Alveolar Organoids in Lung Disease Modeling
Abstract
1. Introduction
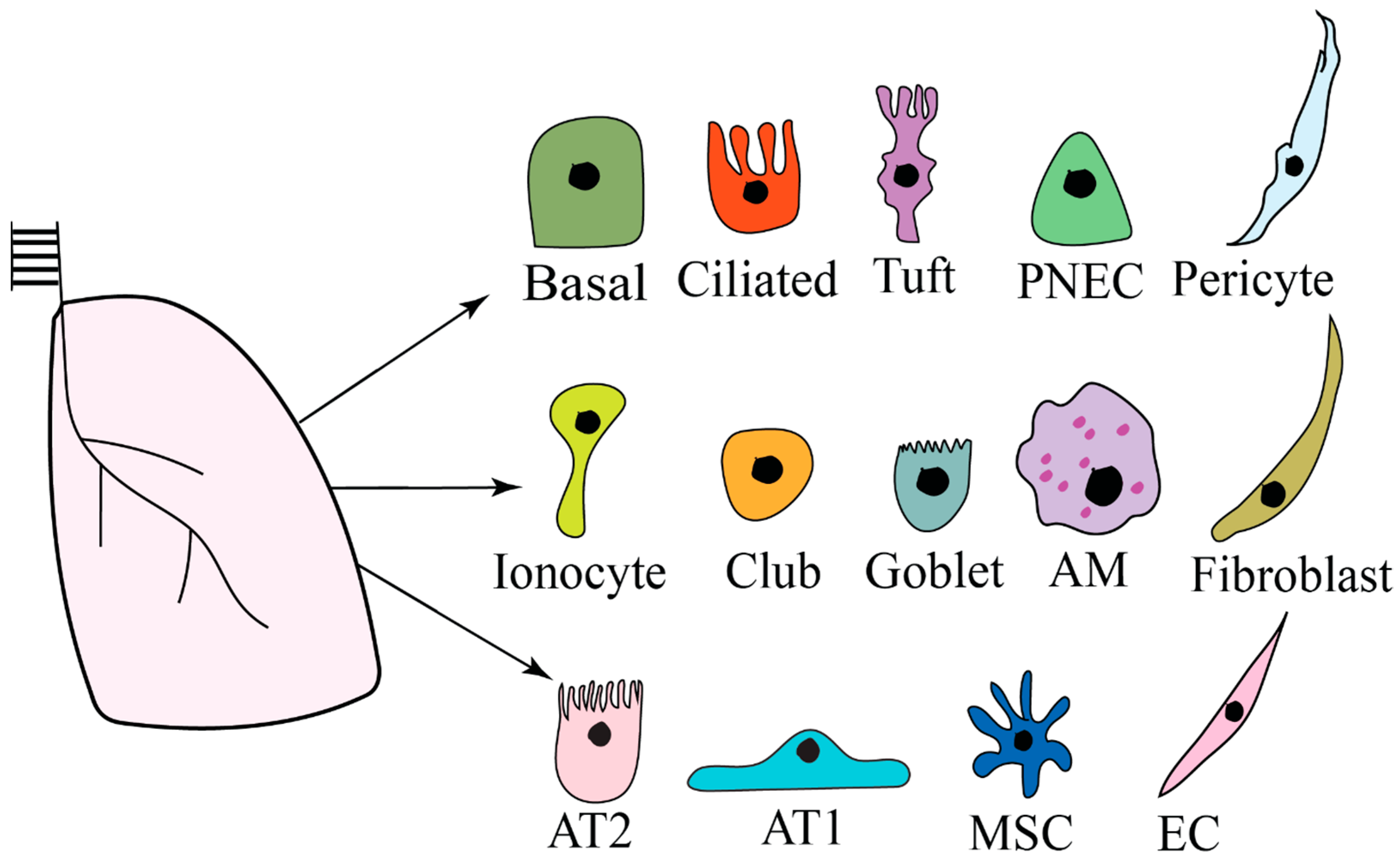
2. Lung Microenvironment
3. Lung Organoids
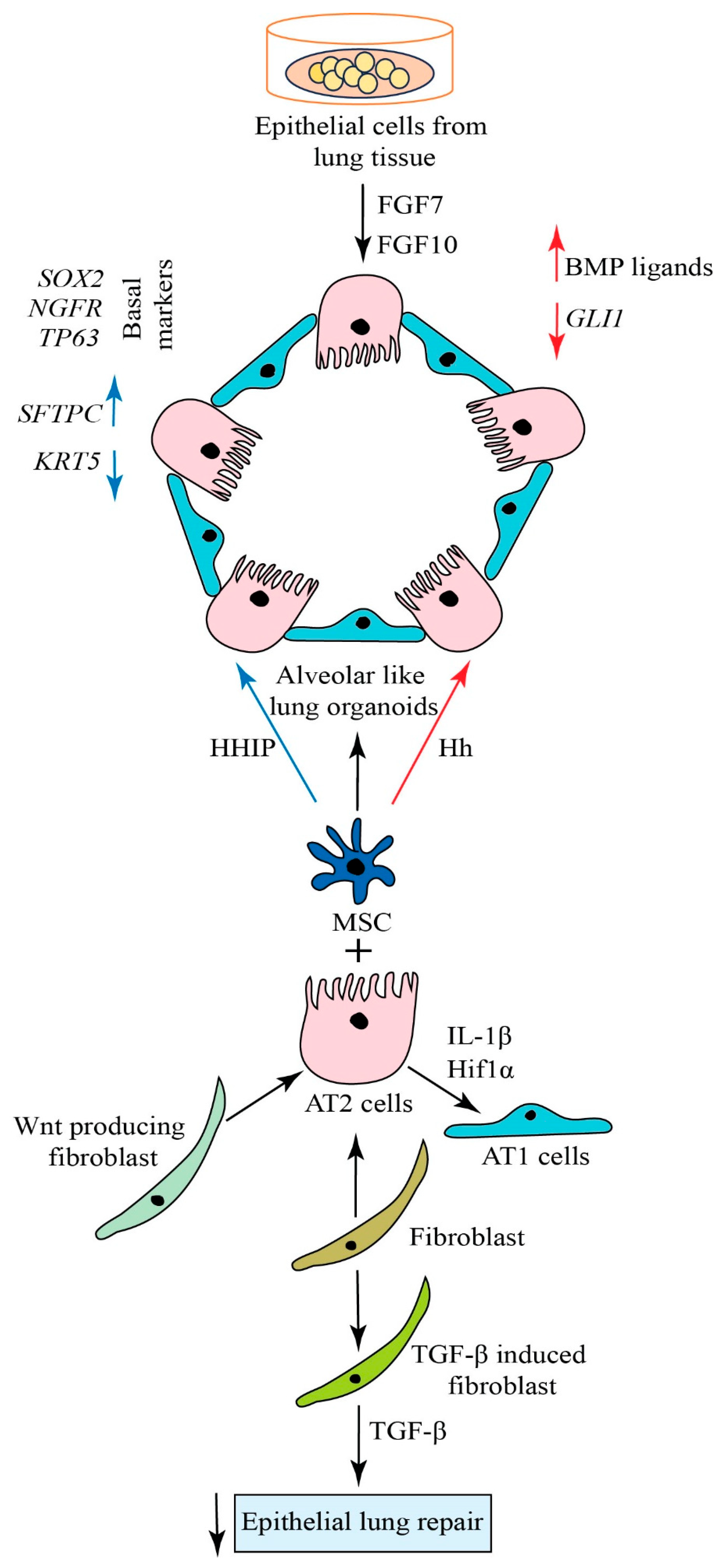
4. Generation of Alveolar Organoids
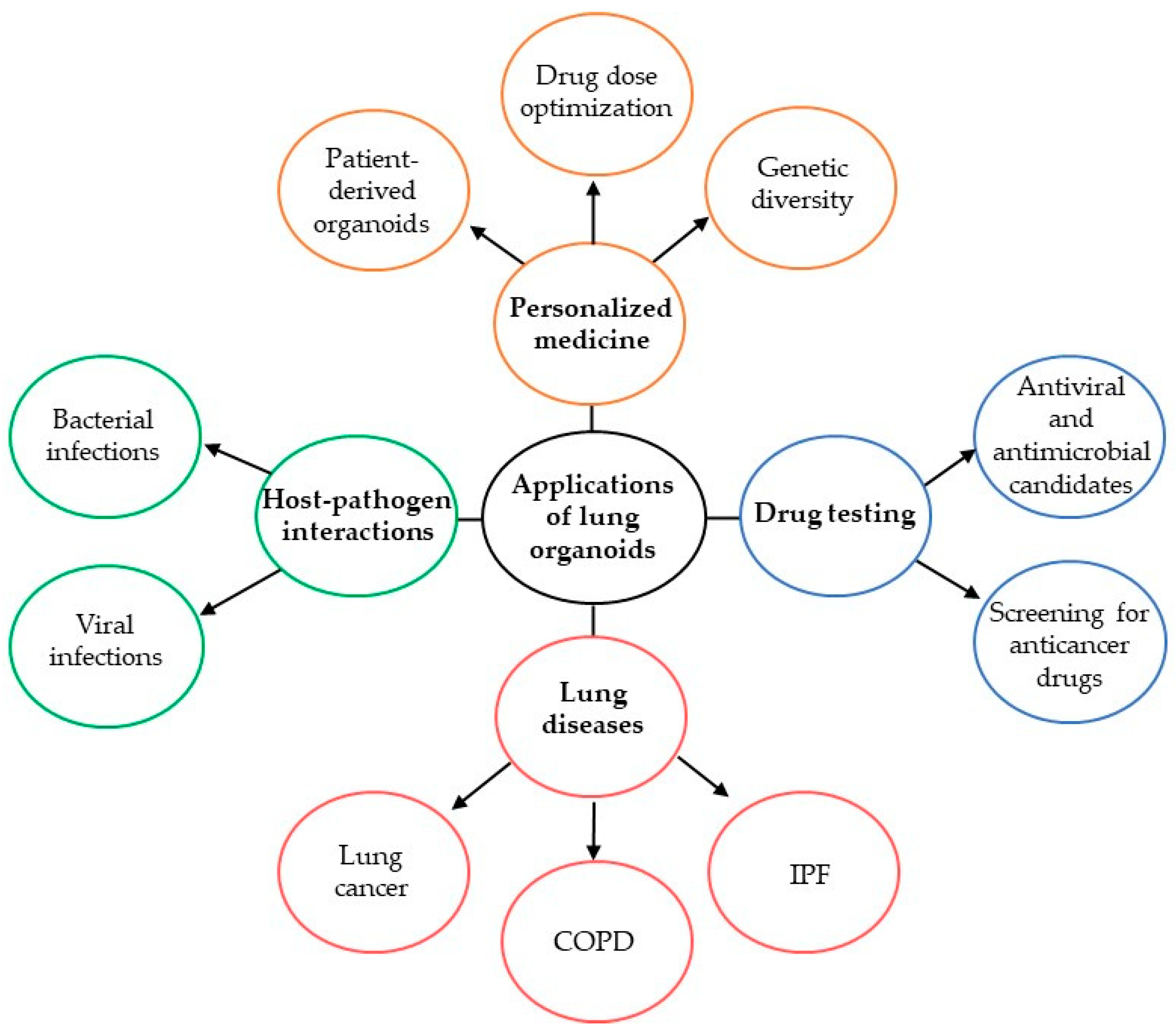
5. Approaches for Lung Regeneration
6. Signaling Pathways in Lung Regeneration
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, T.; Zhou, C.; Shao, Y.; Xiong, Z.; Weng, D.; Pang, Y.; Sun, W. Construction and Application of in vitro Alveolar Models Based on 3D Printing Technology. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tata, A.; Konkimalla, A.; Katsura, H.; Lee, R.F.; Ou, J.; Banovich, N.E.; Kropski, J.A.; Tata, P.R. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 2020, 22, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Shi, T.; Li, X.; Chowdhury, A.Y.; Jiang, D.; Liu, Y.; Wang, H.; Yan, C.; Wallace, W.D.; Lu, R. Development of human alveolar epithelial cell models to study distal lung biology and disease. iScience 2022, 25, 103780. [Google Scholar] [CrossRef] [PubMed]
- Kalender, M.; Bulbul, M.V.; Kolbasi, B.; Keskin, I. In 2D and 3D Cell Culture Models, Effects of Endothelial Cells on E-cadherin/β-catenin Expression Levels and Spheroid Sizes in Ishikawa Cells. Asian Pac. J. Cancer Prev. 2022, 23, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Pietsch, S.; Pannicke, T.; Thome, U.H.; Fabian, C. Development and Functional Characterization of Fetal Lung Organoids. Front. Med. 2021, 8, 678438. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Q.; Huang, W.; Zhou, S.; Wang, Y.; Zeng, X.; Wang, H.; Xie, W.; Kong, H. NLRP3 Inflammasome Mediates Silica-induced Lung Epithelial Injury and Aberrant Regeneration in Lung Stem/Progenitor Cell-derived Organotypic Models. Int. J. Biol. Sci. 2023, 19, 1875. [Google Scholar] [CrossRef]
- Leach, T.; Gandhi, U.; Reeves, K.D.; Stumpf, K.; Okuda, K.; Marini, F.C.; Walker, S.J.; Boucher, R.; Chan, J.; Cox, L.A. Development of a novel air–liquid interface airway tissue equivalent model for in vitro respiratory modeling studies. Sci. Rep. 2023, 13, 10137. [Google Scholar] [CrossRef]
- Sharma, A.; Schwarzbauer, J.E. Differential regulation of neurite outgrowth and growth cone morphology by 3D fibronectin and fibronectin-collagen extracellular matrices. Mol. Neurobiol. 2022, 59, 1112–1123. [Google Scholar] [CrossRef]
- Germano-Costa, T.; Bilesky-José, N.; Guilger-Casagrande, M.; Pasquoto-Stigliani, T.; Rogério, C.; Abrantes, D.; Maruyama, C.; Oliveira, J.; Fraceto, L.; Lima, R. Use of 2D and co-culture cell models to assess the toxicity of zein nanoparticles loading insect repellents icaridin and geraniol. Colloids Surf. B Biointerfaces 2022, 216, 112564. [Google Scholar] [CrossRef]
- Eilenberger, C.; Rothbauer, M.; Selinger, F.; Gerhartl, A.; Jordan, C.; Harasek, M.; Schädl, B.; Grillari, J.; Weghuber, J.; Neuhaus, W. A microfluidic multisize spheroid array for multiparametric screening of anticancer drugs and blood–brain barrier transport properties. Adv. Sci. 2021, 8, 2004856. [Google Scholar] [CrossRef]
- Chiu, M.C.; Li, C.; Liu, X.; Song, W.; Wan, Z.; Yu, Y.; Huang, J.; Xiao, D.; Chu, H.; Cai, J.-P. Human nasal organoids model SARS-CoV-2 upper respiratory infection and recapitulate the differential infectivity of emerging variants. mBio 2022, 13, e01944-22. [Google Scholar] [CrossRef] [PubMed]
- Klimas, A.; Gallagher, B.R.; Wijesekara, P.; Fekir, S.; DiBernardo, E.F.; Cheng, Z.; Stolz, D.B.; Cambi, F.; Watkins, S.C.; Brody, S.L. Magnify is a universal molecular anchoring strategy for expansion microscopy. Nat. Biotechnol. 2023, 41, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, L.; Engelsen, A.S.; Aanerud, M.; Ramnefjell, M.P.; Salminen, P.R.; Gärtner, F.; Halvorsen, T.; Ræder, H.; Bentsen, M.H. Induction of alveolar and bronchiolar phenotypes in human lung organoids. Physiol. Rep. 2021, 9, e14857. [Google Scholar] [CrossRef] [PubMed]
- Rigauts, C.; Aizawa, J.; Taylor, S.L.; Rogers, G.B.; Govaerts, M.; Cos, P.; Ostyn, L.; Sims, S.; Vandeplassche, E.; Sze, M. Rothia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur. Respir. J. 2022, 59, 2101293. [Google Scholar] [CrossRef]
- Chan, L.L.; Anderson, D.E.; Cheng, H.S.; Ivan, F.X.; Chen, S.; Kang, A.E.; Foo, R.; Gamage, A.M.; Tiew, P.Y.; Koh, M.S. The establishment of COPD organoids to study host-pathogen interaction reveals enhanced viral fitness of SARS-CoV-2 in bronchi. Nat. Commun. 2022, 13, 7635. [Google Scholar]
- Roffel, M.P.; Maes, T.; Brandsma, C.-A.; van den Berge, M.; Vanaudenaerde, B.M.; Joos, G.F.; Brusselle, G.G.; Heijink, I.H.; Bracke, K.R. MiR-223 is increased in lungs of patients with COPD and modulates cigarette smoke-induced pulmonary inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L1091–L1104. [Google Scholar] [CrossRef]
- Spelier, S.; de Poel, E.; Ithakisiou, G.N.; Suen, S.W.; Hagemeijer, M.C.; Muilwijk, D.; Vonk, A.M.; Brunsveld, J.E.; Kruisselbrink, E.; van der Ent, C.K. High-throughput functional assay in cystic fibrosis patient-derived organoids allows drug repurposing. ERJ Open Res. 2023, 9, 00495–2022. [Google Scholar] [CrossRef]
- Sette, G.; Cicero, S.L.; Blaconà, G.; Pierandrei, S.; Bruno, S.M.; Salvati, V.; Castelli, G.; Falchi, M.; Fabrizzi, B.; Cimino, G. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient-derived nasal epithelial conditionally reprogrammed stem cells. Eur. Respir. J. 2021, 58, 2100908. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Sandlin, C.W.; Gu, S.; Xu, J.; Deshpande, C.; Feldman, M.D.; Good, M.C. Epithelial cell size dysregulation in human lung adenocarcinoma. PLoS ONE 2022, 17, e0274091. [Google Scholar] [CrossRef]
- Parker, A.L.; Bowman, E.; Zingone, A.; Ryan, B.M.; Cooper, W.A.; Kohonen-Corish, M.; Harris, C.C.; Cox, T.R. Extracellular matrix profiles determine risk and prognosis of the squamous cell carcinoma subtype of non-small cell lung carcinoma. Genome Med. 2022, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, K.G.; Surolia, R.; Kulkarni, T.; Li, F.J.; Singh, P.; Zeng, H.; Stephens, C.; Kumar, A.; Wang, Z.; Antony, V.B. Use of a pulmosphere model to evaluate drug antifibrotic responses in interstitial lung diseases. Respir. Res. 2023, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Kishimoto, J.; Ando, M.; Kenmotsu, H.; Minegishi, Y.; Horinouchi, H.; Kato, T.; Ichihara, E.; Kondo, M.; Atagi, S. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: A randomised phase 3 trial. Eur. Respir. J. 2022, 60, 2200380. [Google Scholar] [CrossRef]
- Kathiriya, J.J.; Wang, C.; Zhou, M.; Brumwell, A.; Cassandras, M.; Le Saux, C.J.; Cohen, M.; Alysandratos, K.-D.; Wang, B.; Wolters, P. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells. Nat. Cell Biol. 2022, 24, 10–23. [Google Scholar] [CrossRef]
- Alba, G.A.; Samokhin, A.O.; Wang, R.S.; Wertheim, B.M.; Haley, K.J.; Padera, R.F.; Vargas, S.O.; Rosas, I.O.; Hariri, L.P.; Shih, A. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS-CoV-2 infection. Pulm. Circ. 2022, 12, e12071. [Google Scholar] [CrossRef]
- Dmytriw, A.A.; Chibbar, R.; Chen, P.P.Y.; Traynor, M.D.; Kim, D.W.; Bruno, F.P.; Cheung, C.C.; Pareek, A.; Chou, A.C.C.; Graham, J. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2021, 15, 1347–1354. [Google Scholar] [CrossRef]
- Liu, J.; Schiralli-Lester, G.M.; Norman, R.; Dean, D.A. Upregulation of alveolar fluid clearance is not sufficient for Na+, K+-ATPase β subunit-mediated gene therapy of LPS-induced acute lung injury in mice. Sci. Rep. 2023, 13, 6792. [Google Scholar] [CrossRef]
- Bluhmki, T.; Traub, S.; Müller, A.-K.; Bitzer, S.; Schruf, E.; Bammert, M.-T.; Leist, M.; Gantner, F.; Garnett, J.P.; Heilker, R. Functional human iPSC-derived alveolar-like cells cultured in a miniaturized 96-Transwell air–liquid interface model. Sci. Rep. 2021, 11, 17028. [Google Scholar] [CrossRef]
- Salahudeen, A.A.; Choi, S.S.; Rustagi, A.; Zhu, J.; van Unen, V.; de la O, S.M.; Flynn, R.A.; Margalef-Català, M.; Santos, A.J.; Ju, J. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020, 588, 670–675. [Google Scholar] [CrossRef]
- Aizawa, Y.; Takada, K.; Aoyama, J.; Sano, D.; Yamanaka, S.; Seki, M.; Kuze, Y.; Ramilowski, J.A.; Okuda, R.; Ueno, Y. Establishment of experimental salivary gland cancer models using organoid culture and patient-derived xenografting. Cell. Oncol. 2023, 46, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Sun, X.; Zhang, J.; Li, H.; Kuang, J.; Xu, L.; Gao, X.; Zhou, C. Exploratory evaluation of EGFR-targeted anti-tumor drugs for lung cancer based on lung-on-a-chip. Biosensors 2022, 12, 618. [Google Scholar] [CrossRef]
- Luan, Q.; Becker, J.H.; Macaraniag, C.; Massad, M.G.; Zhou, J.; Shimamura, T.; Papautsky, I. Non-small cell lung carcinoma spheroid models in agarose microwells for drug response studies. Lab Chip 2022, 22, 2364–2375. [Google Scholar] [CrossRef]
- Novak, C.M.; Sethuraman, S.; Luikart, K.L.; Reader, B.F.; Wheat, J.S.; Whitson, B.; Ghadiali, S.N.; Ballinger, M.N. Alveolar macrophages drive lung fibroblast function in cocultures of IPF and normal patient samples. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L507–L520. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Bozorgmehry Boozarjomehry, R. Fast and accurate multiscale reduced-order model for prediction of multibreath washout curves of human respiratory system. Ind. Eng. Chem. Res. 2021, 60, 4131–4141. [Google Scholar] [CrossRef]
- de Waal, A.M.; Hiemstra, P.S.; Ottenhoff, T.H.; Joosten, S.A.; van der Does, A.M. Lung epithelial cells interact with immune cells and bacteria to shape the microenvironment in tuberculosis. Thorax 2022, 77, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.D.; Hill, C.L.; Alsudayri, A.; Lallier, S.W.; Wijeratne, S.; Tan, Z.H.; Chiang, T.; Cormet-Boyaka, E. Assemblies of JAG1 and JAG2 determine tracheobronchial cell fate in mucosecretory lung disease. JCI Insight 2022, 7, e157380. [Google Scholar] [CrossRef]
- Shivaraju, M.; Chitta, U.K.; Grange, R.M.; Jain, I.H.; Capen, D.; Liao, L.; Xu, J.; Ichinose, F.; Zapol, W.M.; Mootha, V.K. Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science 2021, 371, 52–57. [Google Scholar] [CrossRef]
- Madissoon, E.; Oliver, A.J.; Kleshchevnikov, V.; Wilbrey-Clark, A.; Polanski, K.; Richoz, N.; Ribeiro Orsi, A.; Mamanova, L.; Bolt, L.; Elmentaite, R. A spatially resolved atlas of the human lung characterizes a gland-associated immune niche. Nat. Genet. 2023, 55, 66–77. [Google Scholar] [CrossRef]
- Ualiyeva, S.; Lemire, E.; Aviles, E.C.; Wong, C.; Boyd, A.A.; Lai, J.; Liu, T.; Matsumoto, I.; Barrett, N.A.; Boyce, J.A. Tuft cell–produced cysteinyl leukotrienes and IL-25 synergistically initiate lung type 2 inflammation. Sci. Immunol. 2021, 6, eabj0474. [Google Scholar] [CrossRef]
- Yamada, Y.; Belharazem-Vitacolonnna, D.; Bohnenberger, H.; Weiß, C.; Matsui, N.; Kriegsmann, M.; Kriegsmann, K.; Sinn, P.; Simon-Keller, K.; Hamilton, G. Pulmonary cancers across different histotypes share hybrid tuft cell/ionocyte-like molecular features and potentially druggable vulnerabilities. Cell Death Dis. 2022, 13, 979. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Gentile, M.E.; Lee, S.; Kotas, M.E.; de Mello Costa, M.F.; Holcomb, N.P.; Jaquish, A.; Palashikar, G.; Soewignjo, M.; McDaniel, M. Injury-induced pulmonary tuft cells are heterogenous, arise independent of key Type 2 cytokines, and are dispensable for dysplastic repair. eLife 2022, 11, e78074. [Google Scholar] [CrossRef]
- Ualiyeva, S.; Hallen, N.; Kanaoka, Y.; Ledderose, C.; Matsumoto, I.; Junger, W.G.; Barrett, N.A.; Bankova, L.G. Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci. Immunol. 2020, 5, eaax7224. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Dang, H.; Kobayashi, Y.; Carraro, G.; Nakano, S.; Chen, G.; Kato, T.; Asakura, T.; Gilmore, R.C.; Morton, L.C. Secretory cells dominate airway CFTR expression and function in human airway superficial epithelia. Am. J. Respir. Crit. Care Med. 2021, 203, 1275–1289. [Google Scholar] [CrossRef]
- Goldfarbmuren, K.C.; Jackson, N.D.; Sajuthi, S.P.; Dyjack, N.; Li, K.S.; Rios, C.L.; Plender, E.G.; Montgomery, M.T.; Everman, J.L.; Bratcher, P.E. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat. Commun. 2020, 11, 2485. [Google Scholar] [CrossRef] [PubMed]
- Rindler, T.N.; Stockman, C.A.; Filuta, A.L.; Brown, K.M.; Snowball, J.M.; Zhou, W.; Veldhuizen, R.; Zink, E.M.; Dautel, S.E.; Clair, G. Alveolar injury and regeneration following deletion of ABCA3. JCI Insight 2017, 2, e97381. [Google Scholar] [CrossRef]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Zepp, J.A.; Morley, M.P.; Loebel, C.; Kremp, M.M.; Chaudhry, F.N.; Basil, M.C.; Leach, J.P.; Liberti, D.C.; Niethamer, T.K.; Ying, Y. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science 2021, 371, eabc3172. [Google Scholar] [CrossRef]
- Kook, S.; Wang, P.; Meng, S.; Jetter, C.S.; Sucre, J.M.; Benjamin, J.T.; Gokey, J.J.; Hanby, H.A.; Jaume, A.; Goetzl, L. AP-3–dependent targeting of flippase ATP8A1 to lamellar bodies suppresses activation of YAP in alveolar epithelial type 2 cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2025208118. [Google Scholar] [CrossRef]
- Jain, K.G.; Zhao, R.; Liu, Y.; Guo, X.; Yi, G.; Ji, H.-L. Wnt5a/β-catenin axis is involved in the downregulation of AT2 lineage by PAI-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 323, L515–L524. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Sucre, J.M.; Bock, F.; Negretti, N.M.; Benjamin, J.T.; Gulleman, P.M.; Dong, X.; Ferguson, K.T.; Jetter, C.S.; Han, W.; Liu, Y. Alveolar repair following lipopolysaccharide-induced injury requires cell-extracellular matrix interactions. JCI Insight 2023, 8, e167211. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Woods, P.S.; Kimmig, L.M.; Sun, K.A.; Meliton, A.Y.; Shamaa, O.R.; Tian, Y.; Cetin-Atalay, R.; Sharp, W.W.; Hamanaka, R.B.; Mutlu, G.M. HIF-1α induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury. eLife 2022, 11, e77457. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.D.; Gennaro, M.D.; Lista, G.; Messina, F.; Valente, T.; Borzacchiello, A. Effect of Composition of Lung Biomimetic Niche on The Mesenchymal Stem Cell Differentiation Toward Alveolar Type II Pneumocytes. Macromol. Biosci. 2023, 23, e2300035. [Google Scholar]
- Hezam, K.; Wang, C.; Fu, E.; Zhou, M.; Liu, Y.; Wang, H.; Zhu, L.; Han, Z.; Han, Z.-C.; Chang, Y. Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation. Stem Cell Res. Ther. 2023, 14, 48. [Google Scholar] [CrossRef]
- Baek, S.-H.; Maiorino, E.; Kim, H.; Glass, K.; Raby, B.A.; Yuan, K. Single cell transcriptomic analysis reveals organ specific pericyte markers and identities. Front. Cardiovasc. Med. 2022, 9, 876591. [Google Scholar] [CrossRef]
- Xie, H.; Gao, Y.M.; Zhang, Y.C.; Jia, M.W.; Peng, F.; Meng, Q.H.; Wang, Y.C. Low let-7d exosomes from pulmonary vascular endothelial cells drive lung pericyte fibrosis through the TGFβRI/FoxM1/Smad/β-catenin pathway. J. Cell. Mol. Med. 2020, 24, 13913–13926. [Google Scholar] [CrossRef]
- Schupp, J.C.; Adams, T.S.; Cosme, C., Jr.; Raredon, M.S.B.; Yuan, Y.; Omote, N.; Poli, S.; Chioccioli, M.; Rose, K.-A.; Manning, E.P. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 2021, 144, 286–302. [Google Scholar] [CrossRef]
- Niethamer, T.K.; Stabler, C.T.; Leach, J.P.; Zepp, J.A.; Morley, M.P.; Babu, A.; Zhou, S.; Morrisey, E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife 2020, 9, e53072. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, H.; Li, S.; Huang, K.; Jiang, L.; Wang, Y. Metformin alleviates LPS-induced acute lung injury by regulating the SIRT1/NF-κB/NLRP3 pathway and inhibiting endothelial cell Pyroptosis. Front. Pharmacol. 2022, 13, 801337. [Google Scholar] [CrossRef] [PubMed]
- Gabasa, M.; Arshakyan, M.; Llorente, A.; Chuliá-Peris, L.; Pavelescu, I.; Xaubet, A.; Pereda, J.; Alcaraz, J. Interleukin-1β modulation of the mechanobiology of primary human pulmonary fibroblasts: Potential implications in lung repair. Int. J. Mol. Sci. 2020, 21, 8417. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Ma, S.; Dou, H.; Liu, F.; Zhang, S.-Y.; Jiang, C.; Xiao, M.; Huang, Y.-X. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp. Cell Res. 2020, 391, 111983. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, K.; Zhang, X.; Huang, G.; Lynn, H.; Rabata, A.; Liang, J.; Noble, P.W.; Jiang, D. Multiple Fibroblast Subtypes Contribute to Matrix Deposition in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 45–56. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Yangın, K.; Özkan, S.N.; Sarıca, S.; Örnek, D.; Solcan, N.; Karaoğlu, I.S.C.; Kızılel, S.; Bulutay, P.; Fırat, P. Different Decellularization Methods in Bovine Lung Tissue Reveals Distinct Biochemical Composition, Stiffness, and Viscoelasticity in Reconstituted Hydrogels. ACS Appl. Bio Mater. 2023, 6, 793–805. [Google Scholar] [CrossRef]
- Rönnberg, E.; Boey, D.Z.H.; Ravindran, A.; Säfholm, J.; Orre, A.-C.; Al-Ameri, M.; Adner, M.; Dahlén, S.-E.; Dahlin, J.S.; Nilsson, G. Immunoprofiling reveals novel mast cell receptors and the continuous nature of human lung mast cell heterogeneity. Front. Immunol. 2022, 12, 804812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, K.; Pu, H.; Wang, Z.; Zhao, H.; Zhang, J.; Wang, Y. An immune-related signature predicts survival in patients with lung adenocarcinoma. Front. Oncol. 2019, 9, 1314. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Xu, X.R.; Chen, S. Drug resistance related genes in lung adenocarcinoma predict patient prognosis and influence the tumor microenvironment. Sci. Rep. 2023, 13, 9682. [Google Scholar] [CrossRef]
- Hey, J.; Paulsen, M.; Toth, R.; Weichenhan, D.; Butz, S.; Schatterny, J.; Liebers, R.; Lutsik, P.; Plass, C.; Mall, M.A. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat. Commun. 2021, 12, 6520. [Google Scholar] [CrossRef]
- Van den Bossche, S.; Ostyn, L.; Vandendriessche, V.; Rigauts, C.; De Keersmaecker, H.; Nickerson, C.A.; Crabbé, A. The development and characterization of in vivo-like three-dimensional models of bronchial epithelial cell lines. Eur. J. Pharm. Sci. 2023, 190, 106567. [Google Scholar] [CrossRef] [PubMed]
- Stroulios, G.; Brown, T.; Moreni, G.; Kondro, D.; Dei, A.; Eaves, A.; Louis, S.; Hou, J.; Chang, W.; Pajkrt, D. Apical-out airway organoids as a platform for studying viral infections and screening for antiviral drugs. Sci. Rep. 2022, 12, 7673. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armendariz, A.I.; Heiner, M.; El Agha, E.; Salwig, I.; Hoek, A.; Hessler, M.C.; Shalashova, I.; Shrestha, A.; Carraro, G.; Mengel, J.P. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020, 39, e103476. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.; Kondrateva, E.; Tabakov, V.; Efremova, A.; Salikhova, D.; Bukharova, T.; Goldshtein, D.; Balyasin, M.; Bulatenko, N.; Amelina, E. Airway and Lung Organoids from Human-Induced Pluripotent Stem Cells Can Be Used to Assess CFTR Conductance. Int. J. Mol. Sci. 2023, 24, 6293. [Google Scholar] [CrossRef]
- Miura, A.; Yamada, D.; Nakamura, M.; Tomida, S.; Shimizu, D.; Jiang, Y.; Takao, T.; Yamamoto, H.; Suzawa, K.; Shien, K. Oncogenic potential of human pluripotent stem cell-derived lung organoids with HER2 overexpression. Int. J. Cancer 2021, 149, 1593–1604. [Google Scholar] [CrossRef]
- Chiu, M.C.; Li, C.; Liu, X.; Yu, Y.; Huang, J.; Wan, Z.; Xiao, D.; Chu, H.; Cai, J.-P.; Zhou, B. A bipotential organoid model of respiratory epithelium recapitulates high infectivity of SARS-CoV-2 Omicron variant. Cell Discov. 2022, 8, 57. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Yu, Y.; Wan, Z.; Chiu, M.C.; Liu, X.; Zhang, S.; Cai, J.-P.; Chu, H.; Li, G. Human airway and nasal organoids reveal escalating replicative fitness of SARS-CoV-2 emerging variants. Proc. Natl. Acad. Sci. USA 2023, 120, e2300376120. [Google Scholar] [CrossRef]
- Mulay, A.; Konda, B.; Garcia, G., Jr.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef]
- Konda, B.; Mulay, A.; Yao, C.; Beil, S.; Israely, E.; Stripp, B.R. Isolation and enrichment of human lung epithelial progenitor cells for organoid culture. JoVE (J. Vis. Exp.) 2020, 161, e61541. [Google Scholar]
- Alysandratos, K.-D.; Garcia-de-Alba, C.; Yao, C.; Pessina, P.; Huang, J.; Villacorta-Martin, C.; Hix, O.T.; Minakin, K.; Burgess, C.L.; Bawa, P. Culture impact on the transcriptomic programs of primary and iPSC-derived human alveolar type 2 cells. JCI Insight 2023, 8, e158937. [Google Scholar] [CrossRef]
- Sano, E.; Suzuki, T.; Hashimoto, R.; Itoh, Y.; Sakamoto, A.; Sakai, Y.; Saito, A.; Okuzaki, D.; Motooka, D.; Muramoto, Y. Cell response analysis in SARS-CoV-2 infected bronchial organoids. Commun. Biol. 2022, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; van der Vaart, J.; Knoops, K.; Riesebosch, S.; Breugem, T.I.; Mykytyn, A.Z.; Beumer, J.; Schipper, D.; Bezstarosti, K.; Koopman, C.D. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021, 40, e105912. [Google Scholar] [CrossRef] [PubMed]
- Boecking, C.A.; Walentek, P.; Zlock, L.T.; Sun, D.I.; Wolters, P.J.; Ishikawa, H.; Jin, B.-J.; Haggie, P.M.; Marshall, W.F.; Verkman, A.S. A simple method to generate human airway epithelial organoids with externally orientated apical membranes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L420–L437. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of normal human bronchial epithelial (NHBE) cell 3D cultures for in vitro lung model studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Do, T.N.D.; Donckers, K.; Vangeel, L.; Chatterjee, A.K.; Gallay, P.A.; Bobardt, M.D.; Bilello, J.P.; Cihlar, T.; De Jonghe, S.; Neyts, J. A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents. Antivir. Res. 2021, 192, 105122. [Google Scholar] [CrossRef]
- Alysandratos, K.-D.; Russo, S.J.; Petcherski, A.; Taddeo, E.P.; Acín-Pérez, R.; Villacorta-Martin, C.; Jean, J.; Mulugeta, S.; Rodriguez, L.R.; Blum, B.C. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. 2021, 36, 109636. [Google Scholar] [CrossRef]
- Tindle, C.; Fuller, M.; Fonseca, A.; Taheri, S.; Ibeawuchi, S.-R.; Beutler, N.; Katkar, G.D.; Claire, A.; Castillo, V.; Hernandez, M. Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. eLife 2021, 10, e66417. [Google Scholar] [CrossRef]
- Choi, S.; Choi, S.; Choi, Y.; Cho, N.; Kim, S.-Y.; Lee, C.H.; Park, H.-J.; Oh, W.K.; Kim, K.K.; Kim, E.-M. Polyhexamethylene guanidine phosphate increases stress granule formation in human 3D lung organoids under respiratory syncytial virus infection. Ecotoxicol. Environ. Saf. 2022, 229, 113094. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jeong, S.Y.; Li, Z.; Kim, H.-Y.; Kim, H.-W.; Yoo, M.J.; Jang, H.J.; Kim, D.-K.; Cho, N.; Yoo, H.M. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Zhao, S.; Rong, Z.; Lin, Y. SOX9 inactivation affects the proliferation and differentiation of human lung organoids. Stem Cell Res. Ther. 2021, 12, 343. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Monkhorst, K.; Schipper, L.J.; Hartemink, K.J.; Smit, E.F.; Kaing, S.; de Groot, R.; Wolkers, M.C.; Clevers, H.; Cuppen, E. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 2020, 31, 107588. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Weaver, A.M.; Aloisio, G.M.; Jelinski, J.; Johnson, H.L.; Venable, S.F.; McBride, T.; Aideyan, L.; Piedra, F.-A.; Ye, X. The human nose organoid respiratory virus model: An ex-vivo human challenge model to study RSV and SARS-CoV-2 pathogenesis and evaluate therapeutics. mBio 2021, 13, e0351121. [Google Scholar]
- Kosmider, B.; Mason, R.J.; Bahmed, K. Isolation and characterization of human alveolar type II cells. Lung Innate Immun. Inflamm. Methods Protoc. 2018, 1809, 83–90. [Google Scholar]
- He, Y.; Rofaani, E.; Huang, X.; Huang, B.; Liang, F.; Wang, L.; Shi, J.; Peng, J.; Chen, Y. Generation of Alveolar Epithelium Using Reconstituted Basement Membrane and hiPSC-Derived Organoids. Adv. Healthc. Mater. 2022, 11, 2101972. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 2020, 27, 890–904.e8. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Lew, J.; Goncin, U.; Radomska, A.; Rout, S.S.; Gray, B.E.; Machtaler, S.; Falzarano, D.; Lavender, K.J. SARS-CoV-2 Variant-Specific Infectivity and Immune Profiles Are Detectable in a Humanized Lung Mouse Model. Viruses 2022, 14, 2272. [Google Scholar] [CrossRef]
- Zhang, M.; Ali, G.; Komatsu, S.; Zhao, R.; Ji, H.-L. Prkg2 regulates alveolar type 2-mediated re-alveolarization. Stem Cell Res. Ther. 2022, 13, 111. [Google Scholar] [CrossRef]
- Lee, J.; Baek, H.; Jang, J.; Park, J.; Cha, S.-R.; Hong, S.-H.; Kim, J.; Lee, J.-H.; Hong, I.-S.; Wang, S.-J. Establishment of a human induced pluripotent stem cell derived alveolar organoid for toxicity assessment. Toxicol. Vitr. 2023, 89, 105585. [Google Scholar] [CrossRef]
- Shiraishi, K.; Nakajima, T.; Shichino, S.; Deshimaru, S.; Matsushima, K.; Ueha, S. In vitro expansion of endogenous human alveolar epithelial type II cells in fibroblast-free spheroid culture. Biochem. Biophys. Res. Commun. 2019, 515, 579–585. [Google Scholar] [CrossRef]
- Loebel, C.; Weiner, A.I.; Eiken, M.K.; Katzen, J.B.; Morley, M.P.; Bala, V.; Cardenas-Diaz, F.L.; Davidson, M.D.; Shiraishi, K.; Basil, M.C. Microstructured Hydrogels to Guide Self-Assembly and Function of Lung Alveolospheres. Adv. Mater. 2022, 34, 2202992. [Google Scholar] [CrossRef]
- Kim, J.H.; An, G.H.; Kim, J.Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.H.; Han, C.; Yang, S.R.; Kim, J.H.; et al. Human pluripotent stem-cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Ptasinski, V.; Monkley, S.J.; Öst, K.; Tammia, M.; Alsafadi, H.N.; Overed-Sayer, C.; Hazon, P.; Wagner, D.E.; Murray, L.A. Modeling fibrotic alveolar transitional cells with pluripotent stem cell-derived alveolar organoids. Life Sci. Alliance 2023, 6, e202201853. [Google Scholar] [CrossRef] [PubMed]
- Suezawa, T.; Kanagaki, S.; Moriguchi, K.; Masui, A.; Nakao, K.; Toyomoto, M.; Tamai, K.; Mikawa, R.; Hirai, T.; Murakami, K. Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 2021, 16, 2973–2987. [Google Scholar] [CrossRef]
- Suzuki, T.; Hisata, S.; Fujita, K.; Fujiwara, S.; Liu, F.; Fukushima, N.; Suzuki, T.; Mato, N.; Hagiwara, K. Patient-derived spheroids and patient-derived organoids simulate evolutions of lung cancer. Heliyon 2023, 9, e13829. [Google Scholar]
- Wang, Y.; Liu, M.; Zhang, L.; Liu, X.; Ji, H.; Wang, Y.; Gui, J.; Yue, Y.; Wen, Z. Cancer CD39 drives metabolic adaption and mal-differentiation of CD4+ T cells in patients with non-small-cell lung cancer. Cell Death Dis. 2023, 14, 804. [Google Scholar] [CrossRef]
- Yokota, E.; Iwai, M.; Yukawa, T.; Yoshida, M.; Naomoto, Y.; Haisa, M.; Monobe, Y.; Takigawa, N.; Guo, M.; Maeda, Y. Clinical application of a lung cancer organoid (tumoroid) culture system. NPJ Precis. Oncol. 2021, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Wu, X.; Bos, I.S.T.; Conlon, T.M.; Ansari, M.; Verschut, V.; Van der Koog, L.; Verkleij, L.A.; D’Ambrosi, A.; Matveyenko, A.; Schiller, H.B. A transcriptomics-guided drug target discovery strategy identifies receptor ligands for lung regeneration. Sci. Adv. 2022, 8, eabj9949. [Google Scholar] [CrossRef]
- Bharat, A.; Querrey, M.; Markov, N.S.; Kim, S.; Kurihara, C.; Garza-Castillon, R.; Manerikar, A.; Shilatifard, A.; Tomic, R.; Politanska, Y. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020, 12, eabe4282. [Google Scholar] [CrossRef]
- Miller, C.L.; Allan, J.S.; Madsen, J.C. Novel approaches for long-term lung transplant survival. Front. Immunol. 2022, 13, 931251. [Google Scholar] [CrossRef]
- Hillel-Karniel, C.; Rosen, C.; Milman-Krentsis, I.; Orgad, R.; Bachar-Lustig, E.; Shezen, E.; Reisner, Y. Multi-lineage lung regeneration by stem cell transplantation across major genetic barriers. Cell Rep. 2020, 30, 807–819.e4. [Google Scholar] [CrossRef]
- Yu, C.; Lv, Y.; Li, X.; Bao, H.; Cao, X.; Huang, J.; Zhang, Z. SOD-Functionalized gold nanoparticles as ROS scavenger and CT contrast agent for protection and imaging tracking of mesenchymal stem cells in Idiopathic pulmonary fibrosis treatment. Chem. Eng. J. 2023, 459, 141603. [Google Scholar] [CrossRef]
- Kim, J.; Guenthart, B.; O’Neill, J.D.; Dorrello, N.V.; Bacchetta, M.; Vunjak-Novakovic, G. Controlled delivery and minimally invasive imaging of stem cells in the lung. Sci. Rep. 2017, 7, 13082. [Google Scholar] [CrossRef]
- Guenthart, B.A.; O’Neill, J.D.; Kim, J.; Fung, K.; Vunjak-Novakovic, G.; Bacchetta, M. Cell replacement in human lung bioengineering. J. Heart Lung Transplant. 2019, 38, 215–224. [Google Scholar] [CrossRef]
- Hussein, M.M.; Mokhtar, D.M. The roles of telocytes in lung development and angiogenesis: An immunohistochemical, ultrastructural, scanning electron microscopy and morphometrical study. Dev. Biol. 2018, 443, 137–152. [Google Scholar] [CrossRef]
- Awad, M.; Gaber, W.; Ibrahim, D. Onset of appearance and potential significance of telocytes in the developing fetal lung. Microsc. Microanal. 2019, 25, 1246–1256. [Google Scholar] [CrossRef]
- Bai, H.; Si, L.; Jiang, A.; Belgur, C.; Zhai, Y.; Plebani, R.; Oh, C.Y.; Rodas, M.; Patil, A.; Nurani, A. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 2022, 13, 1928. [Google Scholar] [CrossRef]
- Li, J.; Wen, A.M.; Potla, R.; Benshirim, E.; Seebarran, A.; Benz, M.A.; Henry, O.Y.; Matthews, B.D.; Prantil-Baun, R.; Gilpin, S.E. AAV-mediated gene therapy targeting TRPV4 mechanotransduction for inhibition of pulmonary vascular leakage. APL Bioeng. 2019, 3, 046103. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Yang, F.; Feng, M.; Zhao, Y.; Chen, X.; Mi, J.; Yao, Y.; Guan, D.; Xiao, Z. Biomimetic collagen biomaterial induces in situ lung regeneration by forming functional alveolar. Biomaterials 2020, 236, 119825. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, W.; Zhang, L.; Fan, C.; Qiu, L.; Zhao, Y.; Chen, B.; Chen, Y.; Shen, H.; Dai, J. A novel leptin receptor binding peptide tethered-collagen scaffold promotes lung injury repair. Biomaterials 2022, 291, 121884. [Google Scholar] [CrossRef]
- Söderlund, Z.; Ibáñez-Fonseca, A.; Hajizadeh, S.; Rodríguez-Cabello, J.; Liu, J.; Ye, L.; Tykesson, E.; Elowsson, L.; Westergren-Thorsson, G. Controlled release of growth factors using synthetic glycosaminoglycans in a modular macroporous scaffold for tissue regeneration. Commun. Biol. 2022, 5, 1349. [Google Scholar] [CrossRef]
- Leibel, S.L.; Winquist, A.; Tseu, I.; Wang, J.; Luo, D.; Shojaie, S.; Nathan, N.; Snyder, E.; Post, M. Reversal of surfactant protein B deficiency in patient specific human induced pluripotent stem cell derived lung organoids by gene therapy. Sci. Rep. 2019, 9, 13450. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Kraft, B.D.; Tata, P.R.; Randell, S.H.; Piantadosi, C.A.; Pendergast, A.M. ABL kinase inhibition promotes lung regeneration through expansion of an SCGB1A1+ SPC+ cell population following bacterial pneumonia. Proc. Natl. Acad. Sci. USA 2019, 116, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Nagre, N.; Cong, X.; Ji, H.-L.; Schreiber, J.M.; Fu, H.; Pepper, I.; Warren, S.; Sill, J.M.; Hubmayr, R.D.; Zhao, X. Inhaled TRIM72 protein protects ventilation injury to the lung through injury-guided cell repair. Am. J. Respir. Cell Mol. Biol. 2018, 59, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Hermida, S.; Autilio, C.; Martínez, P.; Bosch, F.; Pérez-Gil, J.; Blasco, M.A. Telomerase treatment prevents lung profibrotic pathologies associated with physiological aging. J. Cell Biol. 2020, 219, e202002120. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.J.; Gardner, Z.J.; Slovik, K.J.; Liberti, D.C.; Estep, K.N.; Yang, W.; Chen, Q.; Santini, G.T.; Perez, J.V.; Root, S. GSK3 inhibition rescues growth and telomere dysfunction in dyskeratosis congenita iPSC-derived type II alveolar epithelial cells. eLife 2022, 11, e64430. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Cardenas-Diaz, F.L.; Kathiriya, J.J.; Morley, M.P.; Carl, J.; Brumwell, A.N.; Katzen, J.; Slovik, K.J.; Babu, A.; Zhou, S. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 2022, 604, 120–126. [Google Scholar] [CrossRef]
- Cao, S.; Feng, H.; Yi, H.; Pan, M.; Lin, L.; Zhang, Y.S.; Feng, Z.; Liang, W.; Cai, B.; Li, Q. Single-cell RNA sequencing reveals the developmental program underlying proximal–distal patterning of the human lung at the embryonic stage. Cell Res. 2023, 33, 421–433. [Google Scholar] [CrossRef]
- Ferdinand, J.R.; Morrison, M.I.; Andreasson, A.; Charlton, C.; Chhatwal, A.K.; Scott, W.E., III; Borthwick, L.A.; Clatworthy, M.R.; Fisher, A.J. Transcriptional analysis identifies potential novel biomarkers associated with successful ex-vivo perfusion of human donor lungs. Clin. Transplant. 2022, 36, e14570. [Google Scholar] [CrossRef]
- Rabata, A.; Fedr, R.; Soucek, K.; Hampl, A.; Koledova, Z. 3D cell culture models demonstrate a role for FGF and WNT signaling in regulation of lung epithelial cell fate and morphogenesis. Front. Cell Dev. Biol. 2020, 8, 574. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.-E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.-K.; Han, N.; Lee, J.-H. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 2020, 27, 366–382.e7. [Google Scholar] [CrossRef] [PubMed]
- Ng-Blichfeldt, J.-P.; de Jong, T.; Kortekaas, R.K.; Wu, X.; Lindner, M.; Guryev, V.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. TGF-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L14–L28. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ng-Blichfeldt, J.-P.; Ota, C.; Ciminieri, C.; Ren, W.; Hiemstra, P.S.; Stolk, J.; Gosens, R.; Königshoff, M. Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells 2020, 38, 1467–1478. [Google Scholar] [PubMed]
- Hyun, S.Y.; Min, H.-Y.; Lee, H.J.; Cho, J.; Boo, H.-J.; Noh, M.; Jang, H.-J.; Lee, H.-J.; Park, C.-S.; Park, J.-S. Ninjurin1 drives lung tumor formation and progression by potentiating Wnt/β-Catenin signaling through Frizzled2-LRP6 assembly. J. Exp. Clin. Cancer Res. 2022, 41, 133. [Google Scholar] [PubMed]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lai, X.; Yang, L.; Ye, F.; Huang, C.; Qiu, Y.; Lin, S.; Pu, L.; Wang, Z.; Huang, W. Asporin promotes TGF-β–induced lung myofibroblast differentiation by facilitating Rab11-dependent recycling of TβRI. Am. J. Respir. Cell Mol. Biol. 2022, 66, 158–170. [Google Scholar] [CrossRef]
- Garcia, A.N.; Casanova, N.G.; Kempf, C.L.; Bermudez, T.; Valera, D.G.; Song, J.H.; Sun, X.; Cai, H.; Moreno-Vinasco, L.; Gregory, T. eNAMPT is a novel damage-associated molecular pattern protein that contributes to the severity of radiation-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 497–509. [Google Scholar] [CrossRef]
- Choi, J.; Jang, Y.J.; Dabrowska, C.; Iich, E.; Evans, K.V.; Hall, H.; Janes, S.M.; Simons, B.D.; Koo, B.-K.; Kim, J. Release of Notch activity coordinated by IL-1β signalling confers differentiation plasticity of airway progenitors via Fosl2 during alveolar regeneration. Nat. Cell Biol. 2021, 23, 953–966. [Google Scholar] [CrossRef]
- Kim, M.N.; Hong, J.Y.; Kim, E.G.; Lee, J.W.; Lee, S.Y.; Kim, K.W.; Shim, H.S.; Lee, C.G.; Elias, J.A.; Lee, Y.J. A novel regulatory role of activated leukocyte cell-adhesion molecule in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 415–427. [Google Scholar] [CrossRef]
- Mitchell, A.; Yu, C.; Zhao, X.; Pearmain, L.; Shah, R.; Hanley, K.P.; Felton, T.; Wang, T. Rapid Generation of Pulmonary Organoids from Induced Pluripotent Stem Cells by Co-Culturing Endodermal and Mesodermal Progenitors for Pulmonary Disease Modelling. Biomedicines 2023, 11, 1476. [Google Scholar] [CrossRef]
- Lim, M.J.; Jo, A.; Kim, S.-W. A novel method for generating induced pluripotent stem cell (iPSC)-derived alveolar organoids: A comparison of their ability depending on iPSC origin. Organoid. 2023, 3, e11. [Google Scholar] [CrossRef]
- Chen, J.H.; Chu, X.P.; Zhang, J.T.; Nie, Q.; Tang, W.F.; Su, J.; Yan, H.H.; Zheng, H.P.; Chen, Z.X.; Chen, X. Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac. Cancer 2020, 11, 2279–2290. [Google Scholar] [CrossRef]
- Hawkins, F.J.; Suzuki, S.; Beermann, M.L.; Barillà, C.; Wang, R.; Villacorta-Martin, C.; Berical, A.; Jean, J.; Le Suer, J.; Matte, T. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell 2021, 28, 79–95.e8. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Ferren, M.; Chen, Y.-W.; Siu, Y.; Makhsous, N.; Rima, B.; Briese, T.; Greninger, A.; Snoeck, H.-W.; Moscona, A. Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung organoids. mBio 2019, 10, e00723-19. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.; Moreira Teixeira, L.; Barata, D.; Tahmasebi Birgani, Z.; King, J.; Van Riet, S.; Pasman, T.; Poot, A.A.; Stamatialis, D.; Rottier, R.J. 3D lung-on-chip model based on biomimetically microcurved culture membranes. ACS Biomater. Sci. Eng. 2022, 8, 2684–2699. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shi, J.; Xu, Z.; Zhang, Y.; Cao, X.; Yu, J.; Li, J.; Xu, S. Identification of solamargine as a cisplatin sensitizer through phenotypical screening in cisplatin-resistant NSCLC organoids. Front. Pharmacol. 2022, 13, 802168. [Google Scholar] [CrossRef] [PubMed]
- van Riet, S.; van Schadewijk, A.; Khedoe, P.P.S.; Limpens, R.W.; Bárcena, M.; Stolk, J.; Hiemstra, P.S.; van der Does, A.M. Organoid-based expansion of patient-derived primary alveolar type 2 cells for establishment of alveolus epithelial Lung-Chip cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L526–L538. [Google Scholar] [CrossRef]
- Jung, O.; Tung, Y.-T.; Sim, E.; Chen, Y.-C.; Lee, E.; Ferrer, M.; Song, M.J. Development of human-derived, three-dimensional respiratory epithelial tissue constructs with perfusable microvasculature on a high-throughput microfluidics screening platform. Biofabrication 2022, 14, 025012. [Google Scholar] [CrossRef]
- Wilkinson, D.C.; Alva-Ornelas, J.A.; Sucre, J.M.; Vijayaraj, P.; Durra, A.; Richardson, W.; Jonas, S.J.; Paul, M.K.; Karumbayaram, S.; Dunn, B. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl. Med. 2017, 6, 622–633. [Google Scholar] [CrossRef]
- Chiu, M.C.; Li, C.; Yu, Y.; Liu, X.; Huang, J.; Wan, Z.; Yuen, K.Y.; Zhou, J. Establishing Bipotential Human Lung Organoid Culture System and Differentiation to Generate Mature Alveolar and Airway Organoids. Bio-protocol 2023, 13, e4657. [Google Scholar] [CrossRef]
| Lung Organoids | Cell Types | Applications | References |
|---|---|---|---|
| AT2 cell-derived organoids iPSC-derived AT2 cells organoids | AT2 cells and MRC5 cells | SARS-CoV-2 infection in vitro model Organoid generations | [78,79,80] |
| Bronchial organoids | Normal bronchial epithelial cells | SARS-CoV-2 infection | [81] |
| Small airway and lung bud tip organoids | Small airway stem cells and fetal lung epithelial bud tips | SARS-CoV-2 infection | [82] |
| Tracheobronchial organoids | Tracheobronchial epithelial cells Cystic fibrosis bronchial epithelial cells | Functional studies | [83] |
| Lung Disease Origin | Type of Lung Organoids | References |
|---|---|---|
| IPF | hPSCs- derived alveolar organoids, iPSC-derived alveolar organoids | [101,102] |
| COPD | Nasopharyngeal and bronchial organoids Alveolar organoids | [15,103] |
| Lung cancer | Small cell carcinoma and adenocarcinoma Primary cancer tissues Patient-derived tumoroid | [104,105,106] |
| Cystic fibrosis | Tracheobronchial epithelial organoids Airway organoids from CF patients | [83,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purev, E.; Bahmed, K.; Kosmider, B. Alveolar Organoids in Lung Disease Modeling. Biomolecules 2024, 14, 115. https://doi.org/10.3390/biom14010115
Purev E, Bahmed K, Kosmider B. Alveolar Organoids in Lung Disease Modeling. Biomolecules. 2024; 14(1):115. https://doi.org/10.3390/biom14010115
Chicago/Turabian StylePurev, Enkhee, Karim Bahmed, and Beata Kosmider. 2024. "Alveolar Organoids in Lung Disease Modeling" Biomolecules 14, no. 1: 115. https://doi.org/10.3390/biom14010115
APA StylePurev, E., Bahmed, K., & Kosmider, B. (2024). Alveolar Organoids in Lung Disease Modeling. Biomolecules, 14(1), 115. https://doi.org/10.3390/biom14010115





