Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mouse Model of Aripiprazole and Trazodone Exposure
2.3. Tissue Collection and Preparation for Sterol and Medications Measurements
2.4. (N,N-Dimethylglycyl) DMG Ester Derivatization Method for Sterol Measurements and LC-MS/MS Analysis
2.5. Immunohistochemistry
2.6. Total RNA Extraction, cDNA Synthesis, and qPCR Analysis
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
3.1. ARI, TRZ, and Metabolites Measurements in Serum and Tissues
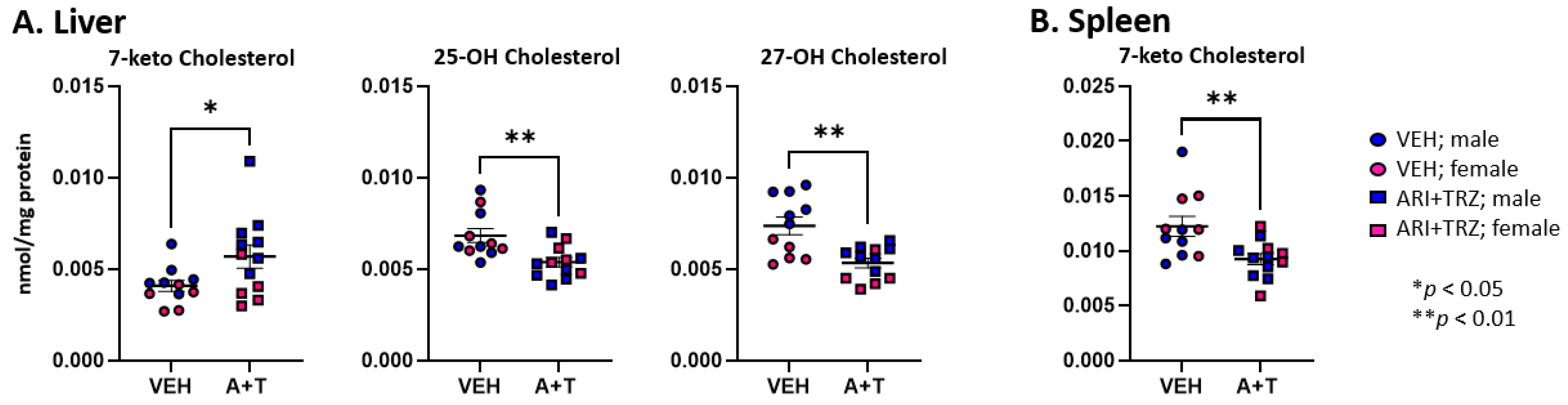
3.2. ARI+TRZ Polypharmacy Alters the Cholesterol Synthesis Pathway in Serum, Liver, and Spleen
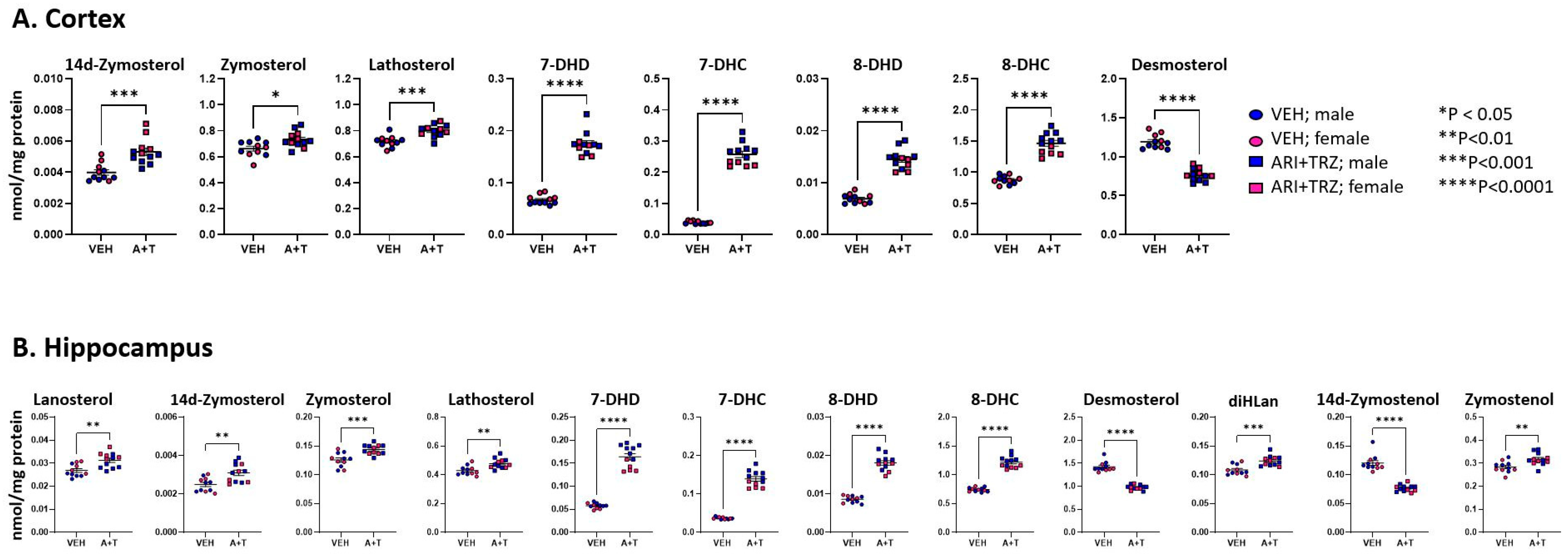
3.3. ARI+TRZ Polypharmacy Alters Cholesterol Synthesis Pathway in the Brain
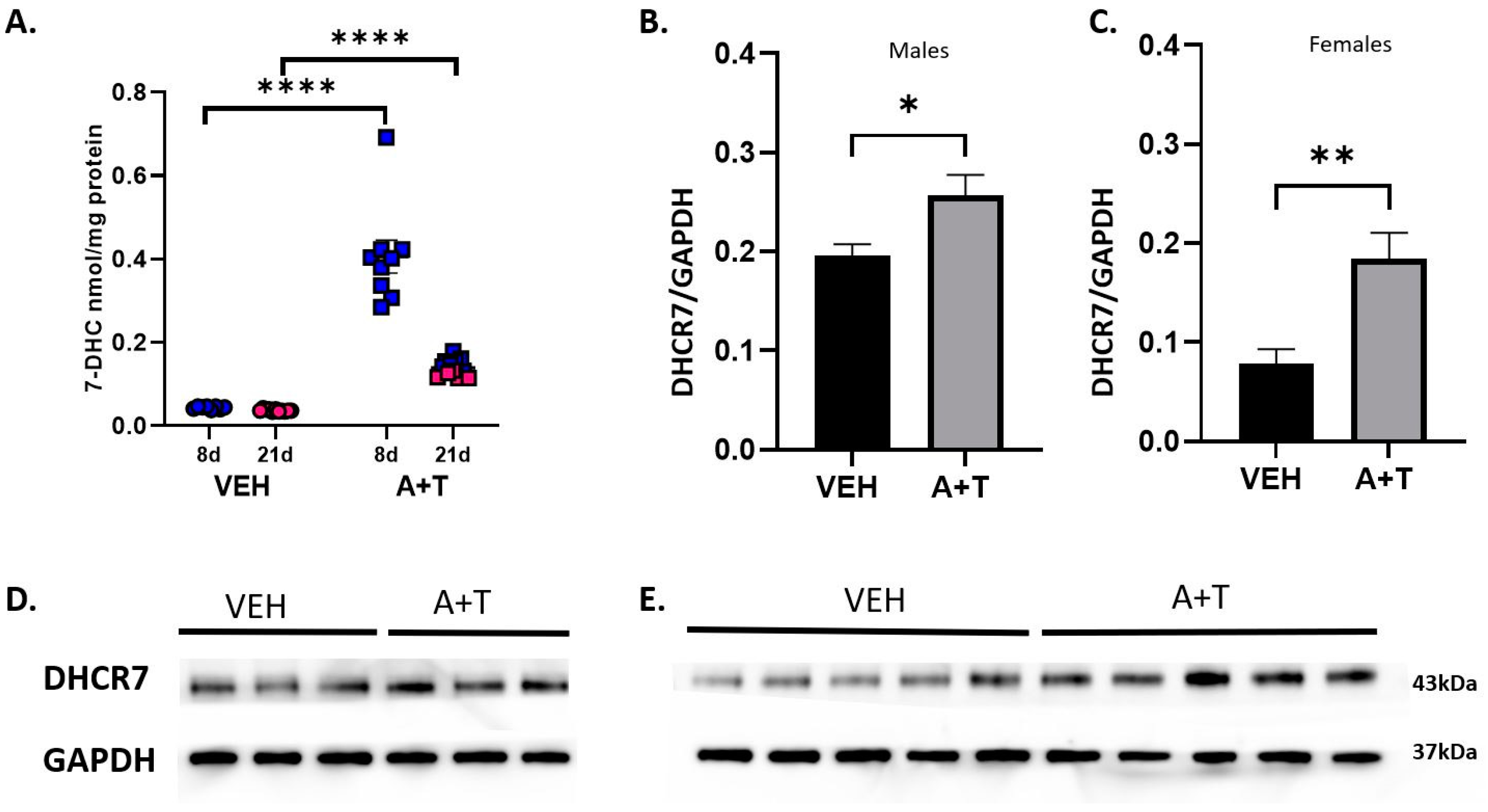
3.4. Long-Term ARI+TRZ Polypharmacy Increases DHCR7 Enzyme Expression in the Brain
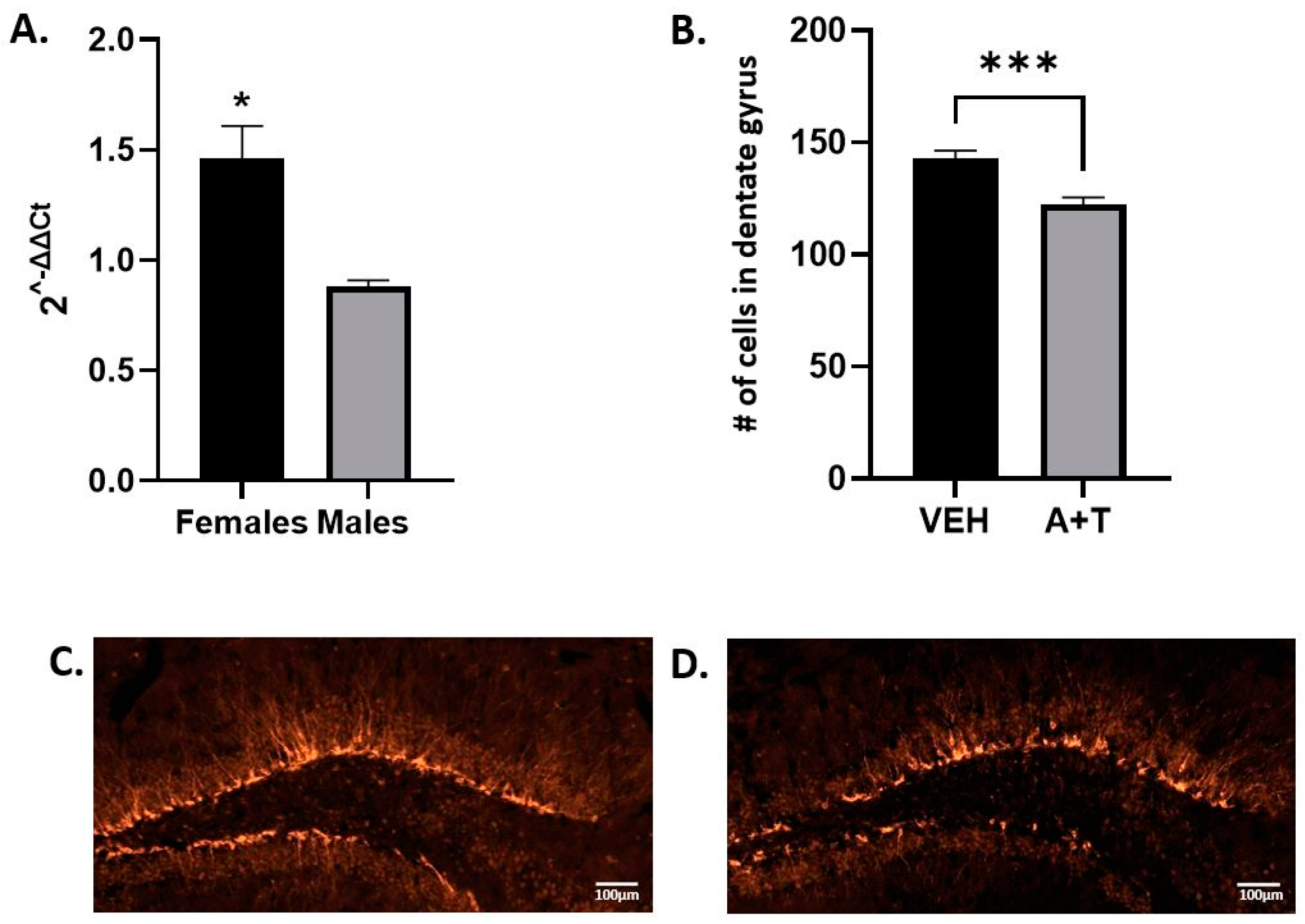
3.5. ARI+TRZ Polypharmacy Effects on Hippocampal Neurogenesis
3.6. ARI+TRZ Polypharmacy Decreases IBA1 Protein Levels in the Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-DHC | 7-dehydrocholesterol |
| ARI | aripiprazole |
| BHT | butylated hydroxytoluene |
| CHOL | cholesterol |
| DCX | doublecortin |
| DES | desmosterol |
| DHCEO | 3β,5α-dihydroxycholest-7-en-6-one |
| Dhcr7 or DHCR7 | 7-dehydrocholesterol reductase |
| DHCR24 | 24-dehydrocholesterol reductase |
| IBA1 | ionized calcium-binding adaptor molecule 1 |
| LAN | lanosterol |
| SLOS | Smith-Lemli-Opitz syndrome |
| SRM | selective reaction monitoring |
| TRZ | trazodone |
| 8-DHC | 8-dehydrocholesterol |
| Lath | lathosterol |
| Zym | zymosterol |
| Zyme | zymostenol |
| 7-DHD | 7-dehydrodesmosterol |
| 8-DHD | 8-dehydrodesmosterol |
| 14d-Zym | 14d-zymosterol |
| DHL | dehydrolathosterol |
| 14d-Zyme | 14d-zymostenol |
| diHLan | dihydrolanosterol |
| 7keto-Chol | 7ketocholesterol |
| 25OH-Chol | 25-hydroxycholesterol |
| β epChol | beta epoxycholesterol |
| α epChol | alpha epoxycholesterol |
| DHCAO | DHCAO |
| 24OH-Chol | 24-hydroxycholesterol |
| 27OH-Chol | 27-hydroxycholesterol |
| d-ARI | dehydroaripiprazole |
| 2,3-DCPP | 2,3-dichlorophenylpiperazine |
| m-CPP | meta-chlorophenylpiperazine |
References
- Quinn, K.J.; Shah, N.H. A dataset quantifying polypharmacy in the United States. Sci. Data 2017, 4, 170167. [Google Scholar] [CrossRef]
- Kukreja, S.; Kalra, G.; Shah, N.; Shrivastava, A. Polypharmacy in psychiatry: A review. Mens. Sana Monogr. 2013, 11, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.K. Antipsychotic Polypharmacy: A Dirty Little Secret or a Fashion? Int. J. Neuropsychopharmacol. 2020, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Marcum, Z.A.; Schmader, K.E.; Hanlon, J.T. Update on Medication Use Quality and Safety in Older Adults, 2017. J. Am. Geriatr. Soc. 2018, 66, 2254–2258. [Google Scholar] [CrossRef]
- Lohr, W.D.; Jawad, K.; Feygin, Y.; Le, J.; Creel, L.; Pasquenza, N.; Williams, P.G.; Jones, V.F.; Myers, J.; Davis, D.W. Antipsychotic Medications for Low-Income Preschoolers: Long Duration and Psychotropic Medication Polypharmacy. Psychiatr. Serv. 2022, 73, 510–517. [Google Scholar] [CrossRef]
- Mojtabai, R.; Olfson, M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch. Gen. Psychiatry 2010, 67, 26–36. [Google Scholar] [CrossRef]
- Argo, T.R.; Carnahan, R.M.; Perry, P.J. Aripiprazole, a novel atypical antipsychotic drug. Pharmacotherapy 2004, 24, 212–228. [Google Scholar] [CrossRef]
- Aihara, K.; Shimada, J.; Miwa, T.; Tottori, K.; Burris, K.D.; Yocca, F.D.; Horie, M.; Kikuchi, T. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004, 1003, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.I.; Gomez, J.; Constain, C.; Rey, J.; Santacruz, H. Controlled clinical trial with trazodone, a novel antidepressant. A South American experience. J. Clin. Pharmacol. 1980, 20, 124–130. [Google Scholar] [CrossRef]
- Shin, J.J.; Saadabadi, A. Trazodone; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Khouzam, H.R. A review of trazodone use in psychiatric and medical conditions. Postgrad. Med. 2017, 129, 140–148. [Google Scholar] [CrossRef]
- Haria, M.; Fitton, A.; McTavish, D. Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders. Drugs Aging 1994, 4, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Anderson, A.; Genaro-Mattos, T.C.; Korade, Z.; Mirnics, K. Individual and simultaneous treatment with antipsychotic aripiprazole and antidepressant trazodone inhibit sterol biosynthesis in the adult brain. J. Lipid Res. 2022, 63, 100249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Liao, D.L.; Hsiung, C.A.; Chen, C.Y.; Liao, Y.C.; Chen, C.H. Chronic treatment with aripiprazole induces differential gene expression in the rat frontal cortex. Int. J. Neuropsychopharmacol. 2008, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Grajales, D.; Vazquez, P.; Ruiz-Rosario, M.; Tuduri, E.; Mirasierra, M.; Ferreira, V.; Hitos, A.B.; Koller, D.; Zubiaur, P.; Cigudosa, J.C.; et al. The second-generation antipsychotic drug aripiprazole modulates the serotonergic system in pancreatic islets and induces beta cell dysfunction in female mice. Diabetologia 2022, 65, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Badran, A.; Tul-Wahab, A.; Zafar, H.; Mohammad, N.; Imad, R.; Ashfaq Khan, M.; Baydoun, E.; Choudhary, M.I. Antipsychotics drug aripiprazole as a lead against breast cancer cell line (MCF-7) in vitro. PLoS ONE 2020, 15, e0235676. [Google Scholar] [CrossRef]
- Koller, D.; Belmonte, C.; Saiz-Rodriguez, M.; Zubiaur, P.; Roman, M.; Ochoa, D.; Abad-Santos, F. Effects of aripiprazole on circadian prolactin secretion related to pharmacogenetics in healthy volunteers. Basic. Clin. Pharmacol. Toxicol. 2020, 126, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Borras, L.; de Timary, P.; Constant, E.L.; Huguelet, P.; Eytan, A. Successful treatment of alcohol withdrawal with trazodone. Pharmacopsychiatry 2006, 39, 232. [Google Scholar] [CrossRef] [PubMed]
- Cenik, B.; Palka, J.M.; Thompson, B.M.; McDonald, J.G.; Tamminga, C.A.; Cenik, C.; Brown, E.S. Desmosterol and 7-dehydrocholesterol concentrations in post mortem brains of depressed people: The role of trazodone. Transl. Psychiatry 2022, 12, 139. [Google Scholar] [CrossRef]
- Fink, H.A.; MacDonald, R.; Rutks, I.R.; Wilt, T.J. Trazodone for erectile dysfunction: A systematic review and meta-analysis. BJU Int. 2003, 92, 441–446. [Google Scholar] [CrossRef]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar]
- La, A.L.; Walsh, C.M.; Neylan, T.C.; Vossel, K.A.; Yaffe, K.; Krystal, A.D.; Miller, B.L.; Karageorgiou, E. Long-Term Trazodone Use and Cognition: A Potential Therapeutic Role for Slow-Wave Sleep Enhancers. J. Alzheimers Dis. 2019, 67, 911–921. [Google Scholar] [CrossRef]
- Gil, C.H.; Kim, Y.R.; Lee, H.J.; Jung, D.H.; Shin, H.K.; Choi, B.T. Aripiprazole exerts a neuroprotective effect in mouse focal cerebral ischemia. Exp. Ther. Med. 2018, 15, 745–750. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Son, Y.; Park, H.J.; Oh, S.J.; Choi, J.Y.; Ko, Y.G.; Lee, H.J. Therapeutic Effects of Aripiprazole in the 5xFAD Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2021, 22, 9374. [Google Scholar] [CrossRef]
- Balog, M.; Anderson, A.C.; Heffer, M.; Korade, Z.; Mirnics, K. Effects of Psychotropic Medication on Somatic Sterol Biosynthesis of Adult Mice. Biomolecules 2022, 12, 1535. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Genaro-Mattos, T.C.; Porter, N.A.; Mirnics, K. Trazodone effects on developing brain. Transl. Psychiatry 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 2019, 24, 491–500. [Google Scholar] [CrossRef]
- Tulenko, T.N.; Boeze-Battaglia, K.; Mason, R.P.; Tint, G.S.; Steiner, R.D.; Connor, W.E.; Labelle, E.F. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 2006, 47, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Gondre-Lewis, M.C.; Petrache, H.I.; Wassif, C.A.; Harries, D.; Parsegian, A.; Porter, F.D.; Loh, Y.P. Abnormal sterols in cholesterol-deficiency diseases cause secretory granule malformation and decreased membrane curvature. J. Cell Sci. 2006, 119, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; Backlund, P.S.; Wassif, C.A.; Yergey, A.L.; Porter, F.D. Quantitative proteomics analysis of inborn errors of cholesterol synthesis: Identification of altered metabolic pathways in DHCR7 and SC5D deficiency. Mol. Cell. Proteom. 2010, 9, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.A.; Wassif, C.A.; Kratz, L.; Cozma, D.; Kovarova, M.; Harris, G.; Grinberg, A.; Yang, Y.; Hunter, A.G.; Tsokos, M.; et al. Lathosterolosis: An inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum. Mol. Genet. 2003, 12, 1631–1641. [Google Scholar] [CrossRef]
- Shrivastava, S.; Paila, Y.D.; Kombrabail, M.; Krishnamoorthy, G.; Chattopadhyay, A. Role of Cholesterol and Its Immediate Biosynthetic Precursors in Membrane Dynamics and Heterogeneity: Implications for Health and Disease. J. Phys. Chem. B 2020, 124, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.A.; Allen, L.B.; Klingelsmith, K.B.; Anderson, A.; Genaro-Mattos, T.C.; Mirnics, K.; Porter, N.A.; Korade, Z. Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem. Neurosci. 2021, 12, 735–745. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jaramillo-Madrid, A.C.; Abbriano, R.; Ashworth, J.; Fabris, M.; Pernice, M.; Ralph, P.J. Overexpression of Key Sterol Pathway Enzymes in Two Model Marine Diatoms Alters Sterol Profiles in Phaeodactylum tricornutum. Pharmaceuticals 2020, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Scott, N.A.; Capell-Hattam, I.M.; Draper, E.A.; Fenton, N.M.; Luu, W.; Sharpe, L.J.; Brown, A.J. Cholesterol synthesis enzyme SC4MOL is fine-tuned by sterols and targeted for degradation by the E3 ligase MARCHF6. J. Lipid Res. 2023, 64, 100362. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Luu, W.; Li, D.; Sharpe, L.J.; Brown, A.J. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 2016, 64, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.J.; Coates, H.W.; Brown, A.J. Post-translational control of the long and winding road to cholesterol. J. Biol. Chem. 2020, 295, 17549–17559. [Google Scholar] [CrossRef]
- Yoneyama, M.; Hasebe, S.; Kawamoto, N.; Shiba, T.; Yamaguchi, T.; Kikuta, M.; Shuto, M.; Ogita, K. Beneficial in vivo effect of aripiprazole on neuronal regeneration following neuronal loss in the dentate gyrus: Evaluation using a mouse model of trimethyltin-induced neuronal loss/self-repair in the dentate gyrus. J. Pharmacol. Sci. 2014, 124, 99–111. [Google Scholar] [CrossRef]
- Bortolotto, V.; Mancini, F.; Mangano, G.; Salem, R.; Xia, E.; Del Grosso, E.; Bianchi, M.; Canonico, P.L.; Polenzani, L.; Grilli, M. Proneurogenic Effects of Trazodone in Murine and Human Neural Progenitor Cells. ACS Chem. Neurosci. 2017, 8, 2027–2038. [Google Scholar] [CrossRef]
- Daniele, S.; Zappelli, E.; Martini, C. Trazodone regulates neurotrophic/growth factors, mitogen-activated protein kinases and lactate release in human primary astrocytes. J. Neuroinflammation 2015, 12, 225. [Google Scholar] [CrossRef]
- Walker, T.L.; Yasuda, T.; Adams, D.J.; Bartlett, P.F. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J. Neurosci. 2007, 27, 3734–3742. [Google Scholar] [CrossRef]
- Zare, I.; Paul, D.; Moody, S. Doublecortin Mutation in an Adolescent Male. Child Neurol. Open 2019, 6, 2329048X19836589. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2001, 32, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Harry, G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, P.; Michels, V.; Gavrilov, D.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 2013, 110, 176–178. [Google Scholar] [CrossRef]
- Goyal, S.; Xiao, Y.; Porter, N.A.; Xu, L.; Guengerich, F.P. Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1. J. Lipid Res. 2014, 55, 1933–1943. [Google Scholar] [CrossRef]
- Pratt, D.A.; Mills, J.H.; Porter, N.A. Theoretical calculations of carbon-oxygen bond dissociation enthalpies of peroxyl radicals formed in the autoxidation of lipids. J. Am. Chem. Soc. 2003, 125, 5801–5810. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Korade, Z.; Porter, N.A. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: Product and mechanistic studies. J. Am. Chem. Soc. 2010, 132, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Korade, Z.; Rosado, D.A., Jr.; Liu, W.; Lamberson, C.R.; Porter, N.A. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1222–1233. [Google Scholar] [CrossRef]
- Xu, L.; Korade, Z.; Rosado, D.A., Jr.; Mirnics, K.; Porter, N.A. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 2013, 54, 1135–1143. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef]
- Xu, L.; Porter, N.A. Free radical oxidation of cholesterol and its precursors: Implications in cholesterol biosynthesis disorders. Free Radic. Res. 2015, 49, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Correa-Cerro, L.S.; Wassif, C.A.; Kratz, L.; Miller, G.F.; Munasinghe, J.P.; Grinberg, A.; Fliesler, S.J.; Porter, F.D. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum. Mol. Genet. 2006, 15, 839–851. [Google Scholar] [CrossRef]
- Porter, F.D. RSH/Smith-Lemli-Opitz syndrome: A multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol. Genet. Metab. 2000, 71, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. Smith-Lemli-Opitz syndrome: Pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008, 16, 535–541. [Google Scholar] [CrossRef]
- Korade, Z.; Xu, L.; Shelton, R.; Porter, N.A. Biological activities of 7-dehydrocholesterol-derived oxysterols: Implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2010, 51, 3259–3269. [Google Scholar] [CrossRef]
- Xu, L.; Mirnics, K.; Bowman, A.B.; Liu, W.; Da, J.; Porter, N.A.; Korade, Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 2012, 45, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, Y.; Gong, W.; Su, Y.; Zhang, Y. A pan-cancer analysis of the oncogenic role of 7-dehydrocholesterol reductase (DHCR7) in human tumors. BMC 2022. under review. [Google Scholar] [CrossRef]
- Gunda, V.; Genaro-Mattos, T.C.; Kaushal, J.B.; Chirravuri-Venkata, R.; Natarajan, G.; Mallya, K.; Grandgenett, P.M.; Mirnics, K.; Batra, S.K.; Korade, Z.; et al. Ubiquitous Aberration in Cholesterol Metabolism across Pancreatic Ductal Adenocarcinoma. Metabolites 2022, 12, 47. [Google Scholar] [CrossRef]
- Zou, J.; Liu, S.; Long, J.; Yan, B. High DHCR7 Expression Predicts Poor Prognosis for Cervical Cancer. Comput. Math. Methods Med. 2022, 2022, 8383885. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Zheng, X.; Qi, L.; Wang, H.; Zhang, C.; Wan, X.; Zheng, Y.; Zhong, R.; Zhou, X.; et al. Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection. Immunity 2020, 52, 109–122.e6. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Tallman, K.A.; Kim, H.H.; Balog, M.; Genaro-Mattos, T.C.; Pattnaik, A.; Mirnics, K.; Pattnaik, A.K.; Porter, N.A. Dose-Response Effects of 7-Dehydrocholesterol Reductase Inhibitors on Sterol Profiles and Vesicular Stomatitis Virus Replication. ACS Pharmacol. Transl. Sci. 2022, 5, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.K.; Lee, J.H.; Lee, J. Aripiprazole repurposed as an inhibitor of biofilm formation and sterol biosynthesis in multidrug-resistant Candida albicans. Int. J. Antimicrob. Agents 2019, 54, 518–523. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Fazeli, G.; Xavier da Silva, T.N.; Friedmann Angeli, J.P. Ferroptosis: Mechanisms and implications for cancer development and therapy response. Trends Cell Biol. 2023. [Google Scholar] [CrossRef]
- Mitsche, M.A.; McDonald, J.G.; Hobbs, H.H.; Cohen, J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. Elife 2015, 4, e07999. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Zhao, C.; Wang, L.; Xiao, Z.; Rong, J.; Xia, X.; Chen, Z.; Pfister, S.K.; Mast, N.; Yutuc, E.; et al. Assessment of cholesterol homeostasis in the living human brain. Sci. Transl. Med. 2022, 14, eadc9967. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korade, Z.; Anderson, A.; Balog, M.; Tallman, K.A.; Porter, N.A.; Mirnics, K. Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis. Biomolecules 2023, 13, 1321. https://doi.org/10.3390/biom13091321
Korade Z, Anderson A, Balog M, Tallman KA, Porter NA, Mirnics K. Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis. Biomolecules. 2023; 13(9):1321. https://doi.org/10.3390/biom13091321
Chicago/Turabian StyleKorade, Zeljka, Allison Anderson, Marta Balog, Keri A. Tallman, Ned A. Porter, and Karoly Mirnics. 2023. "Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis" Biomolecules 13, no. 9: 1321. https://doi.org/10.3390/biom13091321
APA StyleKorade, Z., Anderson, A., Balog, M., Tallman, K. A., Porter, N. A., & Mirnics, K. (2023). Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis. Biomolecules, 13(9), 1321. https://doi.org/10.3390/biom13091321







