Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Polymicrobial Sepsis in Mice
2.2. Enzyme-Linked Immunosorbent Assay
2.3. Double Immunofluorescence Staining of Paraffin Sections
2.4. Statistical Analysis
3. Results
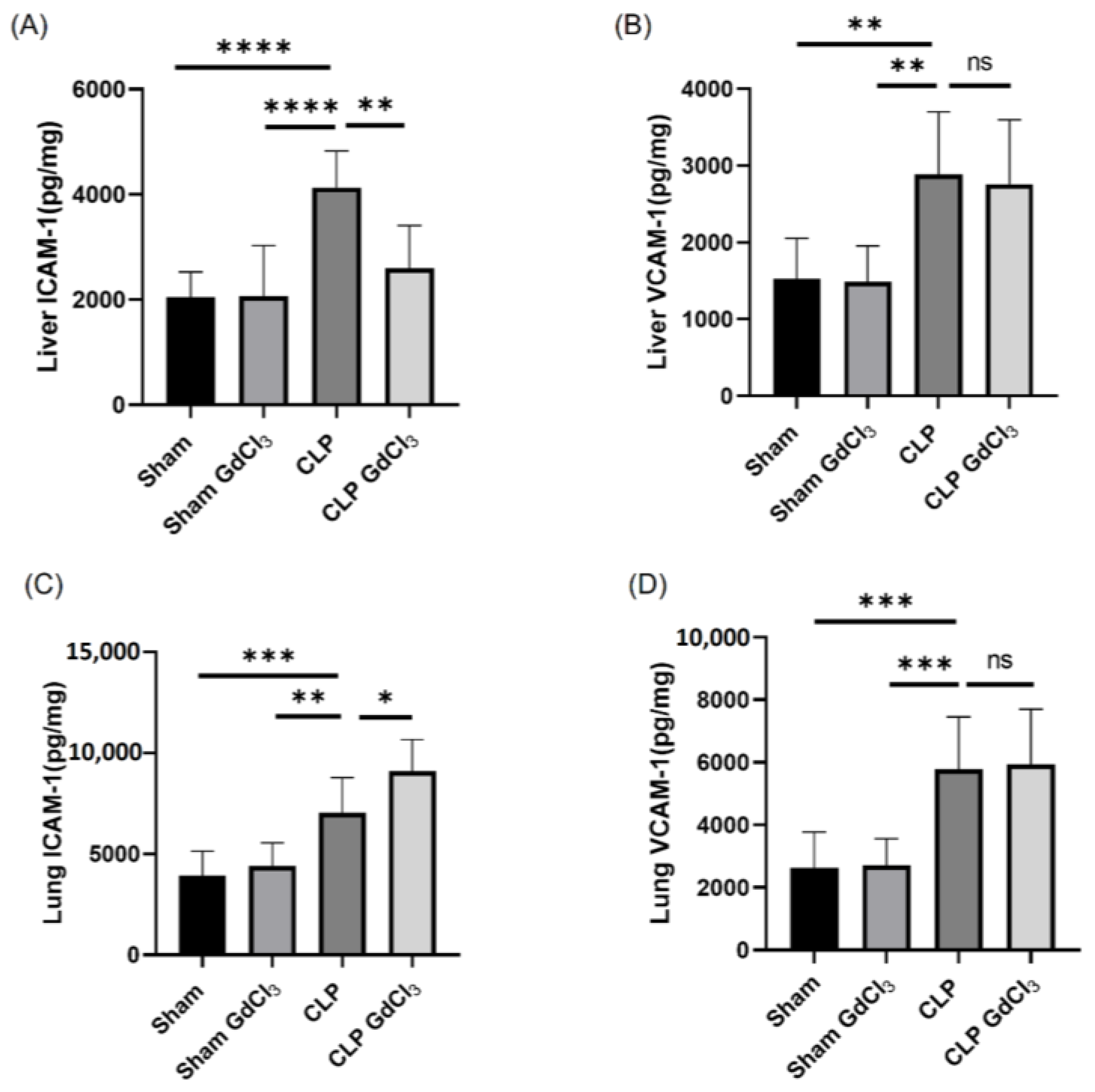
3.1. Effect of Kupffer Cell Inactivation on Adhesion Molecule Expression in the Liver and Lungs Following Cecal Ligation and Puncture (CLP)-Induced Sepsis
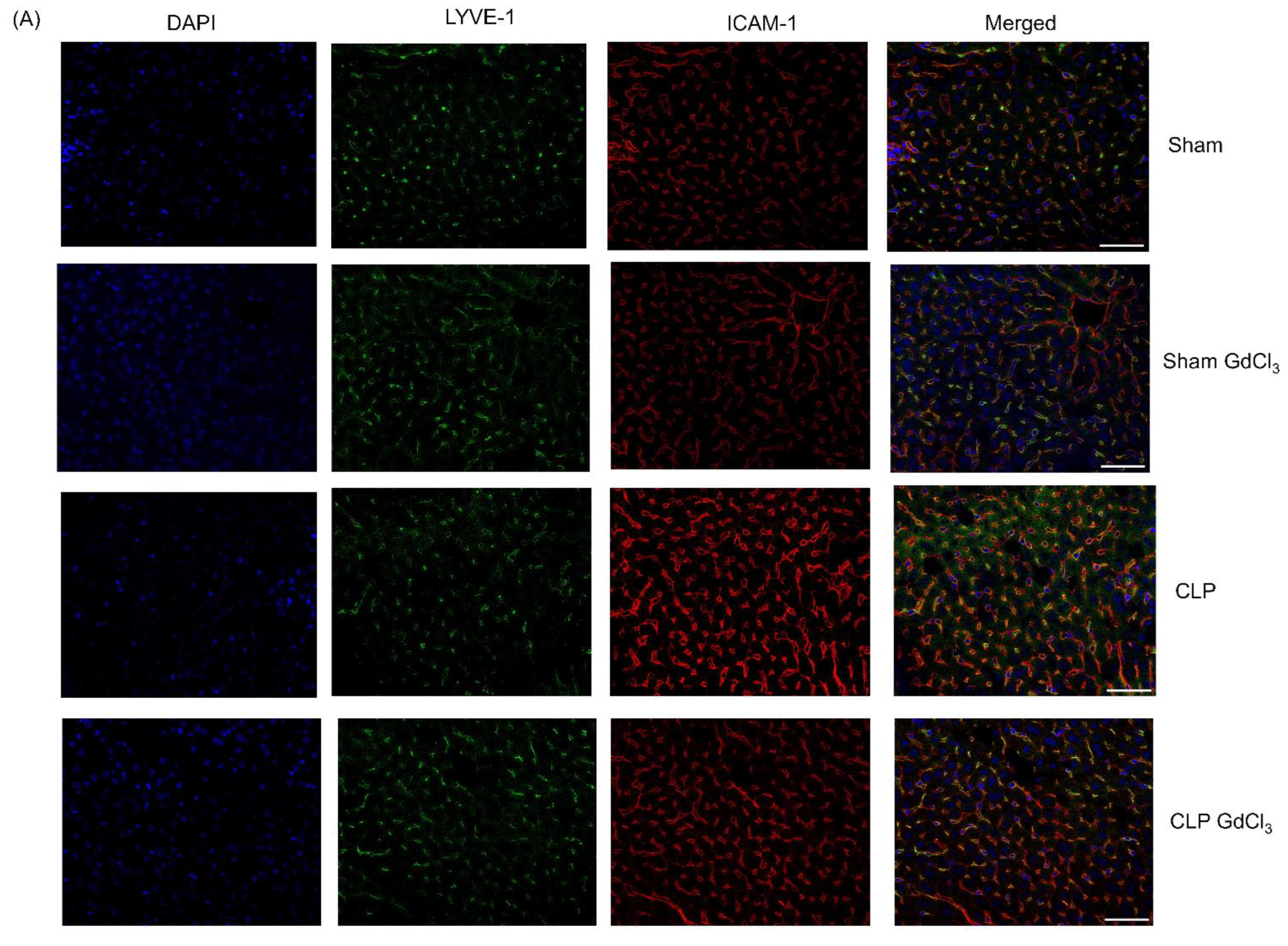
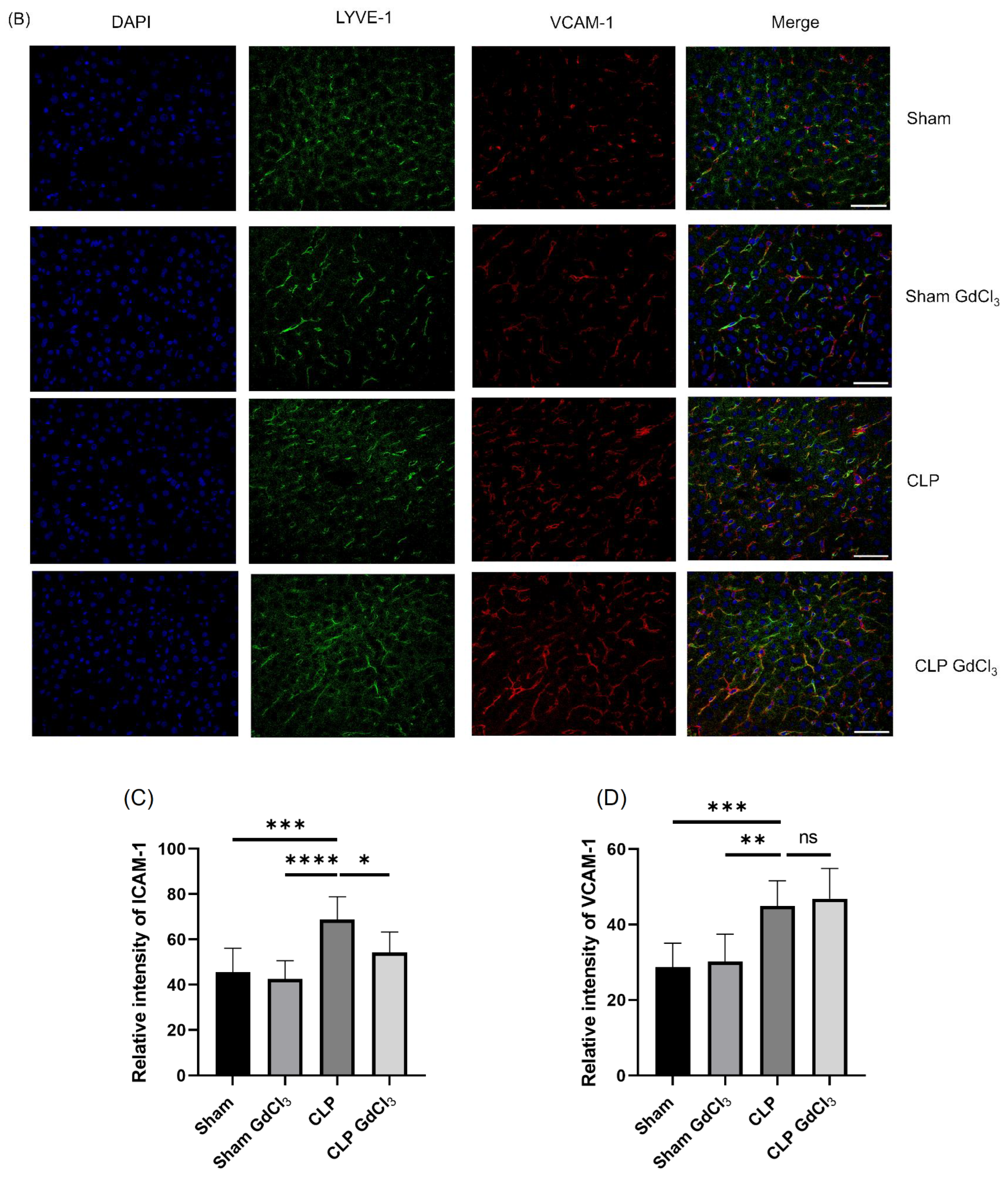
3.2. Effect of Kupffer Cell Inactivation on Immunoreactivity of Liver ICAM-1 and VCAM-1 Co-Localized with Liver Sinusoidal Endothelial Cells
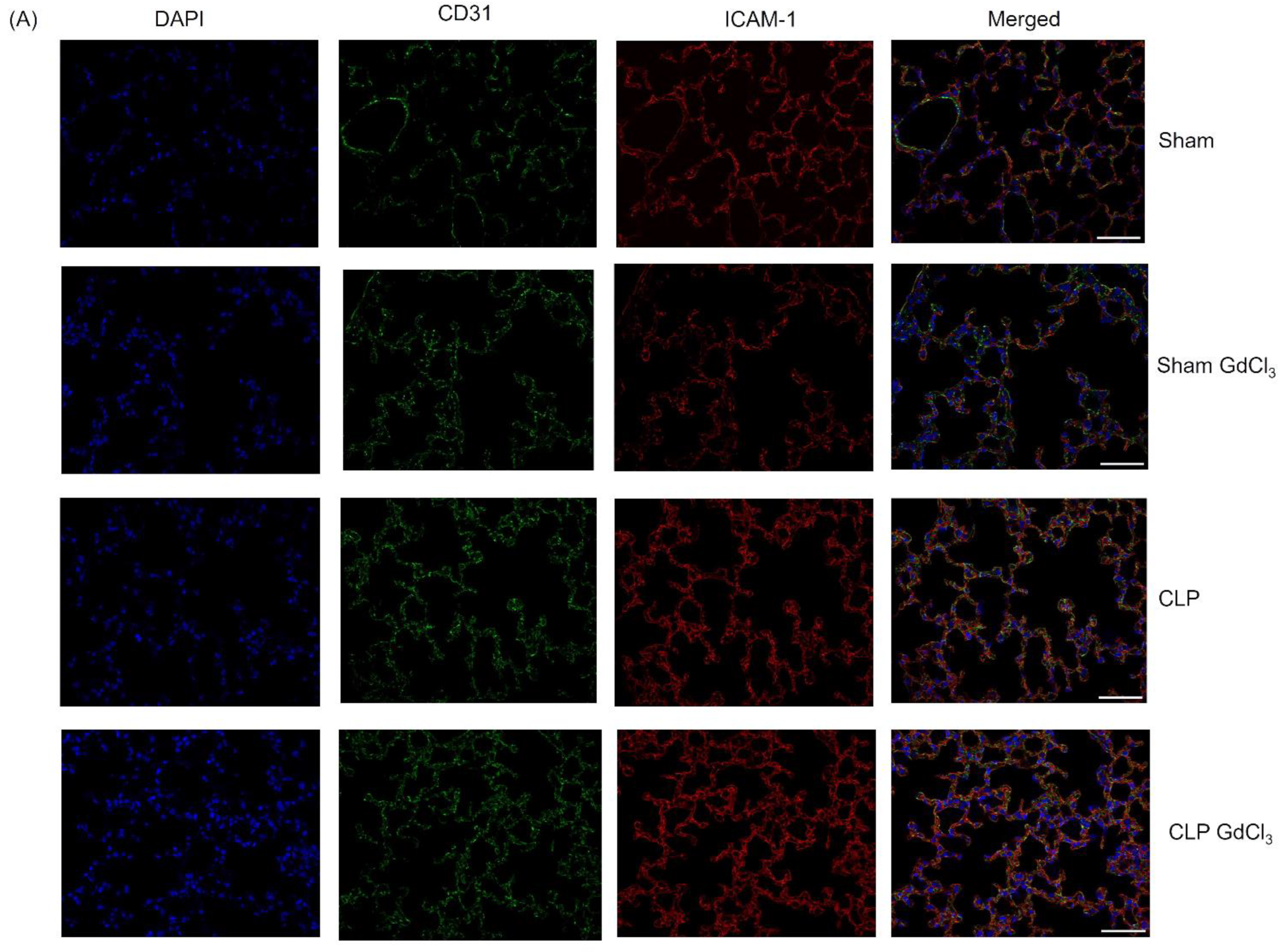
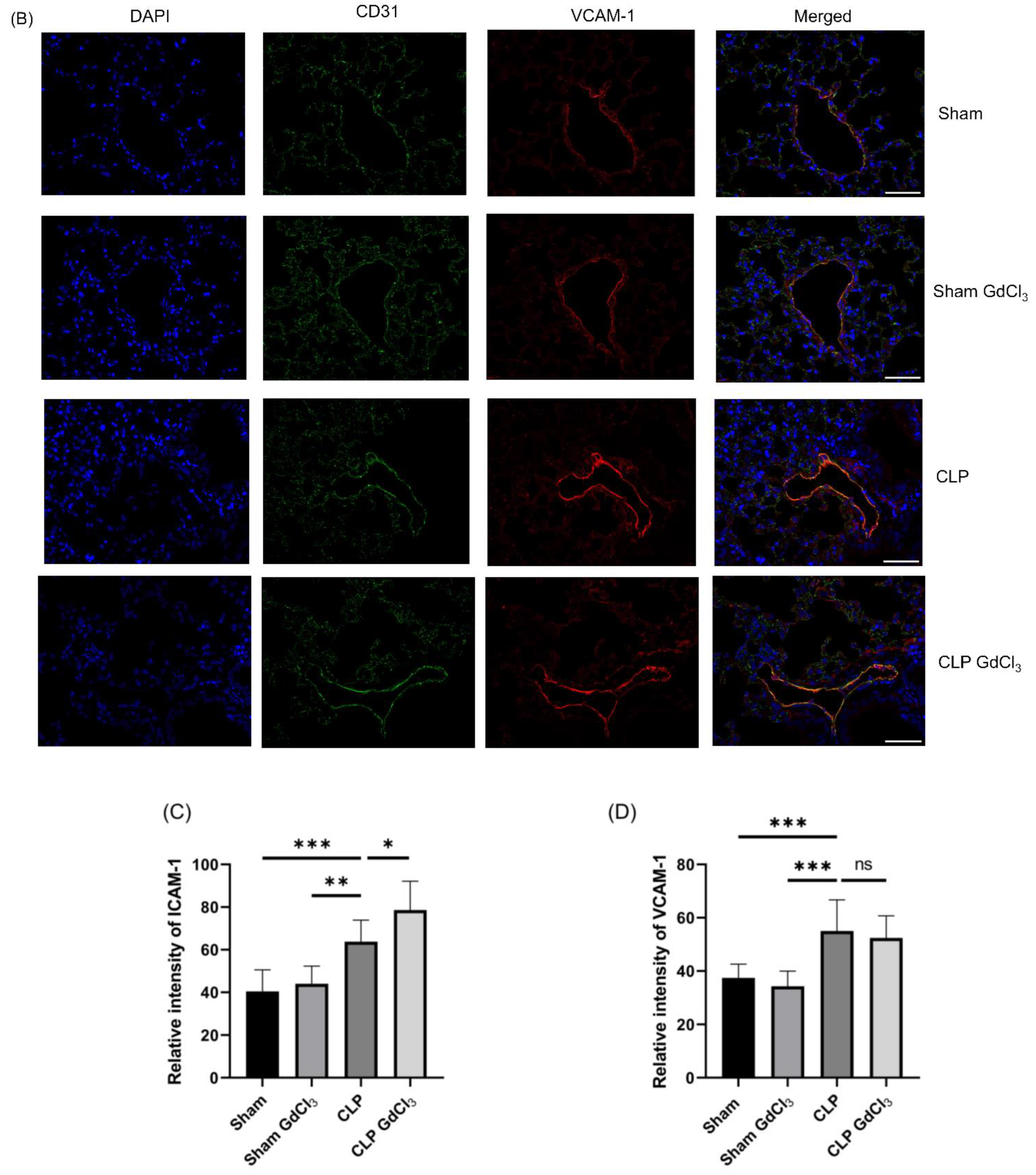
3.3. Effect of Kupffer Cell Inactivation on Immunoreactivity of Lung ICAM-1 and VCAM-1 Co-Localized with Pulmonary Endothelial Cells
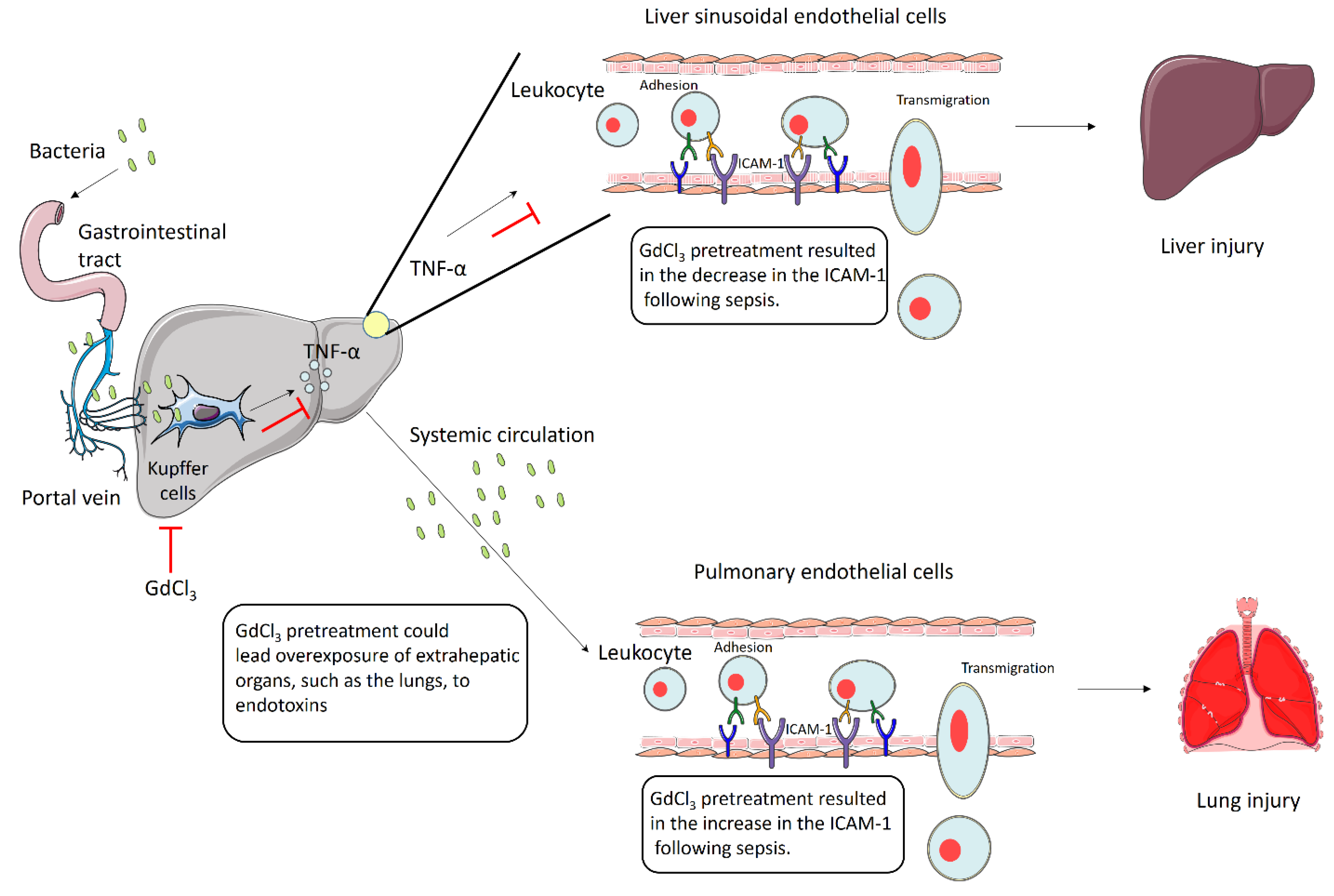
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Liaskou, E.; Wilson, D.V.; Oo, Y.H. Innate immune cells in liver inflammation. Mediat. Inflamm. 2012, 2012, 949157. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, S.H.; Lee, S.M. Role of Kupffer cells in pathogenesis of sepsis-induced drug metabolizing dysfunction. FEBS J. 2011, 278, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; McGuire, G.M.; Fisher, M.A.; Farhood, A.; Smith, C.W.; Jaeschke, H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock 1995, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Matsuda, M.; Yamamoto, M.; Matsumoto, Y. Gadolinium chloride prevents mortality in hepatectomized rats given endotoxin. J. Surg. Res. 2001, 96, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Husztik, E.; Lázár, G.; Párducz, A. Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride. Br. J. Exp. Pathol. 1980, 61, 624–630. [Google Scholar] [PubMed]
- Lazar, G. The reticuloendothelial-blocking effect of rare earth metals in rats. J. Reticuloendothel. Soc. 1973, 13, 231–237. [Google Scholar]
- Bautista, A.P.; Skrepnik, N.; Niesman, M.R.; Bagby, G.J. Elimination of macrophages by liposome-encapsulated dichloromethylene diphosphonate suppresses the endotoxin-induced priming of Kupffer cells. J. Leukoc. Biol. 1994, 55, 321–327. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Qurashi, M.; Shetty, S. The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer Within the Liver. Front. Physiol. 2020, 11, 990. [Google Scholar] [CrossRef]
- DeLisser, H.M.; Albelda, S.M. The function of cell adhesion molecules in lung inflammation: More questions than answers. Am. J. Respir. Cell Mol. Biol. 1998, 19, 533–536. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Luscinskas, F.W.; Gimbrone, M.A., Jr. Endothelial-dependent mechanisms in chronic inflammatory leukocyte recruitment. Annu. Rev. Med. 1996, 47, 413–421. [Google Scholar] [CrossRef]
- Adams, D.H.; Shaw, S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet 1994, 343, 831–836. [Google Scholar] [CrossRef] [PubMed]
- van de Stolpe, A.; van der Saag, P.T. Intercellular adhesion molecule-1. J. Mol. Med. 1996, 74, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Rand, T.H.; Goelz, S.E.; Chi-Rosso, G.; Lobb, R.R. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1991, 88, 7430–7433. [Google Scholar] [CrossRef]
- Bochner, B.S.; Luscinskas, F.W.; Gimbrone, M.A., Jr.; Newman, W.; Sterbinsky, S.A.; Derse-Anthony, C.P.; Klunk, D.; Schleimer, R.P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: Contributions of endothelial cell adhesion molecules. J. Exp. Med. 1991, 173, 1553–1557. [Google Scholar] [CrossRef]
- Dobrina, A.; Menegazzi, R.E.N.Z.O.; Carlos, T.M.; Nardon, E.; Cramer, R.; Zacchi, T.; Harlan, J.M.; Patriarca, P. Mechanisms of eosinophil adherence to cultured vascular endothelial cells. Eosinophils bind to the cytokine-induced ligand vascular cell adhesion molecule-1 via the very late activation antigen-4 integrin receptor. J. Clin. Investig. 1991, 88, 20–26. [Google Scholar] [CrossRef][Green Version]
- Thornhill, M.H.; Wellicome, S.M.; Mahiouz, D.L.; Lanchbury, J.S.S.; Kyan-Aung, U.; Haskard, D.O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J. Immunol. 1991, 146, 592–598. [Google Scholar] [CrossRef]
- Burke-Gaffney, A.; Hellewell, P.G. Tumour necrosis factor-alpha-induced ICAM-1 expression in human vascular endothelial and lung epithelial cells: Modulation by tyrosine kinase inhibitors. Br. J. Pharmacol. 1996, 119, 1149–1158. [Google Scholar] [CrossRef]
- Hoffmann, M.W.; Wonigeit, K.; Steinhoff, G.; Herzbeck, H.; Flad, H.-D.; Pichlmayr, R. Production of cytokines (TNF-alpha, IL-1-beta) and endothelial cell activation in human liver allograft rejection. Transplantation 1993, 55, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Okanoue, T.; Itoh, Y.; Nakagawa, Y.; Nakamura, H.; Morita, A.; Daimon, Y.; Sakamoto, K.; Yoshida, N.; Yoshikawa, T.; et al. Involvement of Kupffer Cells in the Interaction Between Neutrophils and Sinusoidal Endothelial Cells in Rats. Shock 2002, 18, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Fraser, R.; Badiei, A.; Chambers, S.; Cogger, V.C.; Le Couteur, D.G.; Bhatia, M. Differential Effects of Kupffer Cell Inactivation on Inflammation and The Liver Sieve Following Caecal-Ligation and Puncture-Induced Sepsis in Mice. Shock 2017, 47, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, Y.; Chen, M.; Li, J.; Zhang, H.; Yan, S.; Lv, C. Advanced development and mechanism of sepsis-related acute respiratory distress syndrome. Front. Med. 2022, 9, 1043859. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.M.; Cronen, C.; Müller, K.-M.; Kirkpatrick, C.J. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J. Pathol. 2002, 198, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Pendino, K.J.; Meidhof, T.M.; Heck, D.E.; Laskin, J.D.; Laskin, D.L. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 1995, 13, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kishta, O.A.; Goldberg, P.; Husain, S.N. Gadolinium chloride attenuates sepsis-induced pulmonary apoptosis and acute lung injury. ISRN Inflamm. 2012, 2012, 393481. [Google Scholar] [CrossRef]
- Manandhar, S.; Chambers, S.; Miller, A.; Ishii, I.; Bhatia, M. Pharmacological Inhibition and Genetic Deletion of Cystathionine Gamma-Lyase in Mice Protects against Organ Injury in Sepsis: A Key Role of Adhesion Molecules on Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 13650. [Google Scholar] [CrossRef]
- Furutani, M.; Arii, S.; Monden, K.; Adachi, Y.; Funaki, N.; Higashitsuji, H.; Fujita, S.; Mise, M.; Ishiguro, S.; Kitao, T. Immunologic activation of hepatic macrophages in septic rats: A possible mechanism of sepsis-associated liver injury. J. Lab. Clin. Med. 1994, 123, 430–436. [Google Scholar]
- Harty, M.W.; Papa, E.F.; Huddleston, H.M.; Young, E.; Nazareth, S.; Riley, C.A.; Ramm, G.A.; Gregory, S.H.; Tracy, T.F. Hepatic macrophages promote the neutrophil-dependent resolution of fibrosis in repairing cholestatic rat livers. Surgery 2008, 143, 667–678. [Google Scholar] [CrossRef]
- Farrell, G.C.; Teoh, N.C.; McCuskey, R.S. Hepatic microcirculation in fatty liver disease. Anat. Rec. 2008, 291, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Iimuro, Y.; Yamamoto, M.; Kohno, H.; Itakura, J.; Fujii, H.; Matsumoto, Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats—Analysis of mechanisms of lethality in endotoxemia. J. Leukoc. Biol. 1994, 55, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.P.; Villar, J.; Downey, G.P.; Edelson, J.D.; Slutsky, A.S. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: Evidence for TNF-alpha posttranslational regulation. Am. J. Respir. Crit. Care Med. 1996, 154, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Amemiya, H.; Asakawa, M.; Hirai, Y.; Maki, A.; Tsuchiya, M.; Matsuda, M.; Yamamoto, M. Role of Kupffer cells in lung injury in rats administered endotoxin 1. J. Surg. Res. 2005, 129, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Hirai, Y.; Tsuchiya, M.; Amemiya, H.; Asakawa, M.; Maki, A.; Matsuda, M.; Yamamoto, M. The Kupffer cell protects against acute lung injury in a rat peritonitis model: Role of IL-10. J. Leukoc. Biol. 2006, 79, 809–817. [Google Scholar] [CrossRef]
- Chu, L.Y.; Hsueh, Y.-C.; Cheng, H.-L.; Wu, K.K. Cytokine-induced autophagy promotes long-term VCAM-1 but not ICAM-1 expression by degrading late-phase IκBα. Sci. Rep. 2017, 7, 12472. [Google Scholar] [CrossRef]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.U.S.H.T.A.Q.; Alexander, R.W.; Medford, R.M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Invest. 1993, 92, 1866–1874. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.-K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats preferentially inhibit TNF-alpha-mediated induction of VCAM-1 over ICAM-1 through the regulation of GATAs and IRF-1. J. Agric. Food Chem. 2009, 57, 7324–7330. [Google Scholar] [CrossRef]
| Product | Antibody/Type | Source Catalogue No. | Dilution |
|---|---|---|---|
| Immunofluorescence | |||
| ICAM-1 | Primary/Goat polyclonal | R&D System, Minneapolis, MN, USA/AF796 | 1:1000 |
| VCAM-1 | Primary/Goat polyclonal | R&D System, Minneapolis, MN, USA/AF643 | 1:200 |
| LYVE-1 | Primary/Rabbit polyclonal | Abcam, Cambridge, UK/ab14917 | 1:100 |
| CD31 | Primary/Rabbit polyclonal | Abcam, Cambridge, UK/ab124432 | 1:500 |
| Donkey anti-goat | Secondary/Texas Red | Abcam, Cambridge, UK/ab6883 | 1:1000 |
| Donkey anti-rabbit | Secondary/FITC | Abcam, Cambridge, UK/ab6798 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manandhar, S.; Gaddam, R.R.; Chambers, S.; Bhatia, M. Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis. Biomolecules 2024, 14, 84. https://doi.org/10.3390/biom14010084
Manandhar S, Gaddam RR, Chambers S, Bhatia M. Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis. Biomolecules. 2024; 14(1):84. https://doi.org/10.3390/biom14010084
Chicago/Turabian StyleManandhar, Sumeet, Ravinder Reddy Gaddam, Stephen Chambers, and Madhav Bhatia. 2024. "Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis" Biomolecules 14, no. 1: 84. https://doi.org/10.3390/biom14010084
APA StyleManandhar, S., Gaddam, R. R., Chambers, S., & Bhatia, M. (2024). Kupffer Cell Inactivation Alters Endothelial Cell Adhesion Molecules in Cecal Ligation and Puncture-Induced Sepsis. Biomolecules, 14(1), 84. https://doi.org/10.3390/biom14010084







