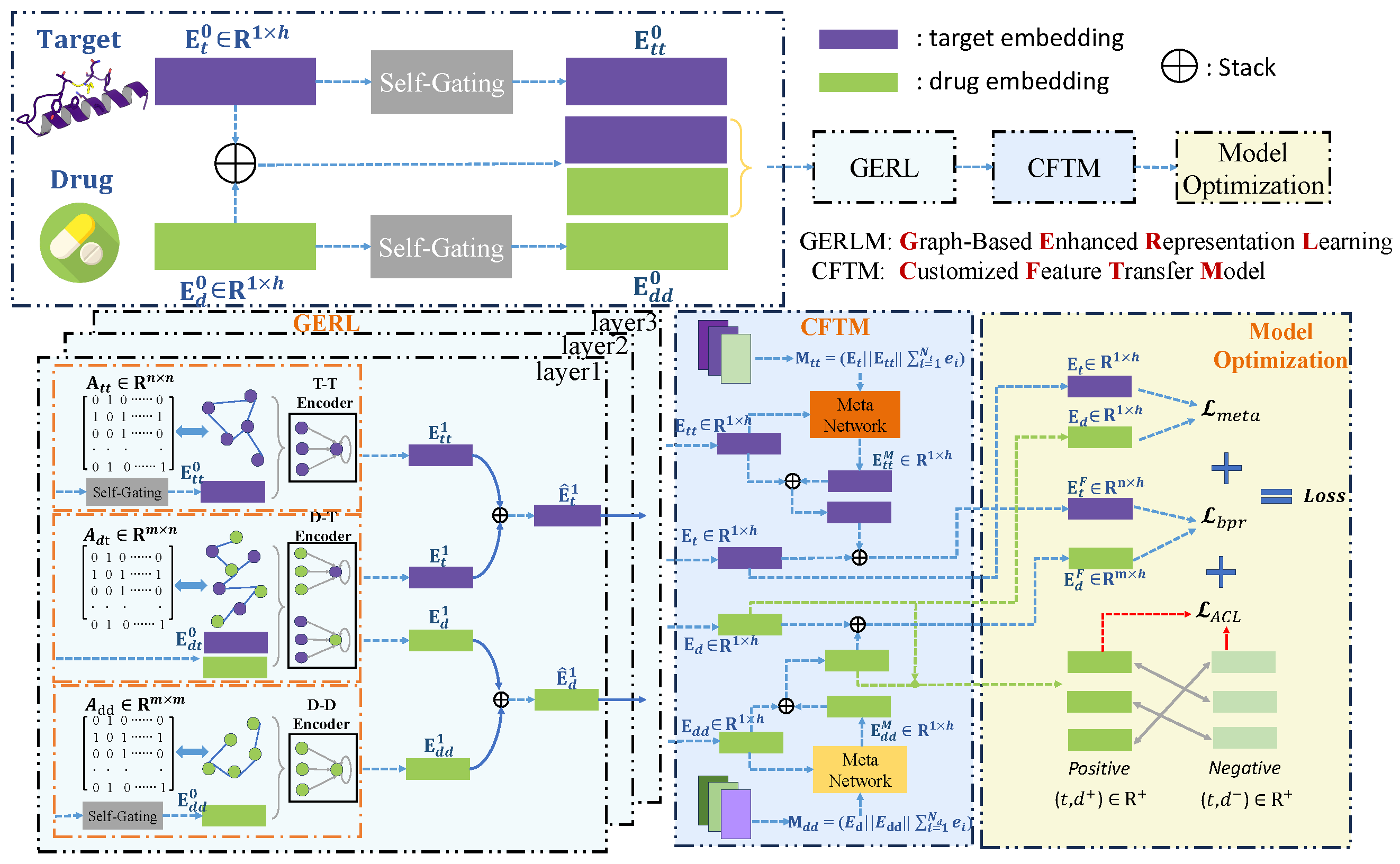
2. Methods
2.1. Preliminaries
2.2. Graph-Based Enhanced Representation Learning
2.2.1. Embedding Initialization with Relational Context Awareness
2.2.2. Heterogeneous Relation-Aware Signal Propagation
2.2.3. Message Integration in Heterogeneous Contexts
2.3. Meta-Knowledge Transfer Network
2.3.1. Multi-Faceted Meta-Knowledge Extraction
2.3.2. Local Connectivity Knowledge Transfer
2.3.3. Meta-Learner Optimization
2.4. DTI Association-Aware Contrastive Learning
2.4.1. Multi-View Augmentation for DTI
2.4.2. Association-Aware Contrastive Learning
2.5. Optimization of MGACL
3. Results
3.1. Experimental Settings
3.1.1. Datasets
3.1.2. Baselines
- DTINet [27]: This method uses a random walk algorithm and a dimensionality reduction scheme to derive low-dimensional feature vectors of nodes.
- NeoDTI [33]: This method extracts complex hidden features of various types of nodes through information passing and aggregation operations.
- GCN-DTI [34]: This method constructs a drug- and protein-based network, and considers the DTI prediction problem as a node classification problem.
- EEG-DTI [25]: This method makes predictions by learning low-dimensional representations of different nodes.
- IMCHGAN [37]: This method proposes a two-level graph attention network (GAT) to learn node latent features from the heterogeneous network and uses inductive matrix completion to predict DTIs.
- HampDTI [38]: This method uses a learnable attention mechanism to automatically extract useful meta-paths, learning multi-channel node embeddings via a GCN without relying on domain knowledge.
- AMGDTI [39]: This method constructs adaptive meta-graphs for drugs and proteins separately using a GCN to extract potential features.
- SGCL-DTI [13]: This method proposes a new positive and negative sample selection strategy to guide model optimization in a supervised manner.
- SHGCL-DTI [40]: This method proposes an auxiliary graph contrastive learning task for DTI prediction.
- MSI-DTI [35]: This method obtains feature representations from different views by integrating biometric features and knowledge graph representations from multiple sources of information.
- HMSA-DTI [36]: This method utilizes a layered multimodal self-attention mechanism to achieve deep fusion of multimodal features of drugs and proteins, thereby capturing the interactions between drugs and proteins.
3.1.3. Hyper-Parameter Settings
3.2. Performance Comparison
- Our MGACL consistently achieves significant performance improvements over the most representative techniques based on heterogeneous networks. We believe that these improvements all stem from the design of the meta-knowledge transfer network combined with the association-aware contrastive learning paradigm: (1) MGACL allows node representations to efficiently transfer knowledge between heterogeneous relationships to aid in DTI prediction; (2) adaptive DTI association-aware contrastive learning significantly improves prediction performance by leveraging self-supervised signals between heterogeneous relationship views to enhance feature extraction.
- Compared to the fine-grained feature fusion method , which is based on machine learning, MGACL has made great progress, especially in the AUPRC performance. The AUPRC provides a more objective evaluation of highly skewed data. The proposed novel contrastive learning strategy utilizes self-supervised signals and can learn effective feature representations from highly skewed data to extract deeper, abstract features, thus improving the feature learning capability of the model.
- Although meta-path-based/adaptive meta-graph-based approaches obtain good performance gains and are more flexible than fixed meta-path methods relying on domain expert knowledge definitions, they cannot effectively learn multiplex relational signals between multiple types of nodes in a multiplex heterogeneous network. For MGACL, by effectively integrating multiple knowledge sources, meta-knowledge transfer networks can capture complex data relations more comprehensively, thus providing more robust performance across different tasks and datasets.
- The superior performance of SGCL-DTI and SHGCL-DTI justifies the use of self-supervised learning to enhance drug–protein interaction coding. The performance gains shown by our MGACL when comparing the above methods validate that the node representations learned from the tilted graph structure contain biased information, and considering how to reduce negative migration further improves the performance.
3.3. Ablation Study
- : MGACL does not contain meta-networks that allow for personalized knowledge transfer in contrastive learning across relational views.
- : In this variant, we do not include the drug–local connectivity relational graph to capture knowledge-aware dependencies between drugs to guide the learning process for drug–target interaction prediction.
- : In this variant, we do not include the target local connectivity relational map to consider biological or functional interrelationships between targets to help encode target–drug interaction patterns.
- : It indicates that we have no contrastive learning between the learned auxiliary map and the original map.
- : It indicates that we implement contrastive learning on the graph of MGACL but do not consider DTI associations (i.e., deleting in Equation (13)).
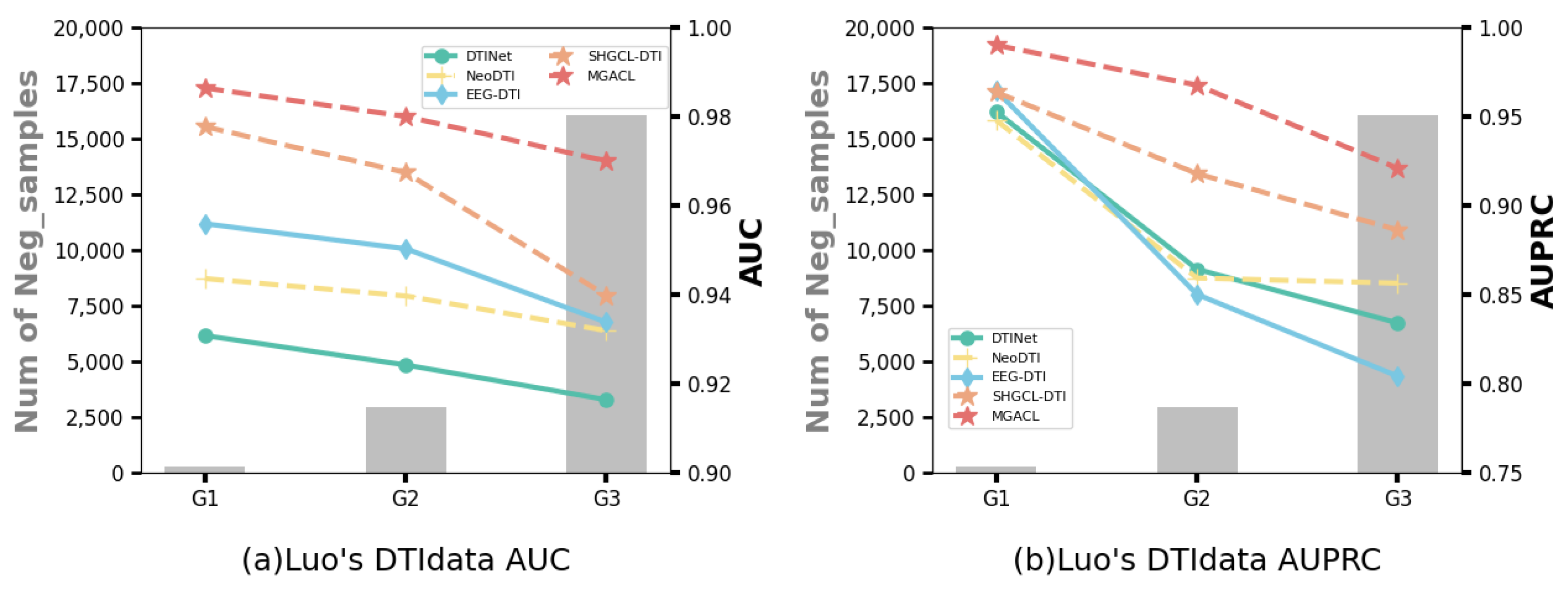
3.4. Performance Variations in Data Imbalance Scenarios
- Group G1: Balanced positive and negative samples in the test data.
- Group G2: The negative samples in the test data are ten times the positive samples.
- Group G3: The negative samples in the test data are all remaining non-interacting pairs not present in the training data.
3.5. Generalization Performance Evaluation
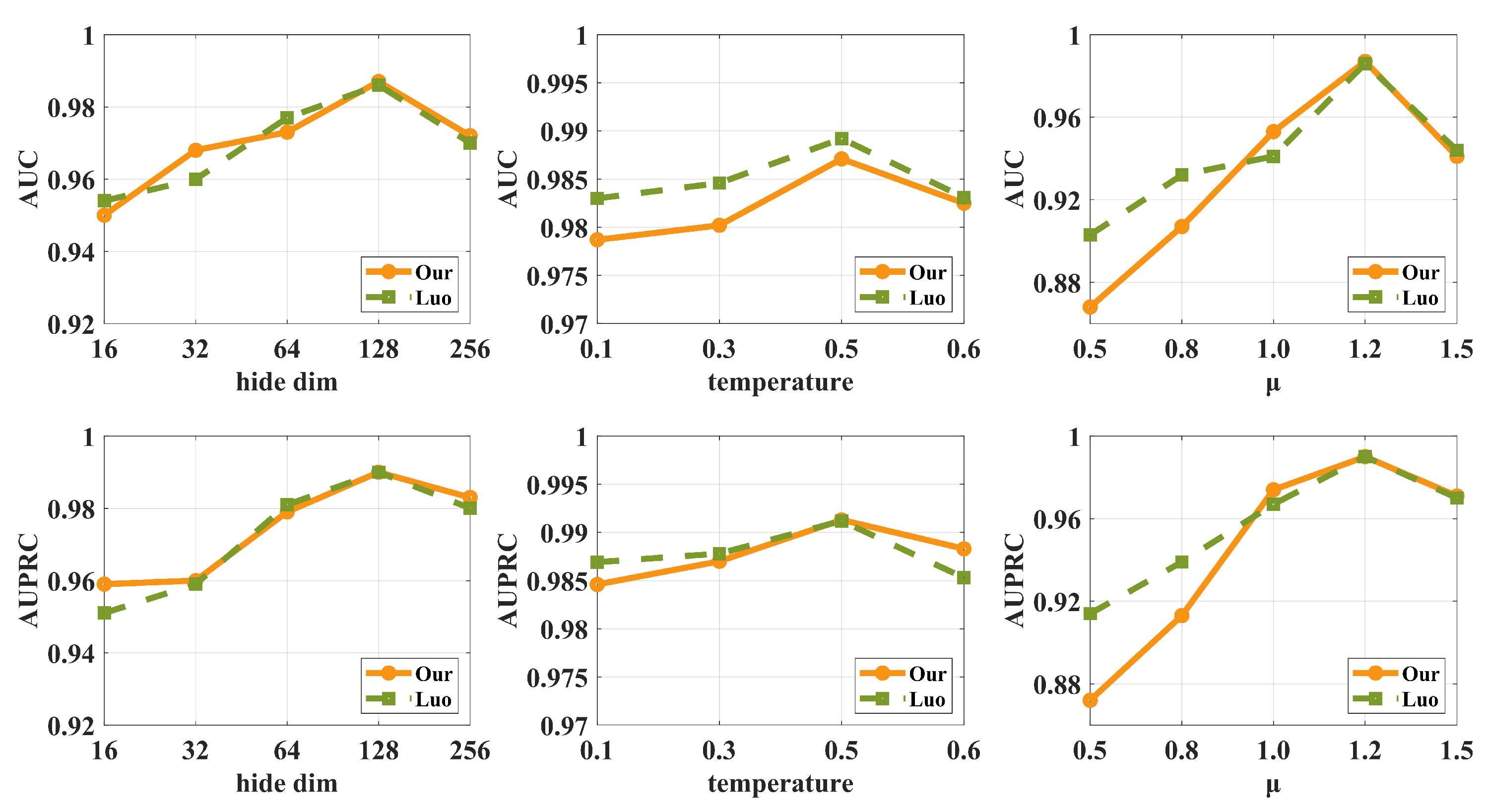
3.6. Hyper-Parameter Analysis
- The selection of the hidden layer dimension h ranges from 16 to 256. We observe that the model reaches its optimal performance when h = 128 and then starts to decline. Therefore, increasing the embedding dimension appropriately can improve the model performance. Still, there may be a risk of overfitting when the model is too complex and the embedding dimensions are too high.
- For the value of , we observe that the model performance is optimal when the is 0.5, while the performance decreases at 0.6. This suggests that an appropriate temperature parameter allows the model to identify the correct category accurately, but too large a temperature parameter may cause the model to start focusing too much on the noise in the training data or features that are not important, leading to performance degradation.
- For the contrastive learning hyper-parameter, we observe that the model reaches its optimal performance when is 1.2, while there is a decreasing trend at 1.5. This suggests that the decrease in performance when is increased to 1.5 may be a sign of over-tuning. This could mean that higher values of cause the model to over-adapt to the specific features of the current dataset, thus affecting its generalization ability.
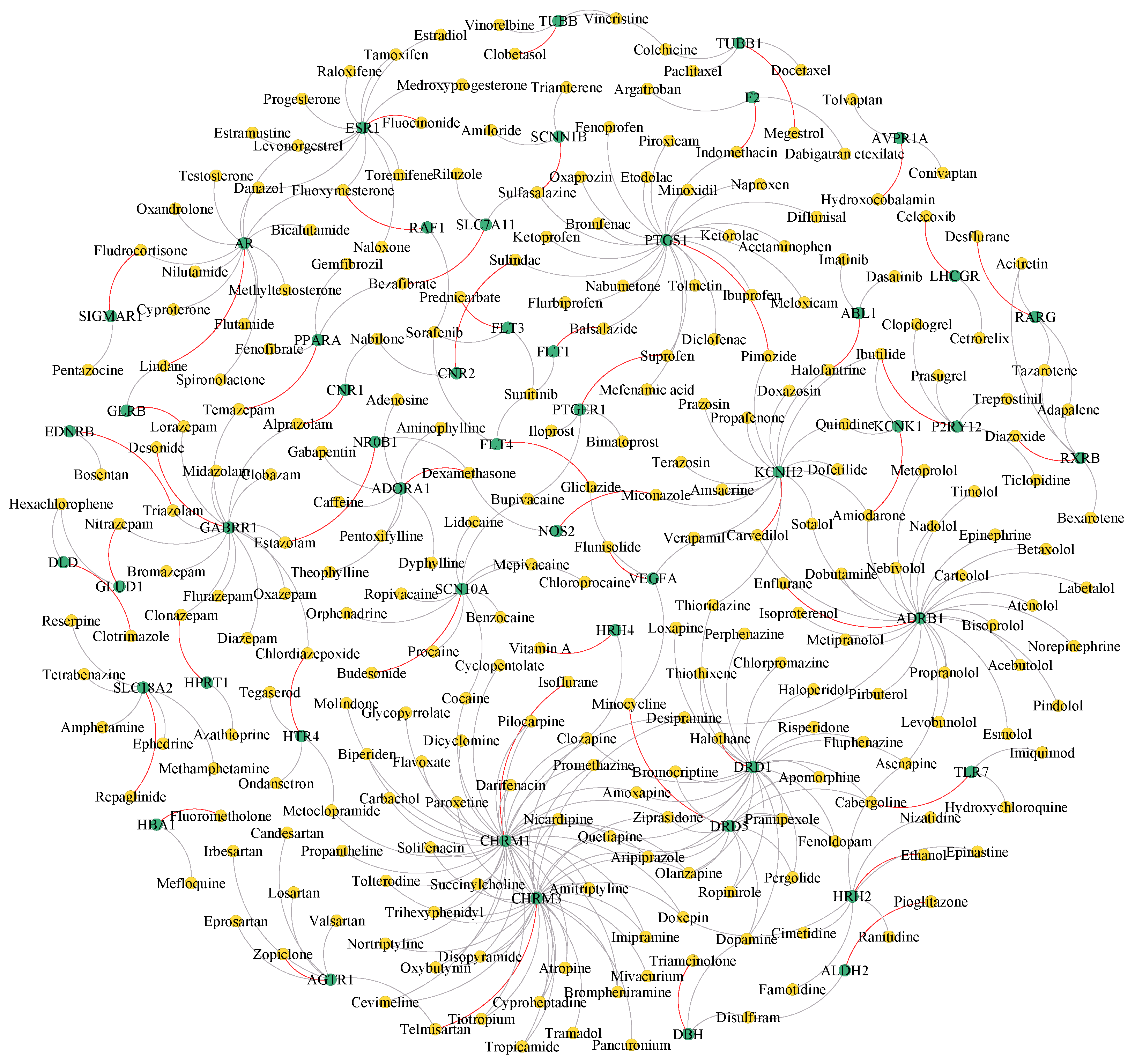
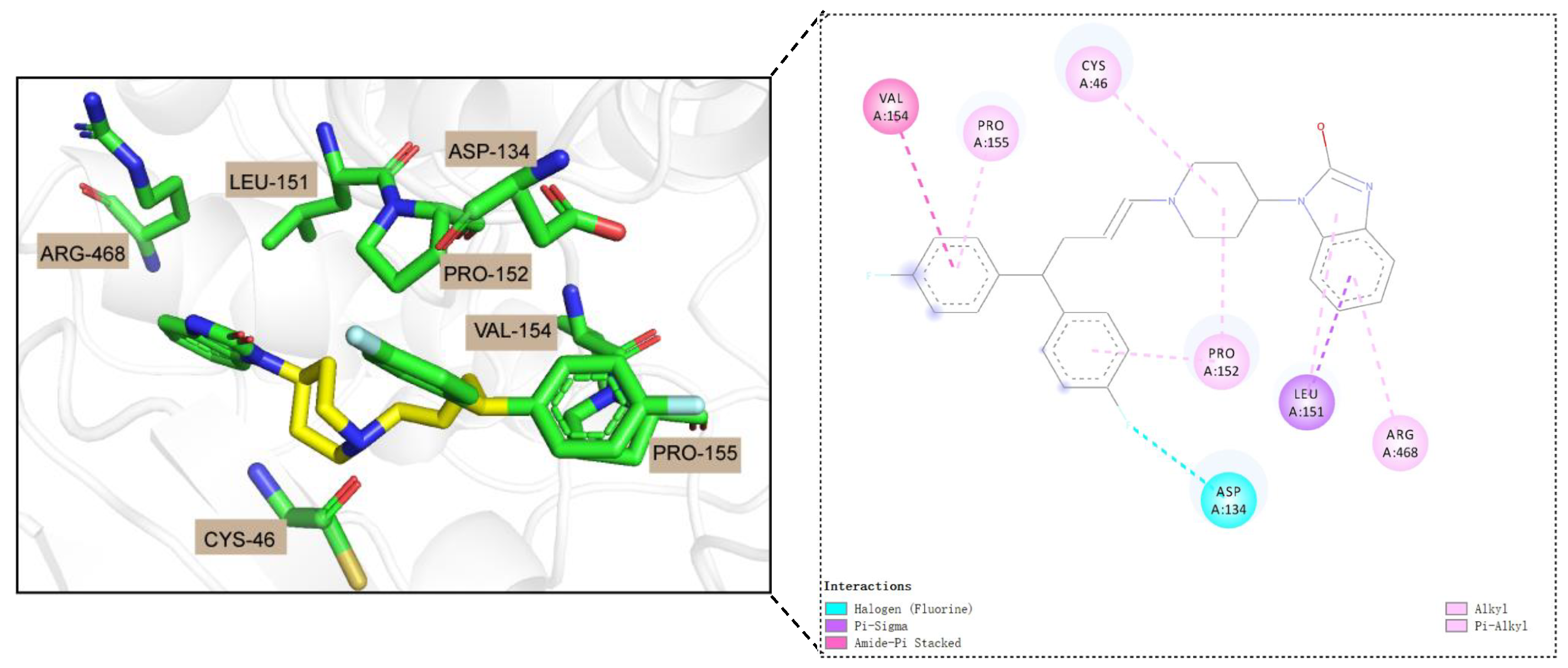
3.7. Case Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Wang, X.; Jang, S.K.; Quach, B.C.; Weissenkampen, J.D.; Khunsriraksakul, C.; Yang, L.; Sauteraud, R.; Albert, C.M.; Allred, N.D.; et al. Multi-ancestry transcriptome-wide association analyses yield insights into tobacco use biology and drug repurposing. Nat. Genet. 2023, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.; Zemmel, R. Prospects for productivity. Nat. Rev. Drug Discov. 2004, 3, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. 2023 FDA approvals. Nat. Rev. Drug Discov. 2024, 23, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Sun, W.; Ren, C.; Wang, L. Bridging-BPs: A novel approach to predict potential drug–target interactions based on a bridging heterogeneous graph and BPs2vec. Briefings Bioinform. 2022, 23, bbab557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.W.; Hu, L.; You, Z.H.; Wang, L.; Su, X.R. HINGRL: Predicting drug–disease associations with graph representation learning on heterogeneous information networks. Briefings Bioinform. 2022, 23, bbab515. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, H.; Zheng, K.; Wang, J. HyperAttentionDTI: Improving drug–protein interaction prediction by sequence-based deep learning with attention mechanism. Bioinformatics 2022, 38, 655–662. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Sun, K.; Tsoumakas, G. Fine-grained selective similarity integration for drug–target interaction prediction. Briefings Bioinform. 2023, 24, bbad085. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Ma, X. Contrastive and adversarial regularized multi-level representation learning for incomplete multi-view clustering. Neural Netw. 2024, 172, 106102. [Google Scholar] [CrossRef]
- Yu, L.; Wang, B.; Ma, X.; Gao, L. The extraction of drug-disease correlations based on module distance in incomplete human interactome. BMC Syst. Biol. 2016, 10, 111. [Google Scholar] [CrossRef]
- Yu, L.; Ma, X.; Zhang, L.; Zhang, J.; Gao, L. Prediction of new drug indications based on clinical data and network modularity. Sci. Rep. 2016, 6, 32530. [Google Scholar] [CrossRef]
- Wei, C.; Liang, J.; Liu, D.; Dai, Z.; Li, M.; Wang, F. Meta Graph Learning for Long-tail Recommendation. In Proceedings of the 29th ACM SIGKDD Conference on Knowledge Discovery and Data Mining, Long Beach, CA, USA, 6–10 August 2023; pp. 2512–2522. [Google Scholar]
- Chen, M.; Huang, C.; Xia, L.; Wei, W.; Xu, Y.; Luo, R. Heterogeneous graph contrastive learning for recommendation. In Proceedings of the Sixteenth ACM International Conference on Web Search and Data Mining, Singapore, 27 February–3 March 2023; pp. 544–552. [Google Scholar]
- Li, Y.; Qiao, G.; Gao, X.; Wang, G. Supervised graph co-contrastive learning for drug–target interaction prediction. Bioinformatics 2022, 38, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Che, C.; Jin, B.; Yuan, J.; Li, R.; Zhu, Y. NCH-DDA: Neighborhood contrastive learning heterogeneous network for drug–disease association prediction. Expert Syst. Appl. 2024, 238, 121855. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, X. Graph Contrastive Learning for Clustering of Multi-Layer Networks. IEEE Trans. Big Data 2024, 10, 429–441. [Google Scholar] [CrossRef]
- Natarajan, N.; Dhillon, I.S. Inductive matrix completion for predicting gene–disease associations. Bioinformatics 2014, 30, i60–i68. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Z.; Yi, X.; Yang, J.; Chen, M.; Tang, J.; Hong, L.; Chi, E.H. Off-policy learning in two-stage recommender systems. In Proceedings of the Web Conference 2020, Taipei, Taiwan, 20–24 April 2020; pp. 463–473. [Google Scholar]
- Shao, K.; Zhang, Y.; Wen, Y.; Zhang, Z.; He, S.; Bo, X. DTI-HETA: Prediction of drug–target interactions based on GCN and GAT on heterogeneous graph. Briefings Bioinform. 2022, 23, bbac109. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015; pp. 1026–1034. [Google Scholar]
- Yu, J.; Yin, H.; Li, J.; Wang, Q.; Hung, N.Q.V.; Zhang, X. Self-supervised multi-channel hypergraph convolutional network for social recommendation. In Proceedings of the Web Conference 2021, Virtual, 19–23 April 2021; pp. 413–424. [Google Scholar]
- Dauphin, Y.N.; Fan, A.; Auli, M.; Grangier, D. Language modeling with gated convolutional networks. In Proceedings of the International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017; pp. 933–941. [Google Scholar]
- He, X.; Deng, K.; Wang, X.; Li, Y.; Zhang, Y.; Wang, M. Lightgcn: Simplifying and powering graph convolution network for recommendation. In Proceedings of the 43rd International ACM SIGIR Conference on Research and Development in Information Retrieval, Virtual, 25–30 July 2020; pp. 639–648. [Google Scholar]
- Hu, Z.; Dong, Y.; Wang, K.; Sun, Y. Heterogeneous graph transformer. In Proceedings of the Web Conference 2020, Taipei, Taiwan, 20–24 April 2020; pp. 2704–2710. [Google Scholar]
- Xu, B.; Wang, N.; Chen, T.; Li, M. Empirical evaluation of rectified activations in convolutional network. arXiv 2015, arXiv:1505.00853. [Google Scholar]
- Peng, J.; Wang, Y.; Guan, J.; Li, J.; Han, R.; Hao, J.; Wei, Z.; Shang, X. An end-to-end heterogeneous graph representation learning-based framework for drug–target interaction prediction. Briefings Bioinform. 2021, 22, bbaa430. [Google Scholar] [CrossRef]
- Rendle, S.; Freudenthaler, C.; Gantner, Z.; Schmidt-Thieme, L. BPR: Bayesian personalized ranking from implicit feedback. arXiv 2012, arXiv:1205.2618. [Google Scholar]
- Luo, Y.; Zhao, X.; Zhou, J.; Yang, J.; Zhang, Y.; Kuang, W.; Peng, J.; Chen, L.; Zeng, J. A network integration approach for drug-target interaction prediction and computational drug repositioning from heterogeneous information. Nat. Commun. 2017, 8, 573. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Kotera, M.; Kanehisa, M.; Goto, S. Drug-target interaction prediction from chemical, genomic and pharmacological data in an integrated framework. Bioinformatics 2010, 26, i246–i254. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef]
- Wan, F.; Hong, L.; Xiao, A.; Jiang, T.; Zeng, J. NeoDTI: Neural integration of neighbor information from a heterogeneous network for discovering new drug–target interactions. Bioinformatics 2019, 35, 104–111. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, Y.; Valsdottir, L.R.; Zang, T.; Peng, J. Identifying drug–target interactions based on graph convolutional network and deep neural network. Briefings Bioinform. 2021, 22, 2141–2150. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, Y.; Liu, G.; Liang, Y.; Xu, D.; Feng, X.; Guan, R. MSI-DTI: Predicting drug-target interaction based on multi-source information and multi-head self-attention. Briefings Bioinform. 2024, 25, bbae238. [Google Scholar] [CrossRef]
- Bian, J.; Lu, H.; Dong, G.; Wang, G. Hierarchical multimodal self-attention-based graph neural network for DTI prediction. Briefings Bioinform. 2024, 25, bbae293. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Lv, H.; Zhang, Z.; Wang, Z. IMCHGAN: Inductive matrix completion with heterogeneous graph attention networks for drug-target interactions prediction. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 655–665. [Google Scholar] [CrossRef]
- Wang, H.; Huang, F.; Xiong, Z.; Zhang, W. A heterogeneous network-based method with attentive meta-path extraction for predicting drug–target interactions. Briefings Bioinform. 2022, 23, bbac184. [Google Scholar] [CrossRef]
- Su, Y.; Hu, Z.; Wang, F.; Bin, Y.; Zheng, C.; Li, H.; Chen, H.; Zeng, X. AMGDTI: Drug–target interaction prediction based on adaptive meta-graph learning in heterogeneous network. Briefings Bioinform. 2024, 25, bbad474. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wang, X.; Li, W.; Zhu, H.; Jiang, Y.; Li, Y.; Tian, T.; Yang, Z.; Liu, Q.; Liu, Q. Semi-supervised heterogeneous graph contrastive learning for drug–target interaction prediction. Comput. Biol. Med. 2023, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, A.; Zhao, P.; Wu, M.; Li, X.L.; Kwoh, C.K. Drug-target interaction prediction with graph regularized matrix factorization. IEEE/ACM Trans. Comput. Biol. Bioinform. 2016, 14, 646–656. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Olson, A.J. Automated docking of substrates to proteins by simulated annealing. Proteins 1990, 8, 195–202. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The AxPyMOL Molecular Graphics Plugin for Microsoft PowerPoint, Version 1.8. Available online: https://www.schrodinger.com (accessed on 7 October 2024).
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef]
- Finder, R.; Brogden, R.; Sawyer, P.R.; Speight, T.; Spencer, R.; Avery, G. Pimozide: A review of its pharmacological properties and therapeutic uses in psychiatry. Drugs 1976, 12, 1–40. [Google Scholar]
- Bai, P.; Miljković, F.; John, B.; Lu, H. Interpretable bilinear attention network with domain adaptation improves drug–target prediction. Nat. Mach. Intell. 2023, 5, 126–136. [Google Scholar] [CrossRef]
| Our DTIdata | Luo’s DTIdata | Yamanishi’s DTIdata | |||
|---|---|---|---|---|---|
| GPCR | Enzyme | IC | |||
| Drug | 1269 | 708 | 223 | 445 | 210 |
| Protein | 1615 | 1512 | 95 | 664 | 204 |
| Known Interaction | 5225 | 1923 | 635 | 2926 | 1476 |
| Sparsity | 99.745% | 99.820% | 97.003% | 99.010% | 96.555% |
| Model | Our DTIdata | Luo’s DTIdata | Yamanishi’s DTIdata | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | AUPRC | AUC | AUPRC | GPCR | Enzyme | IC | ||||
| AUC | AUPRC | AUC | AUPRC | AUC | AUPRC | |||||
| DTINet | 0.8634 | 0.8849 | 0.9308 | 0.9526 | 0.8833 | 0.8789 | 0.9338 | 0.9518 | 0.9139 | 0.9089 |
| NeoDTI | 0.9218 | 0.9346 | 0.9436 | 0.9478 | 0.9258 | 0.9077 | 0.9874 | 0.9829 | 0.9431 | 0.9567 |
| GCN-DTI | 0.8859 | 0.8962 | 0.9192 | 0.8764 | 0.9183 | 0.9046 | 0.9712 | 0.9834 | 0.9741 | 0.9759 |
| EEG-DTI | 0.9036 | 0.8973 | 0.9559 | 0.9645 | 0.961 | 0.9615 | 0.9834 | 0.9858 | 0.9841 | 0.9832 |
| 0.9041 | 0.8262 | 0.9397 | 0.8082 | 0.9134 | 0.8347 | 0.9546 | 0.8573 | 0.9357 | 0.8489 | |
| IMCHGAN | 0.9329 | 0.9546 | 0.9563 | 0.9872 | 0.9485 | 0.9479 | 0.9637 | 0.9518 | 0.9542 | 0.9483 |
| HampDTI | 0.9236 | 0.9149 | 0.9279 | 0.9263 | / | / | / | / | / | / |
| AMGDTI | 0.936 | 0.9441 | 0.978 | 0.979 | / | / | / | / | / | / |
| SHGCL | 0.9563 | 0.9742 | 0.9577 | 0.9636 | 0.9531 | 0.9458 | 0.9836 | 0.9751 | 0.9863 | 0.9849 |
| MSI-DTI | 0.9632 | 0.9769 | 0.9689 | 0.9746 | 0.9601 | 0.9589 | 0.9852 | 0.9760 | 0.9869 | 0.9851 |
| SGCL * | / | / | 0.9771 | 0.9768 | 0.9741 | 0.9812 | 0.9892 | 0.9895 | 0.9857 | 0.9852 |
| HMSA-DTI | 0.9794 | 0.9770 | 0.9799 | 0.9809 | 0.9772 | 0.9839 | 0.9890 | 0.9805 | 0.9877 | 0.9864 |
| MGACL | 0.9871 | 0.9907 | 0.9892 | 0.9912 | 0.9807 | 0.9845 | 0.9939 | 0.995 | 0.9941 | 0.9954 |
| Model | Our DTIdata | Luo’s DTIdata | ||
|---|---|---|---|---|
| AUC | UPRC | AUC | AUPRC | |
| w/o-meta | 0.9670 | 0.9767 | 0.9653 | 0.9753 |
| w/o-dd | 0.9749 | 0.9772 | 0.9755 | 0.9864 |
| w/o-tt | 0.9642 | 0.9749 | 0.9715 | 0.9802 |
| 0.9704 | 0.9796 | 0.9726 | 0.9807 | |
| 0.9758 | 0.9824 | 0.9784 | 0.9846 | |
| MGACL | 0.9871 | 0.9907 | 0.9892 | 0.9912 |
| Model | AUC | AUPRC |
|---|---|---|
| DTINet | 0.9167 | 0.9105 |
| NeoDTI | 0.9357 | 0.9302 |
| HampDTI | 0.9209 | 0.8972 |
| MGACL | 0.9713 | 0.9682 |
| Rank | Drug ID | Drug Name | Target ID | Target Name | Evidence |
|---|---|---|---|---|---|
| 1 | DB00988 | Dopamine | P09172 | DBH | DrugBank |
| 2 | DB01223 | Aminophylline | P30542 | ADORA1 | DrugBank |
| 3 | DB00619 | Imatinib | P00519 | ABL1 | DrugBank |
| 4 | DB00988 | Dopamine | P21728 | DRD1 | DrugBank |
| 5 | DB01268 | Sunitinib | P35916 | FLT4 | DrugBank |
| 6 | DB00384 | Triamterene | P51168 | SCNN1B | DrugBank |
| 7 | DB01248 | Docetaxel | Q9H4B7 | TUBB1 | DrugBank |
| 8 | DB01100 | Pimozide | P23219 | PTGS1 | Unknown |
| 9 | DB01268 | Sunitinib | P17948 | FLT1 | DrugBank |
| 10 | DB00740 | Riluzole | Q9UPY5 | SLC7A11 | DrugBank |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Lin, P.; Li, D.; Wang, W.; Qi, X.; Li, J.; Xiong, J. MGACL: Prediction Drug–Protein Interaction Based on Meta-Graph Association-Aware Contrastive Learning. Biomolecules 2024, 14, 1267. https://doi.org/10.3390/biom14101267
Zhang P, Lin P, Li D, Wang W, Qi X, Li J, Xiong J. MGACL: Prediction Drug–Protein Interaction Based on Meta-Graph Association-Aware Contrastive Learning. Biomolecules. 2024; 14(10):1267. https://doi.org/10.3390/biom14101267
Chicago/Turabian StyleZhang, Pinglu, Peng Lin, Dehai Li, Wanchun Wang, Xin Qi, Jing Li, and Jianshe Xiong. 2024. "MGACL: Prediction Drug–Protein Interaction Based on Meta-Graph Association-Aware Contrastive Learning" Biomolecules 14, no. 10: 1267. https://doi.org/10.3390/biom14101267
APA StyleZhang, P., Lin, P., Li, D., Wang, W., Qi, X., Li, J., & Xiong, J. (2024). MGACL: Prediction Drug–Protein Interaction Based on Meta-Graph Association-Aware Contrastive Learning. Biomolecules, 14(10), 1267. https://doi.org/10.3390/biom14101267







