Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins
Abstract
1. Introduction
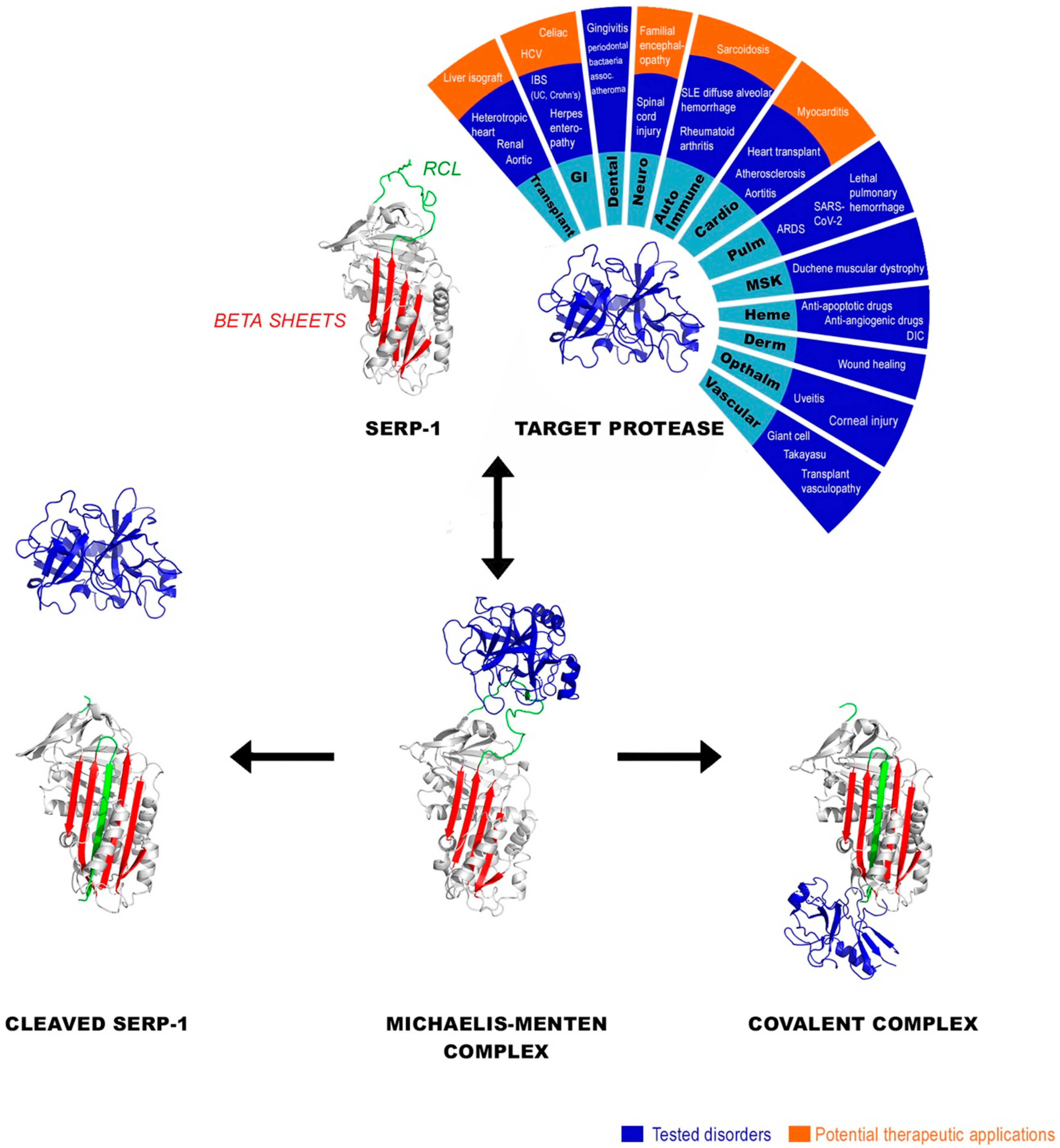
1.1. Serpins—Serine Protease Inhibitors
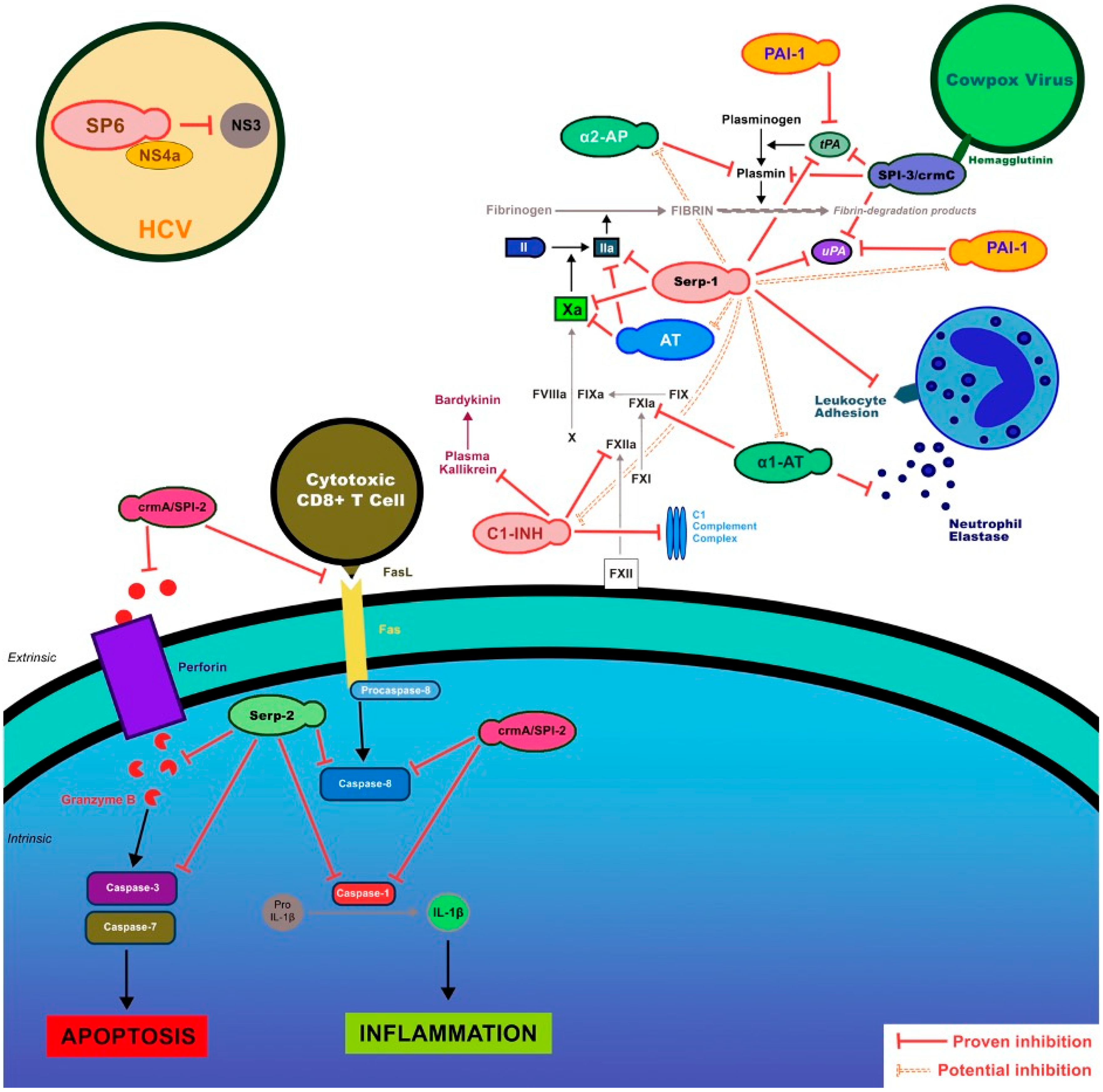
1.2. Serpin Regulation of Coagulation and Immune Responses
| Viral Serpin | Inflammatory Disorder | Target/Outcomes | Subjects Studied | Reference |
|---|---|---|---|---|
| Serp-1 | Atherosclerotic Plaque Acute coronary syndromes with stent implant |
|
| [20,24] |
| Angioplasty injury and intimal plaque restenosis | Efficacy and Mechanism extensively studied | Rabbits, Rats, Mice, Swine/Reduced intimal hyperplasia and inflammation in rodents and rabbits | [20,21,22] | |
| Aortic Allograft Transplant | Established uPAR as central for Serp-1 therapeutic efficacy | Mouse and rat | [21,33,42] | |
| MHV68—Mouse Herpes virus aortitis and lung inflammation | Serp-1 reduced mortality, whereas NSP did not Reduction in monocytes and pro-inflammatory cytokines, reduced lung hemorrhage, NSP not effective | Mice | [26,43] | |
| Carotid compression—Atherosclerotic Plaque | Reduction in carotid arterial inflammation and plaque | Mice | [22] | |
| Giant Cell Arteritis Human Temporal artery biopsy implants | Reduction in monocytes and pro-inflammatory cytokines | SCID Mice | [25] | |
| Ebola infection | Reduced liver damage and improved survival | Mice | [26] | |
| Diffuse Alveolar Hemorrhage (DAH) in Pristane induced Systemic Lupus Erythematosus | Reduced uPAR and Complement and reduced macrophage infiltrates | Mice | [17,44] | |
| SARS-CoV-2 | Reduction in M1 macrophage recruitment in cardiac and pulmonary tissue | Mouse adapted SARS models in C57Bl/6 and BALB/c mice | [39] | |
| Colitis model | Serp-1 reduced mortality | Mice | In preparation | |
| Collagen induced arthritis | Reduced joint swelling without antibodies to Serp-1 treatment | Rats | [28] | |
| Retinal Inflammation—Uveitis Corneal wound healing | Retinal-Intra-vitreal injection of AAV vector expressing Serp-1 Corneal—topical | Mice | [29,45] | |
| Periodontal bacteria with associated increased atherosclerotic plaque | Decreased pro-inflammatory markers and reduced plaque | Mice | [30] | |
| Scar Reduction in Wound Healing | Accelerated wound healing-Improved collagen formation in wound bed Effects blocked by uPAR antibody | Mice | [31] | |
| Transplant Rejection | Rat and Mouse renal, aortic and heterotopic heart allografts Rat to mouse cardiac xenografts | Human, Rat, Mice | [32,33,46,47] | |
| Angiogenesis | Effects blocked by uPAR antibody in wound healing Reduced availability of VEGF | Chicken angiogenesis CAM Model Mouse wound healing model | [31,34] | |
| Pancreatic Cancer subcutaneous implants | Reduced tumor weight for pancreatic cell line implant and reduced macrophage infiltration | Human to Mice cell xenografts in SCID mice | [35] | |
| Spinal Cord Injury Balloon angioplasty crush injury | Improved motor function, reduced inflammation and improved neuronal growth Local infusion | Rats Local infusion | [36,37] | |
| Duchenne Muscular Dystrophy | Reduced leukocyte invasion, Improved myofibril organization Serp-1, PEGSerp-1 | Double knock out DMD Mice | [38] | |
| Serp-2 | Angioplasty injury and intimal hyperplasia | Reduced inflammation and intimal hyperplasia | Mice | [23,40] |
| Carotid cuff compression injury in hyperlipidemic mouse models | Reduction in aortic Atherosclerotic Plaque Development | ApoEnull Mice carotid cuff compression | [40] | |
| Aortic Transplant | Reduced intimal plaque and inflammation Inhibition of Granzyme-B mediated apoptosis | Mouse aortic Allografts (1) WT C57Bl/6 to BALB/c and (2) Granzyme B KO donor allografts | [40] | |
| Liver Transplant | Improved survival, reduced hepatocyte necrosis | Mice | [41] | |
| SPI-1 | Chemotherapy | Multi-pathway inhibitor of apoptosis | In vitro | [48] |
| Viral Host Defense and Vaccination | Mice, in vitro | [48] | ||
| SPI-2 and CrmA | Chemotherapy | Multi-pathway inhibitor of apoptosis | In vitro | [39,40,48] |
| Neurodegeneration | Mice, in vitro | [49] | ||
| Fulminant Liver Failure | Mice, in vitro | [50,51] | ||
| Autoimmune Hepatitis | Reduction in inflammatory cell (CD11), inhibitor of apoptosis | Mice, in vitro | [50,51] | |
| Ischemic-Reperfusion Injury/Chemotherapeutic Cardiotoxicity | Multi-pathway inhibitor of apoptosis | Mice, in vitro | [52,53] | |
| SPI-3 | Hemophilia | Inhibits uPA, plasmin, and tPA | Mice, in vitro–proposed function | [54,55] |
2. Virus-Derived Serpins
2.1. Poxvirus Serpins
- 2-1A).
- Serp-1
- 2.1A)-1.
- Acute and Chronic Inflammatory Diseases
- 2.1A)-1-1.
- Atherosclerotic Disease
- 2.1A)-1-1-1.
- Percutaneous Coronary Intervention (PCI) and Restenosis in Unstable Atherosclerotic Plaque—preclinical testing
- 2.1A)-1-1-2.
- Periodontal Disease and Associated Atherosclerotic Plaque Development
- 2.1A)-1-1-3.
- Acute Coronary Syndrome (ACS)—Clinical Trial
- 2.1A)-1-2.
- Inflammatory Vasculitis Syndromes—Giant Cell Arteritis and Takayasu Disease
- 2.1A)-1-2-1.
- Giant Cell Arteritis
- 2.1A)-1-2-2.
- Mouse Gamma Herpes Viral Infection (MHV68)
- 2.1A)-1-3.
- Autoimmune Disorders
- 2.1A)-1-3-1.
- Transplant Rejection
- 2.1A)-1-3-2.
- Rheumatoid Arthritis
- 2.1A)-1-4.
- Systemic Lupus Erythematosus and Diffuse Alveolar Hemorrhage (DAH)
- 2.1A)-1-5.
- Neurological, Musculoskeletal and Ophthalmological Disorders
- 2.1A)-1-5-1.
- Spinal Cord Injury
- 2.1A)-1-5-2.
- Duchenne Muscular Dystrophy
- 2.1A)-1-5-3.
- Retinal Inflammation
- 2.1A)-1-6.
- Wound Healing
- 2.1A)-1-7.
- Cancer Therapeutics
- 2.1A)-1-8.
- Severe Acute Respiratory Distress syndromes, SARS-CoV-2 Infection
- 2-1B).
- Serp-2 and CrmA
- 2-1B)-1.
- Atherosclerotic Plaque and Restenosis following Angioplasty Injury
- 2-1B)-1-2.
- Transplant Rejection
- 2-1B)-1-2-1.
- Intimal Plaque Hyperplasia in Aortic Transplant
- 2-1B)-1-2-2.
- Ischemia-Reperfusion Injury in Liver Transplant
- 2-1C).
- Orthopoxviral Serpins Spi1, Spi2 and Spi3
- 2-1C)-1.
- Spi1 and Spi2
- 2.1C)-2.
- Spi2/CrmA
- 2.1C)-2-3.
- Spi3
- 2.1C)-2-3-1.
- Inflammation and Vascular Permeability
- 2-2).
- Herpesviral serpins
- 2-3).
- Plant Viral Serpins
- 2-4).
- Arthropod Serpins and Athropod-Viral Serpins—Baculovirus Serpins
2.2. Nucleopolyhedroviral Serpins
2.3. Rice Stripe Virus Serpins (RSV)
3. Modifying Viral Serpins—New Therapeutic Constructs
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Maas, C.; de Maat, S. Therapeutic SERPINs: Improving on Nature. Front. Cardiovasc. Med. 2021, 8, 648349. [Google Scholar] [CrossRef] [PubMed]
- Spence, M.A.; Mortimer, M.D.; Buckle, A.M.; Minh, B.Q.; Jackson, C.J. A Comprehensive Phylogenetic Analysis of the Serpin Superfamily. Mol. Biol. Evol. 2021, 38, 2915–2929. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.C.; Geiger, M.; Sheffield, W.P.; Irving, J.A.; Lomas, D.A.; Song, S.; Satyanarayanan, R.S.; Zhang, L.; McFadden, G.; Lucas, A.R. The under-appreciated world of the serpin family of serine proteinase inhibitors. EMBO Mol. Med. 2023, 15, e17144. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Anticoagulant SERPINs: Endogenous Regulators of Hemostasis and Thrombosis. Front. Cardiovasc. Med. 2022, 9, 878199. [Google Scholar] [CrossRef]
- Lomas, D.A.; Evans, D.L.; Finch, J.T.; Carrell, R.W. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 1992, 357, 605–607. [Google Scholar] [CrossRef]
- Lomas, D.A.; Carrell, R.W. Serpinopathies and conformational dementias. Nat. Rev. Genet. 2002, 3, 759–768. [Google Scholar] [CrossRef]
- Yamasaki, M.; Sendall, T.J.; Pearce, M.C.; Whisstock, J.C.; Huntington, J.A. Molecular basis of α1-antitrypsin deficiency revealed by the structure of a domain-swapped trimer. EMBO Rep. 2011, 12, 1011–1017. [Google Scholar] [CrossRef]
- Faull, S.V.; Elliston, E.L.K.; Gooptu, B.; Jagger, A.M.; Aldobiyan, I.; Redzej, A.; Badaoui, M.; Heyer-Chauhan, N.; Rashid, S.T.; Reynolds, G.M.; et al. The structural basis for Z α1-antitrypsin polymerization in the liver. Sci. Adv. 2020, 6, eabc1370. [Google Scholar] [CrossRef]
- Crowther, D.C.; Belorgey, D.; Miranda, E.; Kinghorn, K.J.; Sharp, L.K.; Lomas, D.A. Practical genetics: Alpha-1-antitrypsin deficiency and the serpinopathies. Eur. J. Hum. Genet. 2004, 12, 167–172. [Google Scholar] [CrossRef]
- Davis, R.L.; Shrimpton, A.E.; Carrell, R.W.; Lomas, D.A.; Gerhard, L.; Baumann, B.; Lawrence, D.A.; Yepes, M.; Kim, T.S.; Ghetti, B.; et al. Association between conformational mutations in neuroserpin and onset and severity of dementia. Lancet 2002, 359, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; López-Lera, A.; Ghannam, A.; López-Trascasa, M.; Cichon, S.; Ponard, D.; Parsopoulou, F.; Grombirikova, H.; Freiberger, T.; Rijavec, M.; et al. SERPING1 Variants and C1-INH Biological Function: A Close Relationship With C1-INH-HAE. Front. Allergy 2022, 3, 835503. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.C.; Beaulieu, L.M.; Huntington, J.A.; Church, F.C. Serpins in thrombosis, hemostasis and fibrinolysis. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 102–115. [Google Scholar] [CrossRef] [PubMed]
- Inthorn, D.; Hoffmann, J.N.; Hartl, W.H.; Mühlbayer, D.; Jochum, M. Antithrombin III supplementation in severe sepsis: Beneficial effects on organ dysfunction. Shock 1997, 8, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T. Antithrombin concentrate use in sepsis-associated disseminated intravascular coagulation: Re-evaluation of a ‘pendulum effect’ drug using a nationwide database. J. Thromb. Haemost. 2018, 16, 458–461. [Google Scholar] [CrossRef]
- Antalis, T.M.; La Linn, M.; Donnan, K.; Mateo, L.; Gardner, J.; Dickinson, J.L.; Buttigieg, K.; Suhrbier, A. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. J. Exp. Med. 1998, 187, 1799–1811. [Google Scholar] [CrossRef]
- Guo, Q.; Yaron, J.R.; Wallen, J.W.; Browder, K.F.; Boyd, R.; Olson, T.L.; Burgin, M.; Ulrich, P.; Aliskevich, E.; Schutz, L.N.; et al. PEGylated Serp-1 Markedly Reduces Pristane-induced Experimental Diffuse Alveolar Hemorrhage, Altering uPAR Distribution and Macrophage Invasion. Front. Cardiovasc. Med. 2021, 8, 633212. [Google Scholar] [CrossRef]
- Viswanathan, K.; Liu, L.; Vaziri, S.; Dai, E.; Richardson, J.; Togonu-Bickersteth, B.; Vatsya, P.; Christov, A.; Lucas, A.R. Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 2006, 95, 499–510. [Google Scholar] [CrossRef]
- Viswanathan, K.; Richardson, J.; Togonu-Bickersteth, B.; Dai, E.; Liu, L.; Pracha Vatsya Sun, Y.; Yu, J.; Munuswamy-Ramanujamg Baker, H.; Lucas, A.R. Myxoma viral serpin, Serp-1, inhibits human monocyte adhesion through regulation of actin-binding protein filamin B. J. Leukoc. Biol. 2009, 85, 418–426. [Google Scholar] [CrossRef]
- Lucas, A.; Liu, L.; Macen, J.; Nash, P.; Dai, E.; Stewart, M.; Graham, K.; Etches, W.; Boshkov, L.; Nation, P.N.; et al. Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation 1996, 94, 2890–2900. [Google Scholar] [CrossRef]
- Dai, E.; Guan, H.; Liu, L.; Little, S.; McFadden, G.; Vaziri, S.; Cao, H.; Ivanova, I.; Bocksch, L.; Lucas, A. Serp-1, a Viral Anti-inflammatory Serpin, Regulates Cellular Serine Proteinase and Serpin Responses to Vascular Injury. J. Biol. Chem. 2003, 278, 18563–18572. [Google Scholar] [CrossRef] [PubMed]
- Bot, I.; von der Thüsen, J.H.; Donners, M.M.; Lucas, A.; Fekkes, M.L.; de Jager, S.C.; Kuiper, J.; Daemen, M.J.; van Berkel, T.J.; Heeneman, S.; et al. Serine protease inhibitor Serp-1 strongly impairs atherosclerotic lesion formation and induces a stable plaque phenotype in ApoE-/-mice. Circ. Res. 2003, 93, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Davids, J.; Fife, N.; Lucas, A. Serp-1 and Serp-2, Viral Anti-inflammatory Proteins, Modulate Signaling in Human Monocytes. In Proceedings of the 17th World Congress on Heart Disease, Toronto, ON, Canada, 27–30 July 2012; pp. 39–42. [Google Scholar]
- Tardif, J.C.; L’Allier, P.L.; Grégoire, J.; Ibrahim, R.; McFadden, G.; Kostuk, W.; Knudtson, M.; Labinaz, M.; Waksman, R.; Pepine, C.J.; et al. A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2010, 3, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, D.; Ambadapadi, S.; Davids, J.; Ryden, S.; Samy, H.; Bartee, M.; Sobel, E.; Dai, E.; Liu, L.; et al. Serpin treatment suppresses inflammatory vascular lesions in temporal artery implants (TAI) from patients with giant cell arteritis. PLoS ONE 2015, 10, e0115482. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Abbott, J.; Liu, L.; Bartee, M.Y.; Long, M.; Davids, J.; Williams, J.; Feldmann, H.; Strong, J.; et al. Myxomavirus-derived serpin prolongs survival and reduces inflammation and hemorrhage in an unrelated lethal mouse viral infection. Antimicrob. Agents Chemother. 2013, 57, 4114–4127. [Google Scholar] [CrossRef]
- Yaron, J.R.; Ambadapadi, S.; Zhang, L.; Chavan, R.N.; Tibbetts, S.A.; Keinan, S.; Varsani, A.; Maldonado, J.; Kraberger, S.; Tafoya, A.M.; et al. Immune protection is dependent on the gut microbiome in a lethal mouse gammaherpesviral infection. Sci. Rep. 2020, 10, 2371. [Google Scholar] [CrossRef]
- Brahn, E.; Lee, S.; Lucas, A.; McFadden, G.; Macaulay, C. Suppression of collagen-induced arthritis with a serine proteinase inhibitor (serpin) derived from myxoma virus. Clin. Immunol. 2014, 153, 254–263. [Google Scholar] [CrossRef]
- White, V.; Ambadapadi, S.; Amontree, M.; Bowman, B.; Ildefesono, C.; Lucas, A.R.; McFadden, G.; Lewin, A. Development of SERP1 and SERP2 anti-inflammatory serpins from Myxoma virus for use in gene therapy for ocular inflammation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1929. [Google Scholar]
- Lucas, A.R.; Verma, R.K.; Dai, E.; Liu, L.; Chen, H.; Kesavalu, S.; Rivera, M.; Velsko, I.; Ambadapadi, S.; Chukkapalli, S.; et al. Myxomavirus anti-inflammatory chemokine binding protein reduces the increased plaque growth induced by chronic Porphyromonas gingivalis oral infection after balloon angioplasty aortic injury in mice. PLoS ONE 2014, 9, e111353. [Google Scholar] [CrossRef]
- Zhang, L.; Yaron, J.R.; Tafoya, A.M.; Wallace, S.E.; Kilbourne, J.; Haydel, S.; Rege, K.; McFadden, G.; Lucas, A.R. A Virus-Derived Immune Modulating Serpin Accelerates Wound Closure with Improved Collagen Remodeling. J. Clin. Med. 2019, 8, 1626. [Google Scholar] [CrossRef]
- Zhang, Z.; Bédard, E.; Luo, Y.; Wang, H.; Deng, S.; Kelvin, D.; Zhong, R. Animal models in xenotransplantation. Expert. Opin. Investig. Drugs 2000, 9, 2051–2068. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Dai, E.; Nash, P.; Liu, L.; Icton, C.; Klironomos, D.; Fan, L.; Nation, P.N.; Zhong, R.; McFadden, G.; et al. Inhibition of transplant vasculopathy in a rat aortic allograft model after infusion of anti-inflammatory viral serpin. Circulation 2000, 101, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Liu, L.; Dunphy, L.; Wong, D.; Sun, Y.; Viswanathan, K.; Singh, G.; Lucas, A. Viral serpin, Serp-1, inhibits endogenous angiogenesis in the chicken chorioallantoic membrane model. Cardiovasc. Pathol. 2007, 16, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, H.; Bartee, M.Y.; Williams, J.; Davids, J.A.; Lomas, D.A.; McFadden, G.; Lucas, A.R. Myxomaviral Anti-Inflammatory Serpin Reduces Myeloid-Derived Suppressor Cells and Human Pancreatic Cancer Cell Growth in Mice. J. Cancer Sci. Ther. 2013, 5, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, J.M.; Dabrowski, W.; Marzec-Kotarska, B.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Lucas, A.R. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019, 57, 41–50. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; Dabrowski, W.; Kwiecien-Delaney, B.J.; Kwiecien-Delaney, C.J.; Siwicka-Gieroba, D.; Yaron, J.R.; Zhang, L.; Delaney, K.H.; Lucas, A.R. Neuroprotective Effect of Subdural Infusion of Serp-1 in Spinal Cord Trauma. Biomedicines 2020, 8, 372. [Google Scholar] [CrossRef]
- Andre, A.B.; Zhang, L.; Nix, J.D.; Elmadbouly, N.; Lucas, A.R.; Wilson-Rawls, J.; Rawls, A. Myxomavirus Serp-1 Protein Ameliorates Inflammation in a Mouse Model of Duchenne Muscular Dystrophy. Biomedicines 2022, 10, 1154. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Kibler, K.; Kraberger, S.; Varsani, A.; Turk, J.; Elmadbouly, N.; Aliskevich, E.; Spaccarelli, L.; Estifanos, B.; et al. Treatment with anti-inflammatory viral serpin modulates immuno-thrombotic responses and improves outcomes in SARS-CoV-2 infected mice. EMBO Mol. Med. 2023, e17376. [Google Scholar] [CrossRef]
- Viswanathan, K.; Bot, I.; Liu, L.; Dai, E.; Turner, P.C.; Togonu-Bickersteth, B.; Richardson, J.; Davids, J.A.; Williams, J.M.; Bartee, M.Y.; et al. Viral cross-class serpin inhibits vascular inflammation and T lymphocyte fratricide; a study in rodent models in vivo and human cell lines in vitro. PLoS ONE 2012, 7, e44694. [Google Scholar] [CrossRef]
- Yaron, J.R.; Chen, H.; Ambadapadi, S.; Zhang, L.; Tafoya, A.M.; Munk, B.H.; Wakefield, D.N.; Fuentes, J.; Marques, B.J.; Harripersaud, K.; et al. Serp-2, a virus-derived apoptosis and inflammasome inhibitor, attenuates liver ischemia-reperfusion injury in mice. J. Inflamm. 2019, 16, 12. [Google Scholar] [CrossRef]
- Dai, E.; Viswanathan, K.; Sun, Y.M.; Li, X.; Liu, L.; Togonu-Bickersteth, B.; Richardson, J.; Macaulay, C.; Nash, P.; Turner, P.; et al. Identification of myxomaviral serpin reactive site loop sequences that regulate innate immune responses. J. Biol. Chem. 2006, 281, 8041–8050. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.; Ambadapadi, S.; Yaron, J.; Lomelino, C.; Pinard, M.; Keinan, S.; Kurnikov, I.; Macaulay, C.; Zhang, L.; Reeves, W.; et al. Crystal structure of Serp-1, a Myxomavirus-derived immune modulating Serpin; structural design of Serpin reactive center loop (RCL) peptides with improved therapeutic function. Biochemistry 2018, 57, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, S.; Lu, L.; Reeves, W.H. Myxomavirus serpin alters macrophage function and prevents diffuse alveolar hemorrhage in pristane-induced lupus. Clin. Immunol. 2021, 229, 108764. [Google Scholar] [CrossRef]
- Ju, B.; Guo, O.; Benissan-Messan, D.Z.; Shawver, M.H.; Chen, P.; Geng, B.; Wei, S.; Yaron, J.R.; Lucas, A.R.; Zhu, H. Serp-1 Promotes Corneal Wound Healing by Facilitating Re-epithelialization and Inhibiting Fibrosis and Angiogenesis. Front. Cardiovasc. Med. 2021, 8, 649124. [Google Scholar] [CrossRef] [PubMed]
- Bédard, E.L.; Jiang, J.; Arp, J.; Qian, H.; Wang, H.; Guan, H.; Liu, L.; Parry, N.; Kim, P.; Garcia, B.; et al. Prevention of chronic renal allograft rejection by SERP-1 protein. Transplantation 2006, 81, 908–914. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; McFadden, G.; Garcia, B.; Lucas, A.R.; Zhong, R. Serp-1, a viral anti-inflammatory serpin, attenuates acute xenograft rejection in a rat-to-mouse cardiac transplantation model. Xenotransplant 2003, 10, 506. [Google Scholar]
- Macen, J.L.; Garner, R.S.; Musy, P.Y.; Brooks, M.A.; Turner, P.C.; Moyer, R.W.; McFadden, G.; Bleackley, R.C. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 9108–9113. [Google Scholar] [CrossRef]
- Roy, M.; Sapolsky, R.M. The neuroprotective effects of virally-derived caspase inhibitors p35 and crmA following a necrotic insult. Neurobiol. Dis. 2003, 14, 1–9. [Google Scholar] [CrossRef]
- Li, X.K.; Fujino, M.; Guo, L.; Okuyama, T.; Funeshima, N.; Hashimoto, M.; Okabe, K.; Yaginuma, H.; Mikoshiba, K.; Enosawa, S.; et al. Inhibition of Fas-mediated fulminant hepatitis in CrmA gene-transfected mice. Biochem. Biophys. Res. Commun. 2000, 273, 101–109. [Google Scholar] [CrossRef]
- Fujino, M.; Kawasaki, M.; Funeshima, N.; Kitazawa, Y.; Kosuga, M.; Okabe, K.; Hashimoto, M.; Yaginuma, H.; Mikoshiba, K.; Okuyama, T.; et al. CrmA gene expression protects mice against concanavalin-A-induced hepatitis by inhibiting IL-18 secretion and hepatocyte apoptosis. Gene Ther. 2003, 10, 1781–1790. [Google Scholar] [CrossRef]
- Krautwald, S.; Ziegler, E.; Rölver, L.; Linkermann, A.; Keyser, K.A.; Steen, P.; Wollert, K.C.; Korf-Klingebiel, M.; Kunzendorf, U. Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J. Biol. Chem. 2010, 285, 19997–20005. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Siu, P.M.; Choudhury, S.; Ke, Q.; Choi, J.H.; Koh, Y.Y.; Kang, P.M. Delayed activation of caspase-independent apoptosis during heart failure in transgenic mice overexpressing caspase inhibitor CrmA. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H1374–H1381. [Google Scholar] [CrossRef]
- Turner, P.C.; Baquero, M.T.; Yuan, S.; Thoennes, S.R.; Moyer, R.W. The cowpox virus serpin SPI-3 complexes with and inhibits urokinase-type and tissue-type plasminogen activators and plasmin. Virology 2000, 272, 267–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brum, L.M.; Turner, P.C.; Devick, H.; Baquero, M.T.; Moyer, R.W. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 2003, 306, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Macen, J.L.; Upton, C.; Nation, N.; McFadden, G. SERP1, a serine proteinase inhibitor encoded by myxoma virus, is a secreted glycoprotein that interferes with inflammation. Virology 1993, 195, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Lomas, D.A.; Evans, D.L.; Upton, C.; McFadden, G.; Carrell, R.W. Inhibition of plasmin, urokinase, tissue plasminogen activator, and C1S by a myxoma virus serine proteinase inhibitor. J. Biol. Chem. 1993, 268, 516–521. [Google Scholar] [CrossRef]
- Davids, J.A.; Dai, E.; Chen, H.; Bartee, M.Y.; Liu, L.; Fortunel, A.; Moyer, R.; McFadden, G.; Lucas, A.R. Viral anti-inflammatory proteins target diverging immune pathways with converging effects on arterial dilatation, plaque, and apoptosis. Eur. J. Inflamm. 2014, 12, 131–145. [Google Scholar] [CrossRef]
- Infante, J.R.; Dees, E.C.; Olszanski, A.J.; Dhuria, S.V.; Sen, S.; Cameron, S.; Cohen, R.B. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 3103–3110. [Google Scholar] [CrossRef]
- Brooks, M.A.; Ali, A.N.; Turner, P.C.; Moyer, R.W. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J. Virol. 1995, 69, 7688–7698. [Google Scholar] [CrossRef]
- Legrand, F.A.; Verardi, P.H.; Jones, L.A.; Chan, K.S.; Peng, Y.; Yilma, T.D. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J. Virol. 2004, 78, 2770–2779. [Google Scholar] [CrossRef]
- Nathaniel, R.; MacNeill, A.L.; Wang, Y.X.; Turner, P.C.; Moyer, R.W. Cowpox virus CrmA, Myxoma virus SERP2 and baculovirus P35 are not functionally interchangeable caspase inhibitors in poxvirus infections. J. Gen. Virol. 2004, 85 Pt 5, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.E.; Rosen, A.; Veliuona, M.A.; Clem, R.J.; Levine, B.; Hardwick, J.M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J. Virol. 1998, 72, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.C.; Scotch, M.; MacIntyre, C.R. Bayesian Phylogeography and Pathogenic Characterization of Smallpox Based on HA, ATI, and CrmB Genes. Mol. Biol. Evol. 2018, 35, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Turner, P.C.; Ness, T.L.; Moon, K.B.; Schoeb, T.R.; Moyer, R.W. The cowpox virus SPI-3 and myxoma virus SERP1 serpins are not functionally interchangeable despite their similar proteinase inhibition profiles in vitro. Virology 2000, 272, 281–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simas, J.P.; Bowden, R.J.; Paige, V.; Efstathiou, S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J. Gen. Virol. 1998, 79 Pt 1, 149–153. [Google Scholar] [CrossRef]
- Lundberg, P.; Welander, P.; Han, X.; Cantin, E. Herpes simplex virus type 1 DNA is immunostimulatory in vitro and in vivo. J. Virol. 2003, 77, 11158–11169. [Google Scholar] [CrossRef]
- Evtushenko, E.A.; Ryabchevskaya, E.M.; Nikitin, N.A.; Atabekov, J.G.; Karpova, O.V. Plant virus particles with various shapes as potential adjuvants. Sci. Rep. 2020, 10, 10365. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Zhao, W.; Liu, Q.; Li, Q.; Lu, L.; Liu, R.; Zhang, X.; Cui, F. Rice stripe virus-derived siRNAs play different regulatory roles in rice and in the insect vector Laodelphax striatellus. BMC Plant Biol. 2018, 18, 219. [Google Scholar] [CrossRef]
- Ardisson-Araujo, D.M.P.; Rohrmann, G.F.; Ribeiro, B.M.; Clem, R.J. Functional characterization of hesp018, a baculovirus-encoded serpin gene. J. Gen. Virol. 2015, 96 Pt 5, 1150–1160. [Google Scholar] [CrossRef]
- Aymonnier, K.; Kawecki, C.; Arocas, V.; Boulaftali, Y.; Bouton, M.C. Serpins, New therapeutic targets for Hemophilia. Thromb. Haemost. 2021, 121, 261–269. [Google Scholar] [CrossRef]
- Dai, E.; Liu, L.Y.; Wang, H.; McIvor, D.; Sun, Y.M.; Macaulay, C.; King, E.; Munuswamy-Ramanujam, G.; Bartee, M.M.; Charo, I.; et al. Chemokine: Glycosaminoglycan Interaction is a Pivotal Regulatory Step in Transplant Vascular Inflammation and Disease. PLoS ONE 2010, 5, e10510. [Google Scholar]
- Bartee, M.Y.; Chen, H.; Dai, E.; Liu, L.Y.; Davids, J.A.; Lucas, A. Defining the anti-inflammatory activity of a potent myxomaviral chemokine modulating protein, M-T7, through site directed mutagenesis. Cytokine 2014, 65, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Stuart, G.S.; Jones, N.C.; Fleming, S.B.; Mercer, A.A. Orf Virus IL-10 and VEGF-E Act Synergistically to Enhance Healing of Cutaneous Wounds in Mice. J. Clin. Med. 2020, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varkoly, K.; Beladi, R.; Hamada, M.; McFadden, G.; Irving, J.; Lucas, A.R. Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins. Biomolecules 2023, 13, 1393. https://doi.org/10.3390/biom13091393
Varkoly K, Beladi R, Hamada M, McFadden G, Irving J, Lucas AR. Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins. Biomolecules. 2023; 13(9):1393. https://doi.org/10.3390/biom13091393
Chicago/Turabian StyleVarkoly, Kyle, Roxana Beladi, Mostafa Hamada, Grant McFadden, James Irving, and Alexandra R. Lucas. 2023. "Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins" Biomolecules 13, no. 9: 1393. https://doi.org/10.3390/biom13091393
APA StyleVarkoly, K., Beladi, R., Hamada, M., McFadden, G., Irving, J., & Lucas, A. R. (2023). Viral SERPINS—A Family of Highly Potent Immune-Modulating Therapeutic Proteins. Biomolecules, 13(9), 1393. https://doi.org/10.3390/biom13091393






