Moderate-High Blood Eosinophilia Is Associated with Increased Hospitalization and Other Asthma Comorbidities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Measurement of Covariates and Outcomes
2.3. Statistical Analyses
2.4. Data Sharing Statement
3. Results
3.1. Study Population
3.2. Asthmatic Patients Have Higher BEC Than Nonasthmatic Subjects in an above 300 BEC/μL Cohort
3.3. History of CRSwNP, Hypertension, and Dyslipidemia Is Associated with Eosinophilia in Asthmatic Patients with above 300 BEC/μL
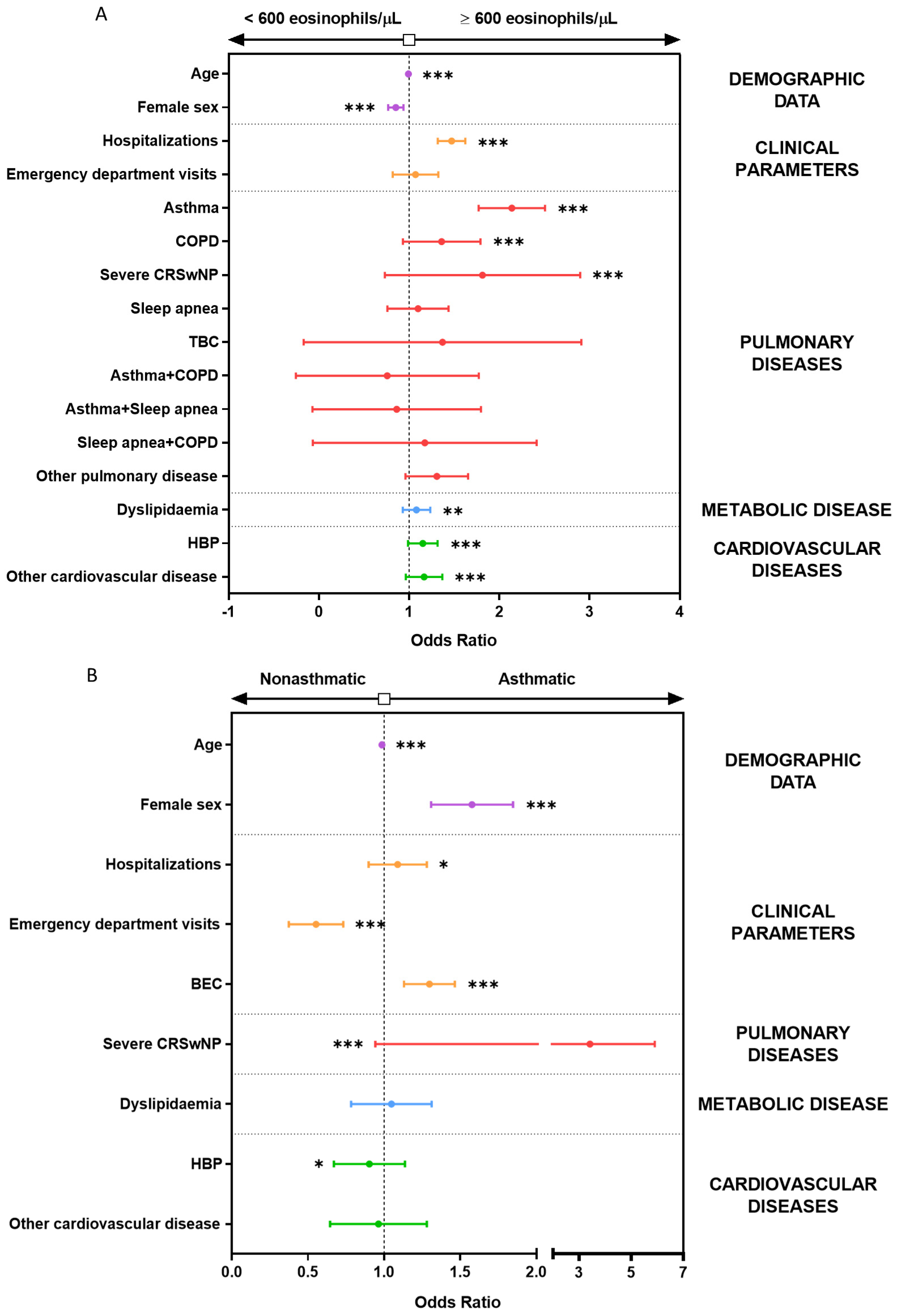
3.4. Multiple Logistic Regression Models Show the Association between BEC Greater Than 600 Eosinophils/µL and Asthma, CRSwNP, COPD, and Hospitalization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| AEs | Asthma exacerbations |
| BEC | Blood eosinophil count |
| COPD | Chronic obstructive pulmonary disease |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| EGPA | Eosinophilic granulomatosis with polyangiitis |
| EMR | Electronic medical record |
| EoE | Eosinophilic esophagitis |
| ESS | Endoscopic sinus surgery |
| GEMA | Guidelines for Asthma Management |
| HBP | High blood pressure |
| HES | Hypereosinophilic syndrome |
| HU | Hospital Universitario |
| IL-5Rα | IL-5 alpha subunit receptor |
| MetS | Metabolic syndrome |
| OR | Odds ratio |
| SEA | Severe eosinophilic asthma |
References
- Sastre, B.; Rodrigo-Muñoz, J.; Garcia-Sanchez, D.; Cañas, J.; del Pozo, V. Eosinophils: Old Players in a New Game. J. Investig. Allergol. Clin. Immunol. 2018, 28, 289–304. [Google Scholar] [CrossRef]
- Ness, T.E.; Erickson, T.A.; Diaz, V.; Grimes, A.B.; Rochat, R.; Anvari, S.; Hajjar, J.; Weatherhead, J. Pediatric Eosinophilia: A Review and Multiyear Investigation into Etiologies. J. Pediatr. 2023, 253, 232–237.e1. [Google Scholar] [CrossRef]
- Klion, A.D. Eosinophilia: A pragmatic approach to diagnosis and treatment. Hematology 2015, 2015, 92–97. [Google Scholar] [CrossRef]
- Kuang, F.L. Approach to Patients with Eosinophilia. Med. Clin. N. Am. 2020, 104, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Ogbogu, P. Hypereosinophilic syndrome. Clin. Rev. Allergy Immunol. 2016, 50, 240–251. [Google Scholar] [CrossRef]
- Chen, Y.-Y.K.; Khoury, P.; Ware, J.M.; Holland-Thomas, N.C.; Stoddard, J.L.; Gurprasad, S.; Waldner, A.J.; Klion, A.D. Marked and persistent eosinophilia in the absence of clinical manifestations. J. Allergy Clin. Immunol. 2014, 133, 1195–1202.e2. [Google Scholar] [CrossRef]
- Helbig, G.; Hus, M.; Francuz, T.; Dziaczkowska-Suszek, J.; Soja, A.; Kyrcz-Krzemień, S. Characteristics and clinical outcome of patients with hypereosinophilia of undetermined significance. Med. Oncol. 2014, 31, 815. [Google Scholar] [CrossRef] [PubMed]
- Hartl, S.; Breyer, M.-K.; Burghuber, O.C.; Ofenheimer, A.; Schrott, A.; Urban, M.H.; Agusti, A.; Studnicka, M.; Wouters, E.F.M.; Breyer-Kohansal, R. Blood eosinophil count in the general population: Typical values and potential confounders. Eur. Respir. J. 2020, 55, 1901874. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Blanchard, C.; Kirby, C.; Buckmeier, B.K.; Cohen, M.B.; Heubi, J.E.; Putnam, P.E.; Rothenberg, M.E. Potential of Blood Eosinophils, Eosinophil-Derived Neurotoxin, and Eotaxin-3 as Biomarkers of Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2006, 4, 1328–1336. [Google Scholar] [CrossRef]
- Bakakos, A.; Loukides, S.; Bakakos, P. Severe Eosinophilic Asthma. J. Clin. Med. 2019, 8, 1375. [Google Scholar] [CrossRef]
- Cardet, J.C.; Bulkhi, A.A.; Lockey, R.F. Nonrespiratory Comorbidities in Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 3887–3897. [Google Scholar] [CrossRef]
- Turrin, M.; Rizzo, M.; Bonato, M.; Bazzan, E.; Cosio, M.G.; Semenzato, U.; Saetta, M.; Baraldo, S. Differences Between Early- and Late-Onset Asthma: Role of Comorbidities in Symptom Control. J. Allergy Clin. Immunol. Pract. 2022, 10, 3196–3203. [Google Scholar] [CrossRef]
- Hekking, P.P.; Amelink, M.; Wener, R.R.; Bouvy, M.L.; Bel, E.H. Comorbidities in Difficult-to-Control Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 108–113. [Google Scholar] [CrossRef]
- Pongdee, T.; Manemann, S.M.; Decker, P.A.; Larson, N.B.; Moon, S.; Killian, J.M.; Liu, H.; Kita, H.; Bielinski, S.J. Rethinking blood eosinophil counts: Epidemiology, associated chronic diseases, and increased risks of cardiovascular disease. J. Allergy Clin. Immunol. Glob. 2022, 1, 233–240. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; de Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Sprekelsen, M.B.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Matsunaga, K. Measurement of Blood Eosinophils in Asthma and Chronic Obstructive Pulmonary Disease. Intern. Med. 2023, 62, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Chen, J.D. The association between eosinophil count, serum lipids and metabolic syndrome in Taiwanese. Am. J. Med. Sci. 2023, 365, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.; Sawant, S.; McDonald, H.; Rye, K.A.; Patel, S.; Ong, K.L.; Cochran, B.J. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: Results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis 2021, 319, 1–9. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, Y.S.; Hong, J.I.; Kim, S.H.; Jung, H.H.; Kim, S.M. Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr. J. 2006, 53, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.K.; Bush, A.; Stokes, J.; Nair, P.; Akuthota, P. Eosinophilic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Kerkhof, M.; Carter, V.; Price, D.B. Persistence of eosinophilic asthma endotype and clinical outcomes: A real-world observational study. J. Asthma Allergy 2021, 14, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Spanish Guidelines on Asthma Management (GEMA 5.3). Available online: https://www.gemasma.com/ (accessed on 11 December 2023).
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.-P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. npj Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Bleecker, E.R.; Curran-everett, D.; Erzurum, S.C.; Ameredes, B.T.; Bacharier, L.; William, J.; Castro, M.; Chung, F.; Clark, M.P.; et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’ s Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 405–413. [Google Scholar] [CrossRef]
- Yancey, S.W.; Keene, O.N.; Albers, F.C.; Ortega, H.; Bates, S.; Bleecker, E.R.; Pavord, I. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1509–1518. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines—Recommendations for severe asthma. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 14–44. [Google Scholar] [CrossRef]
- Hambly, N.; Nair, P. Monoclonal antibodies for the treatment of refractory asthma. Curr. Opin. Pulm. Med. 2014, 20, 87–94. [Google Scholar] [CrossRef]
- Pitlick, M.M.; Li, J.T.; Pongdee, T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ. J. 2022, 15, 100676. [Google Scholar] [CrossRef]
- Patel, S.S.; Casale, T.B.; Cardet, J.C. Biological therapies for eosinophilic asthma. Expert Opin. Biol. Ther. 2018, 18, 747–754. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Santomasi, C.; Buonamico, E.; Dragonieri, S.; Iannuzzi, L.; Portacci, A.; Quaranta, N.; Carpagnano, G.E. Effects of benralizumab in a population of patients affected by severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps: A real-life study. Acta Biomed. 2023, 94, e2023028. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Piñera, P.; Trigueros, J.A.; Calle, M.; Casanova, C.; Cosío, B.G.; López-Campos, J.L.; Molina, J.; Almagro, P.; Gómez, J.T.; et al. Spanish COPD Guidelines (GesEPOC) 2021 Update Diagnosis and Treatment of COPD Exacerbation Syndrome. Arch. Bronconeumol. 2022, 58, 159–170. [Google Scholar] [CrossRef]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International consensus document on obstructive sleep apnea. Arch. Bronconeumol. 2022, 58, T52–T68. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Consolidated Guidelines on Tuberculosis Treatment; WHO: Geneva, Switzerland, 2020; ISBN 9789241550529.
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Colás, C.; Castillo, J.A.; Arismendi, E.; Del Cuvillo, A.; Gómez-Outes, A.; Sastre, J.; Mullol, J.; POLINA Group. Spanish consensus on the management of chronic rhinosinusitis with nasal polyps (POLIposis NAsal POLINA 2.0). J. Investig. Allergol. Clin. Immunol. 2023, 33, 317–331. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, M.J.; Horne, B.D.; Trudo, F.; Kreindler, J.L.; Rea, S.; Blagev, D.P. Blood Eosinophil Count and Hospital Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2629–2641. [Google Scholar]
- Chen, B.; Fu, Y.; Wang, Z.; Rong, Q.; Zhang, Q.; Xie, J.; Kong, X.; Jiang, M. Eosinophilia attention, diagnosis, treatment, and awareness in physicians: A cross-sectional survey. Ther. Adv. Chronic Dis. 2023, 14, 204062232211469. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Wechsler, M.E.; FitzGerald, J.M.; Menzies-Gow, A.; Wu, Y.; Hirsch, I.; Goldman, M.; Newbold, P.; Zangrilli, J.G. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur. Respir. J. 2018, 52, 1800936. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Toledo-Pons, N.; van Boven, J.F.M.; Muncunill, J.; Millán, A.; Román-Rodríguez, M.; López-Andrade, B.; Almonacid, C.; Álvarez, D.V.; Kocks, J.W.H.; Cosío, B.G. Impact of Blood Eosinophil Variability in Asthma: A Real-Life Population Study. Ann. Am. Thorac. Soc. 2022, 19, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gorgojo, I.; Rial, M.J.; Sastre, J. The Influence of Peripheral Blood Eosinophil Counts in Asthma Comorbidities in Adults: A Real Life Study. Appl. Sci. 2022, 12, 4271. [Google Scholar] [CrossRef]
- Benson, V.S.; Hartl, S.; Barnes, N.; Galwey, N.; Van Dyke, M.K.; Kwon, N. Blood eosinophil counts in the general population and airways disease: A comprehensive review and meta-analysis. Eur. Respir. J. 2022, 59, 2004590. [Google Scholar] [CrossRef]
- Coumou, H.; Westerhof, G.A.; De Nijs, S.B.; Amelink, M.; Bel, E.H. Diagnosing persistent blood eosinophilia in asthma with single blood eosinophil or exhaled nitric oxide level. Respir. Med. 2018, 141, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.J.; Tavernier, G.; Niven, R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J. Allergy Clin. Immunol. 2015, 135, 822–824. [Google Scholar] [CrossRef]
- Jin, H.J. Biological treatments for severe asthma. Yeungnam Univ. J. Med. 2020, 37, 262–268. [Google Scholar] [CrossRef]
- Babu, S. Eosinophil Polymorphonuclear Leukocytes in TB: What We Know so. Front. Immunol. 2019, 10, 2639. [Google Scholar] [CrossRef]
- Health, P.; Haftu, H.; Tadese, K.; Gebrehiwot, T.; Gebregziabher, H. How Common is Eosinophilia in Tuberculosis? Case Report. Pediatr. Health Med. Ther. 2020, 11, 59–63. [Google Scholar]
- Garg, G. Persistent Marked Peripheral Eosinophilia due to Tuberculosis: A Case Report. Iran. J. Med. Sci. 2017, 42, 102. [Google Scholar]
- Naidu, V.; Belton, M. Prevalence of Eosinophilia in Patients with Tuberculosis. Eur. Respir. J. 2021, 58, PA1725. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef]
- Takabayashi, T.; Schleimer, R.P. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J. Allergy Clin. Immunol. 2020, 145, 740–750. [Google Scholar] [CrossRef]
- Zhong, B.; Yuan, T.; Du, J.; Tan, K.; Yang, Q.; Liu, F.; Liu, Y.; Ba, L.; Liu, S. The role of preoperative blood eosinophil counts in distinguishing chronic rhinosinusitis with nasal polyps phenotypes. Int. Forum Allergy Rhinol. 2021, 11, 16–23. [Google Scholar] [CrossRef]
- Amini, M.; Bashirova, D.; Prins, B.P.; Corpeleijn, E.; LifeLines Cohort Study; Bruinenberg, M.; Franke, L.; Van Der Harst, P.; Navis, G.; Wolffenbuttel, B.H.R.; et al. Eosinophil count is a common factor for complex metabolic and pulmonary traits and diseases: The lifelines cohort study. PLoS ONE 2016, 11, e0168480. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluña, J.J.; Soriano, J.B.; García-Río, F.; de Lucas, P.; Alfageme, I.; Casanova, C.; Rodríguez González-Moro, J.M.; Sánchez-Herrero, M.G.; Ancochea, J.; et al. Determinants of blood eosinophil levels in the general population and patients with COPD: A population-based, epidemiological study. Respir. Res. 2022, 23, 49. [Google Scholar] [CrossRef]
- Kim, V.; Aaron, S.D. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur. Respir. J. 2018, 52, 1801261. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Pancholi, M.; Venge, P.; Lomas, D.A.; Barer, M.R.; Johnston, S.L.; Pavord, I.D.; et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2012, 186, 48–55. [Google Scholar] [CrossRef]
- Huang, Z.S.; Chien, K.L.; Yang, C.Y.; Tsai, K.S.; Wang, C.H. Peripheral differential leukocyte counts in humans vary with hyperlipidemia, smoking, and body mass index. Lipids 2001, 36, 237–245. [Google Scholar] [CrossRef]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic Rhinosinusitis with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Shrestha Palikhe, N.; Bosonea, A.M.; Laratta, C.; Gandhi, V.D.; Nahirney, D.; Hillaby, A.; Bowen, M.; Bhutani, M.; Mayers, I.; Cameron, L.; et al. Stability of peripheral blood immune markers in patients with asthma. Allergy Asthma Clin. Immunol. 2019, 15, 30. [Google Scholar] [CrossRef]
- Herrett, E.; Thomas, S.L.; Schoonen, W.M.; Smeeth, L.; Hall, A.J. Validation and validity of diagnoses in the General Practice Research Database: A systematic review. Br. J. Clin. Pharmacol. 2010, 69, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Muñoz, J.M.; Sastre, B.; Cañas, J.A.; Gil-Martínez, M.; Redondo, N.; Del Pozo, V. Eosinophil response against classical and emerging respiratory viruses: COVID-19. J. Investig. Allergol. Clin. Immunol. 2021, 31, 94–107. [Google Scholar] [CrossRef]
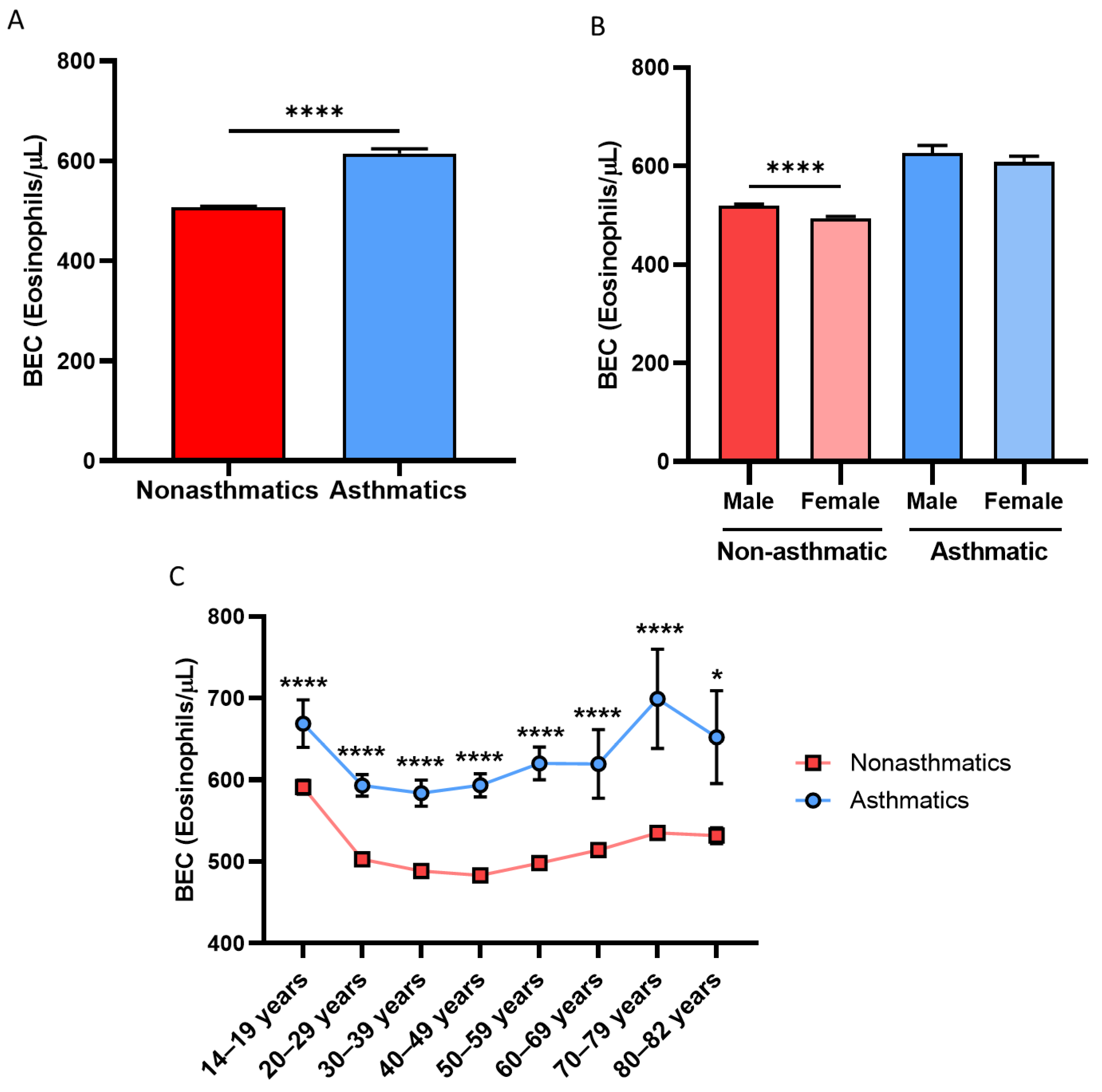
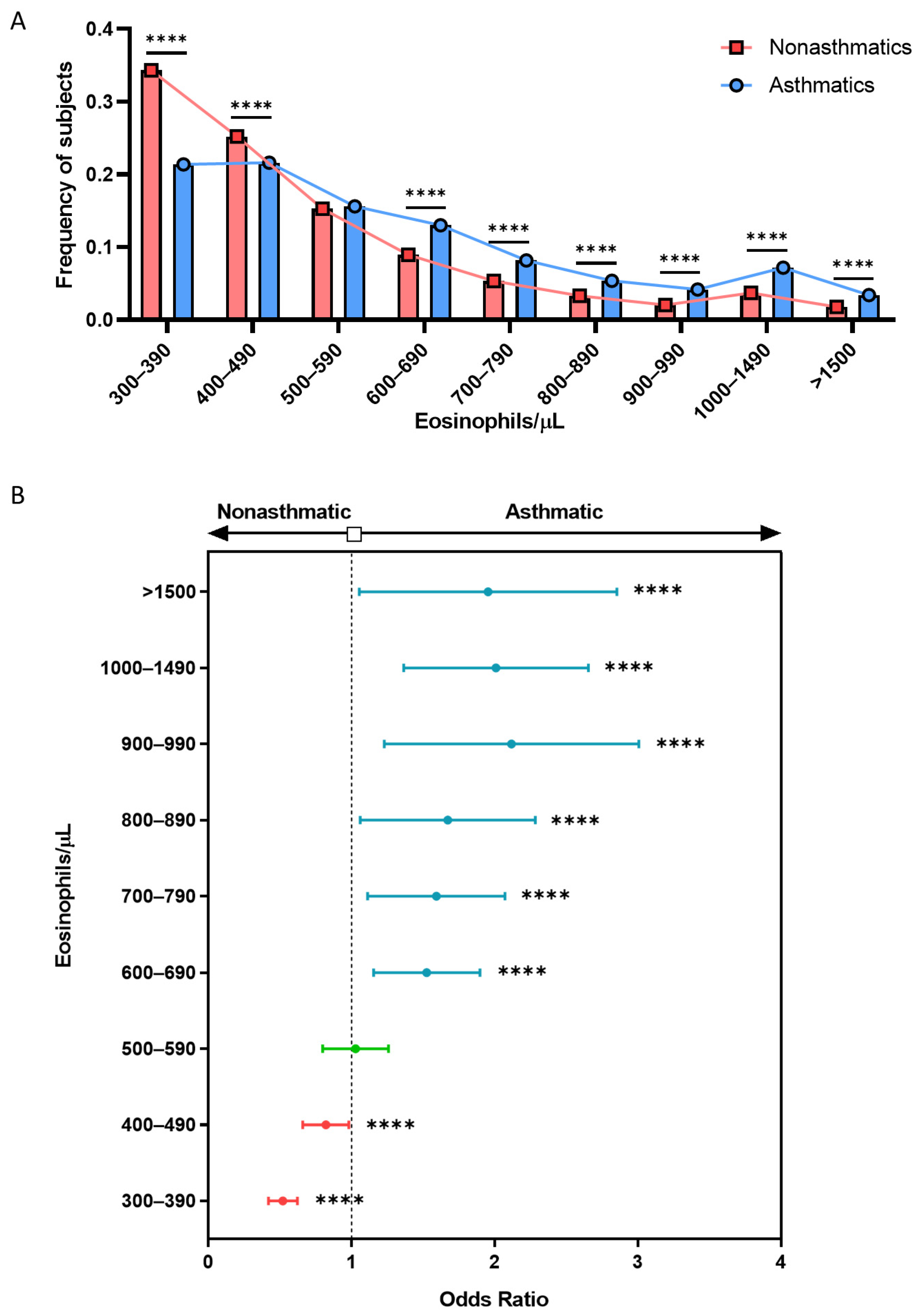
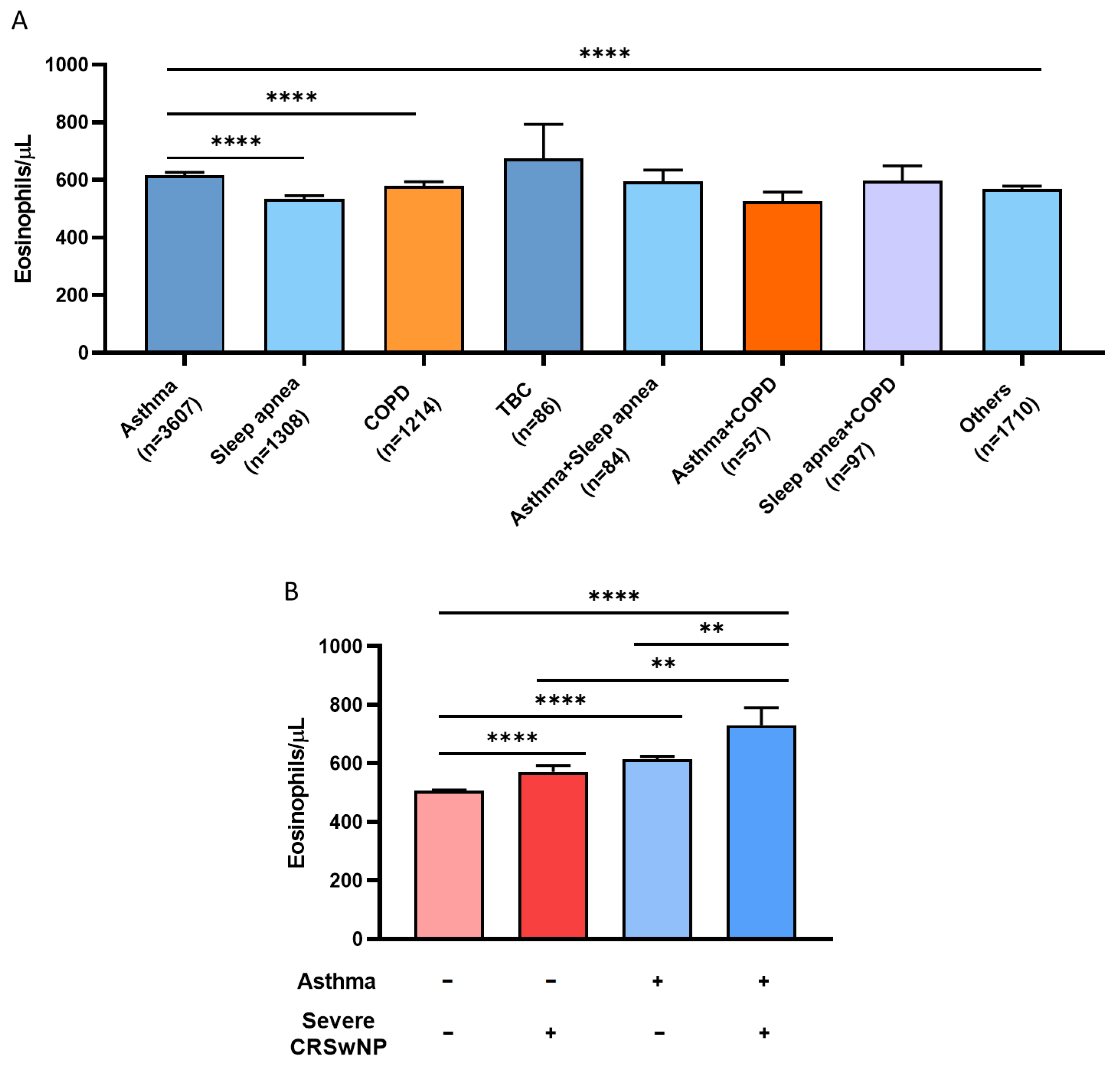
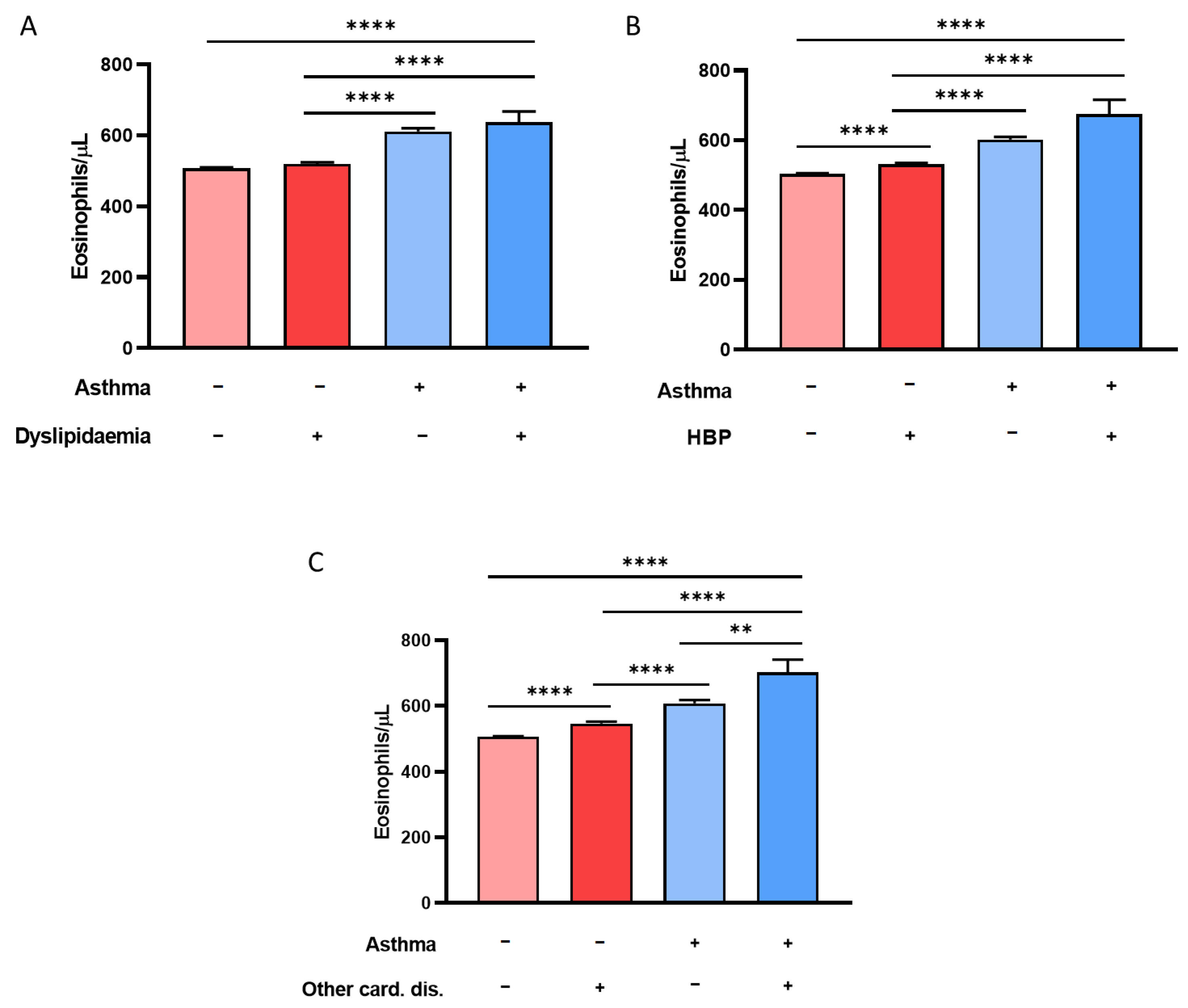
| Total Population >300 BEC (n = 53,788) | Nonasthmatics >300 BEC (n = 49,909) | Asthmatics >300 BEC (n = 3879) | p-Value (Asthma vs. No Asthma) | |
|---|---|---|---|---|
| Age (Mean ± SD) | 48.5 ± 17.8 | 48.7 ± 17.8 | 45.5 ± 17.3 | <0.0001 |
| Female sex (n, %) | 27,188 (50.5) | 24,814 (49.7) | 2374 (61.2) | <0.0001 |
| BEC, eosinophils/μL (Mean ± SD) | 510 ± 490 | 510 ± 480 | 610 ± 600 | <0.0001 |
| Hospitalizations (n, %) | 20,824 (38.7) | 19,278 (38.6) | 1546 (39.9) | <0.0001 |
| Emergency department visits (n, %) | 51,218 (95.2) | 47,626 (95.4) | 3592 (92.6) | <0.0001 |
| Cardiovascular diseases (n, %) | ||||
| HBP | 11,755 (23.3) | 11,076 (23.7) | 679 (17.7) | <0.0001 |
| Others | 5266 (11.6) | 4964 (11.9) | 302 (8.3) | <0.0001 |
| Metabolic diseases (n, %) | ||||
| Dyslipidemia | 10,212 (20.2) | 9574 (20.5) | 638 (16.6) | <0.0001 |
| Respiratory diseases (n, %) | ||||
| Asthma | 3607 (6.7) | 0 (0) | 3879 (100) | <0.0001 |
| Severe CRSwNP | 280 (0.5) | 222 (0.4) | 58 (1.5) | <0.0001 |
| Sleep apnea | 1308 (2.4) | 1308 (2.6) | 84 (2.2) | ns |
| COPD | 1214 (2.3) | 1214 (2.4) | 57 (1.5) | <0.0001 |
| TBC | 86 (0.2) | 86 (0.2) | 4 (0.1) | ns |
| Others | 1710 (3.2) | 1710 (3.4) | 103 (2.7) | 0.0100 |
| Smoking habit (n, %) | ||||
| Smoker | 11,951 (24.0) | 11,196 (24.4) | 755 (19.8) | <0.0001 |
| Nonsmoker | 28,985 (58.3) | 26,653 (58.0) | 2332 (61.2) | 0.0002 |
| Ex-smoker | 8720 (17.5) | 8003 (17.4) | 717 (18.8) | 0.0300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naharro-González, S.; Lorente-Sorolla, C.; Rodrigo-Muñoz, J.M.; Valverde-Monge, M.; Pinillos-Robles, E.J.; Betancor, D.; Fernández-Nieto, M.; Sánchez-Mellado, D.; Gil-Martínez, M.; Santillán-Coello, J.M.; et al. Moderate-High Blood Eosinophilia Is Associated with Increased Hospitalization and Other Asthma Comorbidities. Biomolecules 2024, 14, 126. https://doi.org/10.3390/biom14010126
Naharro-González S, Lorente-Sorolla C, Rodrigo-Muñoz JM, Valverde-Monge M, Pinillos-Robles EJ, Betancor D, Fernández-Nieto M, Sánchez-Mellado D, Gil-Martínez M, Santillán-Coello JM, et al. Moderate-High Blood Eosinophilia Is Associated with Increased Hospitalization and Other Asthma Comorbidities. Biomolecules. 2024; 14(1):126. https://doi.org/10.3390/biom14010126
Chicago/Turabian StyleNaharro-González, Sara, Clara Lorente-Sorolla, José Manuel Rodrigo-Muñoz, Marcela Valverde-Monge, Erwin Javier Pinillos-Robles, Diana Betancor, Mar Fernández-Nieto, Diana Sánchez-Mellado, Marta Gil-Martínez, Jessica Mireya Santillán-Coello, and et al. 2024. "Moderate-High Blood Eosinophilia Is Associated with Increased Hospitalization and Other Asthma Comorbidities" Biomolecules 14, no. 1: 126. https://doi.org/10.3390/biom14010126
APA StyleNaharro-González, S., Lorente-Sorolla, C., Rodrigo-Muñoz, J. M., Valverde-Monge, M., Pinillos-Robles, E. J., Betancor, D., Fernández-Nieto, M., Sánchez-Mellado, D., Gil-Martínez, M., Santillán-Coello, J. M., Villacampa-Aubá, J. M., Mahillo-Fernandez, I., Herrero-González, A., Perez-González, A., Rodríguez-Nieto, M. J., & del Pozo, V. (2024). Moderate-High Blood Eosinophilia Is Associated with Increased Hospitalization and Other Asthma Comorbidities. Biomolecules, 14(1), 126. https://doi.org/10.3390/biom14010126






