Advances in Dystrophinopathy Diagnosis and Therapy
Abstract
1. Introduction
2. Diagnosis Technology
2.1. Serum Creatine Kinase Assay
2.2. Haplotype and Southern Blot Analyses
2.3. Diagnostic Methods on Muscle Tissue
2.4. Multiplex Ligation-Dependent Probe Amplification
2.5. Multiplex PCR
2.6. Point Mutations Screening
2.7. Next Generation DNA Sequencing
3. Pharmacological Therapy
3.1. Skeletal Muscle Care
3.2. Cardiologic Care
4. Standard Multidisciplinary Care
5. Gene Therapy
6. Discussion
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Monaco, A.P.; Bertelson, C.J.; Middlesworth, W.; Colletti, C.A.; Aldridge, J.; Fischbeck, K.H.; Bartlett, R.; Pericak-Vance, M.A.; Roses, A.D.; Kunkel, L.M. Detection of deletions spanning the Duchenne muscular dystro-phy locus using a tightly linked DNA segment. Nature 1985, 316, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.N.; Belfall, B.; Duff, C.; Logan, C.; Kean, V.; Thompson, M.W.; Sylvester, J.E.; Gorski, J.L.; Schmickel, R.D.; Worton, R.G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature 1985, 318, 672–675. [Google Scholar] [CrossRef]
- Koenig, M.; Hoffman, E.P.; Bertelson, C.J.; Monaco, A.P.; Feener, C.; Kunkel, L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Gherardi, S.; Bovolenta, M.; Passarelli, C.; Falzarano, M.S.; Pigini, P.; Scotton, C.; Neri, M.; Armaroli, A.; Osman, H.; Selvatici, R.; et al. Transcriptional and epigenetic analyses of the DMD locus reveal novel cis acting DNA elements that govern muscle dystrophin expression. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Toward the correction of muscular dystrophy by gene editing. Proc. Natl. Acad. Sci. USA 2021, 118, e2004840117. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Kunkel, L.M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron 1989, 2, 1019. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishiguro, T.; Eguchi, C.; Saito, K.; Ozawa, E. Expression of a dystrophin-related protein associated with the skeletal muscle cell membrane. Histochemistry 1991, 96, 1–5. [Google Scholar] [CrossRef]
- Fratter, C.; Dalgleish, R.; Allen, S.K.; Santos, R.; Abbs, S.; Tuffery-Giraud, S.; Ferlini, A. EMQN best practice guidelines for genetic testing in Dystrophinopathies. Eur. J. Hum. Genet. 2020, 28, 1141–1159. [Google Scholar] [CrossRef]
- De Palma, F.D.E.; Nunziato, M.; D’Argenio, V.; Savarese, M.; Esposito, G.; Salvatore, F. Comprehensive Molecular Analysis of DMD Gene Increases the Diagnostic Value of Dystrophinopathies: A Pilot Study in a Southern Italy Cohort of Patients. Diagnostics 2021, 11, 1910. [Google Scholar] [CrossRef]
- Amrani, N.; Sachs, M.S.; Jacobson, A. Early nonsense: mRNA decay solves a translational problem. Nat. Rev. Mol. Cell Biol. 2006, 7, 415–425. [Google Scholar]
- Puisac, B.; Teresa-Rodrigo, M.E.; Arnedo, M.; Gil-Rodríguez, M.C.; Pérez-Cerdá, C.; Ribes, A.; Pié, A.; Bueno, G.; Gómez-Puertas, P.; Pié, J. Analysis of aberrant splicing and nonsense-mediated decay of the stop codon mutations c.109G>T and c.504-505delCT in 7 patients with HMG-CoA lyase deficiency. Mol. Genet. Metab. 2013, 108, 232–240. [Google Scholar] [PubMed]
- Verhaart, I.E.; van Duijn, R.J.; den Adel, B.; Roest, A.A.; Verschuuren, J.J.; Aartsma-Rus, A.; van der Weerd, L. Assessment of cardiac function in three mouse Dystrophinopathies by magnetic resonance imaging. Neuromuscul. Disord. 2012, 22, 418–426. [Google Scholar] [PubMed]
- Del Rio-Pertuz, G.; Morataya, C.; Parmar, K.; Dubay, S.; Argueta-Sosa, E. Dilated cardiomyopathy as the initial presentation of Becker muscular dystrophy: A systematic review of published cases. Orphanet J. Rare Dis. 2022, 17, 194. [Google Scholar]
- Fayssoil, A.; Yaou, R.B.; Ogna, A.; Leturcq, F.; Nardi, O.; Clair, B.; Wahbi, K.; Lofaso, F.; Laforet, P.; Duboc, D.; et al. Clinical profiles and prognosis of acute heart failure in adult patients with Dystrophinopathies on home mechanical ventilation. ESC Heart Fail. 2017, 4, 527–534. [Google Scholar]
- Nigro, G.; Comi, L.I.; Politano, L.; Bain, R.J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 1990, 26, 271–277. [Google Scholar]
- Bello, L.; Campadello, P.; Barp, A.; Fanin, M.; Semplicini, C.; Sorarù, G.; Caumo, L.; Calore, C.; Angelini, C.; Pegoraro, E. Functional changes in Becker muscular dystrophy: Implications for clinical trials in Dystrophinopathies. Sci. Rep. 2016, 6, 32439. [Google Scholar]
- Muntoni, F.; Di Lenarda, A.; Porcu, M.; Sinagra, G.; Mateddu, A.; Marrosu, G.; Ferlini, A.; Cau, M.; Milasin, J.; Melis, M.A.; et al. Dystrophin gene abnormalities in two patients with idiopathic dilated cardiomyopathy. Heart 1997, 78, 608–612. [Google Scholar] [CrossRef]
- Berardo, A.; DiMauro, S.; Hirano, M. A diagnostic algorithm for metabolic myopathies. Curr. Neurol. Neurosci. Rep. 2010, 10, 118–126. [Google Scholar]
- Brandsema, J.F.; Darras, B.T. Dystrophinopathies. In Seminars in Neurology; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 35, pp. 369–384. [Google Scholar]
- Topaloglu, H. Duchenne muscular dystrophy: A short review and treatment update. Iran. J. Child Neurol. 2021, 15, 9–15. [Google Scholar]
- Melacini, P.; Fanin, M.; Danieli, G.A.; Fasoli, G.; Villanova, C.; Angelini, C.; Vitiello, L.; Miorelli, M.; Buja, G.F.; Mostacciuolo, M.L.; et al. Cardiac involvement in Becker muscular dystrophy. J. Am. Coll. Cardiol. 1993, 22, 1927–1934. [Google Scholar]
- Suthar, R.; Kesavan, S.; Sharawat, I.K.; Malviya, M.; Sirari, T.; Sihag, B.K.; Saini, A.G.; Jyothi, V.; Sankhyan, N. The Expanding Spectrum of Dystrophinopathies: HyperCKemia to Manifest Female Carriers. J. Pediatr. Neurosci. 2021, 16, 206–211. [Google Scholar]
- Kamakura, K.; Kawai, M.; Arahata, K.; Koizumi, H.; Watanabe, K.; Sugita, H. A manifesting carrier of Duchenne muscular dystrophy with severe myocardial symptoms. J. Neurol. 1990, 237, 483–485. [Google Scholar]
- Politano, L.; Nigro, V.; Nigro, G.; Petretta, V.R.; Passamano, L.; Papparella, S.; Di Somma, S.; Comi, L.I. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA 1996, 275, 1335–1338. [Google Scholar] [CrossRef]
- Hoogerwaard, E.M.; van der Wouw, P.A.; Wilde, A.A.; Bakker, E.; Ippel, P.F.; Oosterwijk, J.C.; Majoor-Krakauer, D.F.; van Essen, A.J.; Leschot, N.J.; de Visser, M. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 1999, 9, 347–351. [Google Scholar]
- Grain, L.; Cortina-Borja, M.; Forfar, C.; Hilton-Jones, D.; Hopkin, J.; Burch, M. Cardiac abnormalities and skeletal muscle weakness in carriers of Duchenne and Becker muscular dystrophies and controls. Neuromuscul. Disord. 2001, 11, 186–191. [Google Scholar]
- Lim, K.R.Q.; Sheri, N.; Nguyen, Q.; Yokota, T. Cardiac Involvement in Dystrophin-Deficient Females: Current Understanding and Implications for the Treatment of Dystrophinopathies. Genes 2020, 11, 765. [Google Scholar]
- Richterich, R.; Rosin, S.; Aebi, U.; Rossi, E. Progressive Muscular Dystrophy. V. The Identification of the Carrier State in the Duchenne Type by Serum Creatine Kinase Determination. Am. J. Hum. Genet. 1963, 15, 133–154. [Google Scholar]
- Emery, A.E.H. Muscle histology in carriers of Duchenne muscular dystrophy. J. Med. Genet. 1965, 2, 1–7. [Google Scholar]
- Bakker, E.; Hofker, M.H.; Goor, N.; Mandel, J.L.; Wrogemann, K.; Davies, K.E.; Kunkel, L.M.; Willard, H.F.; Fenton, W.A.; Sandkuyl, L.; et al. Prenatal diagnosis and carrier detection of Duchenne muscular dystrophy with closely linked RFLPs. Lancet 1985, 1, 655–658. [Google Scholar]
- Abbs, S.; Roberts, R.G.; Mathew, C.G.; Bentley, D.R.; Bobrow, M. Accurate assessment of intragenic recombination frequency within the Duchenne muscular dystrophy gene. Genomics 1990, 7, 602–606. [Google Scholar]
- Dreyfus, J.C.; Schapira, G.; Schapira, F. Serum enzymes in the physiopathology of muscle. Ann. N. Y. Acad. Sci. 1958, 75, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M.; Pennigton, R.J.; Walton, J.N. Serum enzyme studies in muscle disease, Part III. Serum creatine kinase activity in relatives of patients with Duchenne type of muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 1964, 27, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.; Park, D.C.; Parsons, M.E.; O’Brien, M.D. Serum creatine kinase studies in the detection of carriers of Duchenne dystrophy. J. Neurol. Neurosurg. Psychiatry 1971, 34, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Emery, A.E. Letter: Serum-creatine-kinase levels in carriers of Becker muscular dystrophy. Lancet 1974, 2, 1023–1024. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.W.; Suh, M.R.; Choi, W.A.; Kang, S.W.; Oh, H.J. Correlation of Serum Creatine Kinase Level with Pulmonary Function in Duchenne Muscular Dystrophy. Ann. Rehabil. Med. 2017, 41, 306–312. [Google Scholar] [CrossRef]
- Yasmineh, W.G.; Ibrahim, G.A.; Abbasnezhad, M.; Awad, E.A. Isoenzyme distribution of creatine kinase and lactate dehydrogenase in serum and skeletal muscle in Duchenne muscular dystrophy, collagen disease, and other muscular disorders. Clin. Chem. 1978, 24, 1985–1989. [Google Scholar] [CrossRef]
- Roy, S.; Dubowitz, V. Carrier detection in Duchenne muscular dystrophy. A comparative study of electron microscopy, light microscopy and serum enzymes. J. Neurol. Sci. 1970, 11, 65–67. [Google Scholar] [CrossRef]
- Hofker, M.H.; Wapenaar, M.C.; Goor, N.; Bakker, E.; van Ommen, G.J.; Pearson, P.L. Isolation of probes detecting restriction fragment length polymorphisms from X chromosomespecific libraries: Potential use for diagnosis of Duchenne muscular dystrophy. Hum. Genet. 1985, 70, 148–156. [Google Scholar] [CrossRef]
- Bakker, E.; Bonten, E.J.; De Lange, L.F.; Veenema, H.; Majoor-Krakauer, D.; Hofker, M.H.; Van Ommen, G.J.; Pearson, P.L. DNA probe analysis for carrier detection and prenatal diagnosis of Duchenne muscular dystrophy: A standard diagnostic procedure. J. Med. Genet. 1986, 23, 573–580. [Google Scholar] [CrossRef]
- Southern, E.M. An improved method for transferring nucleotides from electrophoresis strips to thin layers of ion-exchange cellulose. Anal. Biochem. 1974, 62, 317–318. [Google Scholar] [CrossRef]
- Forrest, S.M.; Cross, G.S.; Flint, T.; Speer, A.; Robson, K.J.; Davies, K.E. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics 1988, 2, 109–114. [Google Scholar] [CrossRef]
- Gilgenkrantz, H.; Chelly, J.; Lambert, M.; Recan, D.; Barbot, J.C. Analysis of molecular deletions with cDNA probes in patients with Duchenne and Becker muscular dystrophy. Genomics 1989, 5, 574–580. [Google Scholar] [CrossRef]
- Angelini, C.; Beggs, A.H.; Hoffman, E.P.; Fanin, M.; Kunkel, L.M. Enormous dystrophin in a patient with Becker muscular dystrophy. Neurology 1990, 40, 808–812. [Google Scholar] [CrossRef]
- Herrmann, F.H.; Wulff, K.; Schütz, M.; Wehnert, M. Deletion screening and prenatal diagnosis of Duchenne muscular dystrophy using cDNA probes Cf 23a and Cf 56a. Eur. J. Pediatr. 1990, 149, 263–265. [Google Scholar] [CrossRef]
- Gold, R.; Kress, W.; Bettecken, T.; Reichmann, H.; Müller, C.R. A 400-kb tandem duplication within the dystrophin gene leads to severe Becker muscular dystrophy. J. Neurol. 1994, 241, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Radosavljević, D.; Todorović, D.; Crkvenjakov, R. Deletion analysis of Duchenne muscular dystrophy using cDNA probes and multiplex PCR. Neurol. Croat. 1991, 40, 157–164. [Google Scholar] [PubMed]
- Vitiello, L.; Mostacciuolo, M.L.; Oliviero, S.; Schiavon, F.; Nicoletti, L.; Angelini, C.; Danieli, G.A. Screening for mutations in the muscle promoter region and for exonic deletions in a series of 115 DMD and BMD patients. J. Med. Genet. 1992, 29, 127–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Southern, E.M. Blotting at 25. Trends Biochem. Sci. 2000, 25, 585–588. [Google Scholar] [CrossRef]
- Zubrzycka-Gaarn, E.E.; Bulman, D.E.; Karpati, G.; Burghes, A.H.; Belfall, B.; Klamut, H.J.; Talbot, J.; Hodges, R.S.; Ray, P.N.; Worton, R.G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 1988, 333, 466–469. [Google Scholar] [CrossRef]
- Uchino, M.; Araki, S.; Miike, T. Electrophoretic studies of muscle proteins in Duchenne muscular dystrophy and other neuromuscular disorders--with special reference to the change of dystrophin. Jpn. J. Med. 1989, 28, 170–174. [Google Scholar] [CrossRef]
- Sahashi, K.; Ibi, T.; Suoh, H.; Nakao, N.; Tashiro, M.; Marui, K.; Arahata, K.; Sugita, H. Immunostaining of dystrophin and utrophin in skeletal muscle of dystrophinopathies. Intern. Med. 1994, 33, 277–283. [Google Scholar] [CrossRef]
- Voit, T.; Stuettgen, P.; Cremer, M.; Goebel, H.H. Dystrophin as a diagnostic marker in Duchenne and Becker muscular dystrophy. Correlation of immunofluorescence and western blot. Neuropediatrics 1991, 22, 152–162. [Google Scholar] [CrossRef]
- Chevron, M.P.; Tuffery, S.; Echenne, B.; Demaille, J.; Claustres, M. Becker muscular dystrophy: Demonstration of the carrier status of a female by immunoblotting and immunostaining. Neuromuscul. Disord. 1992, 2, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Kunkel, L.M.; Angelini, C.; Clarke, A.; Johnson, M.; Harris, J.B. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology 1989, 39, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.G.; Bobrow, M.; Bentley, D.R. Point mutations in the dystrophin gene. Proc. Natl. Acad. Sci. USA 1992, 89, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Deburgrave, N.; Daoud, F.; Llense, S.; Barbot, J.C.; Récan, D.; Peccate, C.; Burghes, A.H.; Béroud, C.; Garcia, L.; Kaplan, J.C.; et al. Protein- and mRNA-based phenotype-genotype correlations in DMD/BMD with point mutations and molecular basis for BMD with nonsense and frameshift mutations in the DMD gene. Hum. Mutat. 2007, 28, 183–195. [Google Scholar] [CrossRef]
- Bougé, A.L.; Murauer, E.; Beyne, E.; Miro, J.; Varilh, J.; Taulan, M.; Koenig, M.; Claustres, M.; Tuffery-Giraud, S. Targeted RNA-Seq profiling of splicing pattern in the DMD gene: Exons are mostly constitutively spliced in human skeletal muscle. Sci. Rep. 2017, 7, 39094. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Grilli, A.; Zia, S.; Fang, M.; Rossi, R.; Gualandi, F.; Rimessi, P.; El Dani, R.; Fabris, M.; Lu, Z.; et al. RNA-seq in DMD urinary stem cells recognized muscle-related transcription signatures and addressed the identification of atypical mutations by whole-genome sequencing. HGG Adv. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Gatta, V.; Scarciolla, O.; Gaspari, A.R.; Palka, C.; De Angelis, M.V.; Di Muzio, A.; Guanciali-Franchi, P.; Calabrese, G.; Uncini, A.; Stuppia, L. Identification of deletions and duplications of the DMD gene in affected males and carrier females by multiple ligation probe amplification (MLPA). Hum. Genet. 2005, 117, 92–98. [Google Scholar] [CrossRef]
- Lalic, T.; Vossen, R.H.; Coffa, J.; Schouten, J.P.; Guc-Scekic, M.; Radivojevic, D.; Djurisic, M.; Breuning, M.H.; White, S.J.; den Dunnen, J.T. Deletion and duplication screening in the DMD gene using MLPA. Eur. J. Hum. Genet. 2005, 13, 1231–1234. [Google Scholar] [CrossRef]
- Lai, K.K.; Lo, I.F.; Tong, T.M.; Cheng, L.Y.; Lam, S.T. Detecting exon deletions and duplications of the DMD gene using Multiplex Ligation-dependent Probe Amplification (MLPA). Clin. Biochem. 2006, 39, 367–372. [Google Scholar] [CrossRef]
- Todorova, A.; Todorov, T.; Georgieva, B.; Lukova, M.; Guergueltcheva, V.; Kremensky, I.; Mitev, V. MLPA analysis/complete sequencing of DMD gene in a group of Bulgarian Duchenne/Becker muscular dystrophy patients. Neuromuscul. Disord. 2008, 18, 667–670. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, G.; Fu, K.; Wu, D.; Zhai, Q.; Du, H.; Huang, Z.; Niu, Y. Gene diagnosis for nine Chinese patients with DMD/BMD by multiplex ligation-dependent probe amplification and prenatal diagnosis for one of them. J. Clin. Lab. Anal. 2009, 23, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; Chandramohan, A.; Lakshmi, B.R. Use of multiplex ligation-dependent probe amplification (MLPA) for Duchenne muscular dystrophy (DMD) gene mutation analysis. Indian J. Med. Res. 2010, 132, 303–311. [Google Scholar]
- Sansović, I.; Barišić, I.; Dumić, K. Improved detection of deletions and duplications in the DMD gene using the multiplex ligation-dependent probe amplification (MLPA) method. Biochem. Genet. 2013, 51, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, S.Y.; Lee, M.J.; Kang, K.M.; Park, J.E.; Shim, S.H.; Cha, D.H. Prenatal diagnosis of de novo DMD duplication by multiplex ligation-dependent probe amplification (MLPA) after noninvasive prenatal screening (NIPS) at 11 gestational weeks. Taiwan. J. Obstet. Gynecol. 2021, 60, 570–573. [Google Scholar] [CrossRef]
- Echigoya, Y.; Lim, K.R.Q.; Nakamura, A.; Yokota, T. Multiple exon skipping in the Duchenne muscular dystrophy hot spots: Prospects and challenges. J. Pers. Med. 2018, 8, 41. [Google Scholar] [CrossRef]
- Chamberlain, J.S.; Gibbs, R.A.; Ranier, J.E.; Nguyen, P.N.; Caskey, C.T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988, 16, 11141–11156. [Google Scholar] [CrossRef]
- Beggs, A.H.; Koenig, M.; Boyce, F.M.; Kunkel, L.M. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum. Genet. 1990, 86, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, T.M.; Flanigan, K.M. Reassessing carrier status for dystrophinopathies. Neurol. Genet. 2016, 2, e108. [Google Scholar] [CrossRef]
- Saad, F.A.; Galvagni, F.; Danieli, G.A. Rapid detection of human dystrophin gene mutations by multiplex semi-quantitave PCR. Basic Appl. Myol. 1993, 3, 229–231. [Google Scholar]
- Galvagni, F.; Saad, F.A.; Danieli, G.A.; Miorin, M.; Vitiello, L.; Mostacciuolo, M.L.; Angelini, C. A study on duplications of the dystrophin gene: Evidence of a geographical difference in the distribution of breakpoints by intron. Hum. Genet. 1994, 94, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Coffey, A.J.; Abbs, S.; Vermeesch, J.R.; Dumanski, J.P.; Woodward, K.J.; Andrews, R.M.; Langford, C.; Vetrie, D. Exon array CGH: Detection of copy-number changes at the resolution of individual exons in the human genome. Am. J. Hum. Genet. 2005, 76, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, M.; Neri, M.; Fini, S.; Fabris, M.; Trabanelli, C.; Venturoli, A.; Martoni, E.; Bassi, E.; Spitali, P.; Brioschi, S.; et al. A novel custom high density-comparative genomic hybridization array detects common rearrangements as well as deep intronic mutations in Dystrophinopathies. BMC Genom. 2008, 9, 572. [Google Scholar] [CrossRef]
- Myers, R.M.; Fischer, S.G.; Maniatis, T.; Lerman, L.S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985, 13, 3111–3129. [Google Scholar] [CrossRef][Green Version]
- Lerman, L.S.; Silverstein, K.; Grinfeld, E. Searching for gene defects by denaturing gradient gel electrophoresis. Cold Spring Harb. Symp. Quant. Biol. 1986, 51 Pt 1, 285–297. [Google Scholar] [CrossRef]
- Saeki, Y.; Ueno, S.; Yorifuji, S.; Sugiyama, Y.; Ide, Y.; Matsuzawa, Y. New mutant gene (transthyretin Arg 58) in cases with hereditary polyneuropathy detected by non-isotope method of single-strand conformation polymorphism analysis. Biochem. Biophys. Res. Commun. 1991, 180, 380–385. [Google Scholar] [CrossRef]
- Saijo, T.; Ito, M.; Takeda, E.; Huq, A.H.; Naito, E.; Yokota, I.; Sone, T.; Pike, J.W.; Kuroda, Y. A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: Utility of single-strand conformation polymorphism analysis for heterozygous carrier detection. Am. J. Hum. Genet. 1991, 49, 668–673. [Google Scholar]
- Saad, F.A.; Halliger, B.; Müller, C.R.; Roberts, R.G.; Danieli, G.A. Single base substitutions are detected by double strand conformation analysis. Nucleic Acids Res. 1994, 22, 4352–4353. [Google Scholar] [CrossRef][Green Version]
- Saad, F.A.; Mostacciuolo, M.L.; Trevisan, C.P.; Tomelleri, G.; Angelini, C.; Abdel Salam, E.; Danieli, G.A. Novel mutations and polymorphisms in the human dystrophin gene detected by double-strand conformation analysis. Hum. Mutat. 1997, 9, 188–190. [Google Scholar] [CrossRef]
- Nürnberg, P.; Roewer, L.; Neitzel, H.; Sperling, K.; Pöpperl, A.; Hundrieser, J.; Pöche, H.; Epplen, C.; Zischler, H.; Epplen, J.T. DNA fingerprinting with the oligonucleotide probe (CAC)5/(GTG)5: Somatic stability and germline mutations. Hum. Genet. 1989, 84, 75–78. [Google Scholar] [CrossRef] [PubMed]
- McClelland, M.; Welsh, J. DNA fingerprinting by arbitrarily primed PCR. Genome Res. 1994, 4, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.L.; Quinn, J.S. Multilocus DNA fingerprinting reveals high rate of heritable genetic mutation in herring gulls nesting in an industrialized urban site. Proc. Natl. Acad. Sci. USA 1996, 93, 12137–12141. [Google Scholar] [CrossRef]
- Flanigan, K.M.; von Niederhausern, A.; Dunn, D.M.; Alder, J.; Mendell, J.R.; Weiss, R.B. Rapid direct sequence analysis of the dystrophin gene. Am. J. Hum. Genet. 2003, 72, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Alame, M.; Lacourt, D.; Zenagui, R.; Mechin, D.; Danton, F.; Koenig, M.; Claustres, M.; Cossée, M. Implementation of a Reliable Next-Generation Sequencing Strategy for Molecular Diagnosis of Dystrophinopathies. J. Mol. Diagn. 2016, 18, 731–740. [Google Scholar] [CrossRef]
- Ebrahimzadeh-Vesal, R.; Teymoori, A.; Azimi-Nezhad, M.; Hosseini, F.S. Next Generation Sequencing approach to molecular diagnosis of Duchenne muscular dystrophy; identification of a novel mutation. Gene 2018, 644, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Deng, H.; Yang, C.; Li, X.; Zhu, Y.; Chen, X.; Li, H.; Li, S.; Cui, H.; Zhang, X.; et al. A resolved discrepancy between multiplex PCR and multiplex ligation-dependent probe amplification by targeted next-generation sequencing discloses a novel partial exonic deletion in the Duchenne muscular dystrophy gene. J. Clin. Lab. Anal. 2018, 32, e22575. [Google Scholar] [CrossRef]
- Alcántara-Ortigoza, M.A.; Reyna-Fabián, M.E.; González-Del Angel, A.; Estandia-Ortega, B.; Bermúdez-López, C.; Cruz-Miranda, G.M.; Ruíz-García, M. Predominance of Dystrophinopathy Genotypes in Mexican Male Patients Presenting as Muscular Dystrophy with A Normal Multiplex Polymerase Chain Reaction DMD Gene Result: A Study Including Targeted Next-Generation Sequencing. Genes 2019, 10, 856. [Google Scholar] [CrossRef]
- Nerakh, G.; Ranganath, P.; Murugan, S. Next-Generation Sequencing in a Cohort of Asian Indian Patients with the Duchenne Muscular Dystrophy Phenotype: Diagnostic Yield and Mutation Spectrum. J. Pediatr. Genet. 2021, 10, 23–28. [Google Scholar] [CrossRef]
- Park, E.W.; Shim, Y.J.; Ha, J.S.; Shin, J.H.; Lee, S.; Cho, J.H. Diagnosis of Duchenne Muscular Dystrophy in a Presymptomatic Infant Using Next-Generation Sequencing and Chromosomal Microarray Analysis: A Case Report. Children 2021, 8, 377. [Google Scholar] [CrossRef]
- Guevara-Fujita, M.L.; Huaman-Dianderas, F.; Obispo, D.; Sánchez, R.; Barrenechea, V.; Rojas-Málaga, D.; Estrada-Cuzcano, A.; Trubnykova, M.; Cornejo-Olivas, M.; Marca, V.; et al. MLPA followed by target-NGS to detect mutations in the dystrophin gene of Peruvian patients suspected of DMD/DMB. Mol. Genet. Genom. Med. 2021, 9, e1759. [Google Scholar] [CrossRef]
- Nallamilli, B.R.R.; Chaubey, A.; Valencia, C.A.; Stansberry, L.; Behlmann, A.M.; Ma, Z.; Mathur, A.; Shenoy, S.; Ganapathy, V.; Jagannathan, L.; et al. A single NGS-based assay covering the entire genomic sequence of the DMD gene facilitates diagnostic and newborn screening confirmatory testing. Hum. Mutat. 2021, 42, 626–638. [Google Scholar] [CrossRef]
- Duchenne, D. The pathology of paralysis with muscular degeneration (paralysie myosclerotique), or paralysis with apparent hypertrophy. Br. Med. J. 1867, 2, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8 (Suppl. S2), S303–S316. [Google Scholar] [CrossRef] [PubMed]
- Wilton-Clark, H.; Yokota, T. Biological and genetic therapies for the treatment of Duchenne muscular dystrophy. Expert Opin. Biol. Ther. 2023, 23, 49–59. [Google Scholar] [CrossRef]
- Saad, F.A.; Saad, J.F.; Siciliano, G.; Merlini, L.; Angelini, C. Duchenne Muscular Dystrophy Gene therapy. Curr. Gene Ther. 2022; in press. [Google Scholar]
- Reeves, E.K.; Rayavarapu, S.; Damsker, J.M.; Nagaraju, K. Glucocorticoid analogues: Potential therapeutic alternatives for treating inflammatory muscle diseases. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, CD003725. [Google Scholar] [CrossRef]
- Srinivasan, M.; Walker, C. Circadian Clock, Glucocorticoids and NF-κB Signaling in Neuroinflammation- Implicating Glucocorticoid Induced Leucine Zipper as a Molecular Link. ASN Neuro 2022, 14, 17590914221120190. [Google Scholar] [CrossRef]
- De Luca, A. Pre-clinical drug tests in the mdx mouse as a model of dystrophinopathies: An overview. Acta Myol. 2012, 31, 40–47. [Google Scholar]
- Goemans, N.; Buyse, G. Current treatment and management of Dystrophinopathies. Curr. Treat. Options Neurol. 2014, 16, 287. [Google Scholar] [CrossRef]
- Muntoni, F.; Fisher, I.; Morgan, J.E.; Abraham, D. Steroids in Duchenne muscular dystrophy: From clinical trials to genomic research. Neuromuscul. Disord. 2002, 12 (Suppl. S1), S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L.; Mazzanti, A.; Galbiati, E.; Saraifoger, S.; Dubini, A.; Cornelio, F.; Morandi, L. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos. Int. 2003, 14, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, B.; Matthews, D.J.; Clayton, G.H.; Carry, T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: Long-term effect. Am. J. Phys. Med. Rehabil. 2005, 84, 843–850. [Google Scholar]
- Angelini, C. The role of corticosteroids in muscular dystrophy: A critical appraisal. Muscle Nerve 2007, 2036, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Viviano, K.R. Glucocorticoids, Cyclosporine, Azathioprine, Chlorambucil, and Mycophenolate in Dogs and Cats: Clinical Uses, Pharmacology, and Side Effects. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 797–817. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, D.M.; Witchel, S.F.; Ermani, M.; Hoffman, E.P.; Angelini, C.; Pegoraro, E. The glucocorticoid receptor N363S polymorphism and steroid response in Duchenne dystrophy. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1177–1179. [Google Scholar] [CrossRef]
- Angelini, C.; Pegoraro, E.; Turella, E.; Intino, M.T.; Pini, A.; Costa, C. Deflazacort in Duchenne dystrophy: Study of long-term effect. Muscle Nerve 1994, 17, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Wissing, E.R.; Millay, D.P.; Vuagniaux, G.; Molkentin, J.D. Debio-025 is more effective than prednisone in reducing muscular pathology in mdx mice. Neuromuscul. Disord. 2010, 20, 753–760. [Google Scholar] [CrossRef]
- Pauly, M.; Daussin, F.; Burelle, Y.; Li, T.; Godin, R.; Fauconnier, J.; Koechlin-Ramonatxo, C.; Hugon, G.; Lacampagne, A.; Coisy-Quivy, M.; et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am. J. Pathol. 2012, 181, 583–592. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gutierrez, J.S.; Damsker, J.M.; Nagaraju, K.; Hoffman, E.P.; Ortlund, E.A. Disruption of a key ligand-H-bond network drives dissociative properties in vamorolone for Duchenne muscular dystrophy treatment. Proc. Natl. Acad. Sci. USA 2020, 117, 24285–24293. [Google Scholar] [CrossRef]
- Smith, E.C.; Conklin, L.S.; Hoffman, E.P.; Clemens, P.R.; Mah, J.K.; Finkel, R.S.; Guglieri, M.; Tulinius, M.; Nevo, Y.; Ryan, M.M.; et al. Efficacy and safety of vamorolone in Duchenne muscular dystrophy: An 18-month interim analysis of a non-randomized open-label extension study. PLoS Med. 2020, 17, e1003222. [Google Scholar]
- Guglieri, M.; Clemens, P.R.; Perlman, S.J.; Smith, E.C.; Horrocks, I.; Finkel, R.S.; Mah, J.K.; Deconinck, N.; Goemans, N.; Haberlova, J.; et al. Efficacy and Safety of Vamorolone vs Placebo and Prednisone among Boys with Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1005–1014. [Google Scholar] [CrossRef]
- Dent, K.M.; Dunn, D.M.; von Niederhausern, A.C.; Aoyagi, A.T.; Kerr, L.; Bromberg, M.B.; Hart, K.J.; Tuohy, T.; White, S.; den Dunnen, J.T.; et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am. J. Med. Genet. Part A 2005, 134, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Wong, B.; Barohn, R.; Campbell, C.; Comi, G.P.; Connolly, A.M.; Day, J.W.; Flanigan, K.M.; Goemans, N.; et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 2014, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, H.M.; Siddiqui, M.A.; Kanneganti, S.; Sharmin, N.; Chowdhury, M.W.; Nasim, M.T. Aminoglycoside-mediated promotion of translation readthrough occurs through a non-stochastic mechanism that competes with translation termination. Hum. Mol. Genet. 2018, 27, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Landfeldt, E.; Lindberg, C.; Sejersen, T. Improvements in health status and utility associated with ataluren for the treatment of nonsense mutation Duchenne muscular dystrophy. Muscle Nerve 2020, 61, 363–368. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Hu, K.Y.; Tian, Q.B.; Wei, L.G.; Zhao, Z.; Shen, H.R.; Hu, J. Early myocardial damage assessment in Dystrophinopathies using (99)Tc(m)-MIBI gated myocardial perfusion imaging. Ther. Clin. Risk Manag. 2015, 11, 1819–1827. [Google Scholar]
- Merlini, L.; Cecconi, I.; Parmeggiani, A.; Cordelli, D.M.; Dormi, A. Quadriceps muscle strength in Duchenne muscular dystrophy and effect of corticosteroid treatment. Acta Myol. 2020, 39, 200–206. [Google Scholar]
- Barp, A.; Bello, L.; Politano, L.; Melacini, P.; Calore, C.; Polo, A.; Vianello, S.; Sorarù, G.; Semplicini, C.; Pantic, B.; et al. Genetic Modifiers of Duchenne Muscular Dystrophy and Dilated Cardiomyopathy. PLoS ONE 2015, 10, e0141240. [Google Scholar] [CrossRef]
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E.; Cripe, L.H.; Duan, D.; Finder, J.D.; Groh, W.J.; Hoffman, E.P.; Judge, D.P.; et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar]
- Lee, H.; Song, J.; Kang, I.S.; Huh, J.; Yoon, J.A.; Shin, Y.B. Early prophylaxis of cardiomyopathy with beta-blockers and angiotensin receptor blockers in patients with Duchenne muscular dystrophy. Clin. Exp. Pediatr. 2022, 65, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Fanin, M.; Angelini, A.; Pegoraro, E.; Livi, U.; Danieli, G.A.; Hoffman, E.P.; Thiene, G.; Dalla Volta, S.; Angelini, C. Cardiac transplantation in a Duchenne muscular dystrophy carrier. Neuromuscul. Disord. 1998, 8, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Connuck, D.M.; Sleeper, L.A.; Colan, S.D.; Cox, G.F.; Towbin, J.A.; Lowe, A.M.; Wilkinson, J.D.; Orav, E.J.; Cuniberti, L.; Salbert, B.A.; et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: A comparative study from the Pediatric Cardiomyopathy Registry. Am. Heart J. 2008, 155, 998–1005. [Google Scholar] [CrossRef]
- Cohn, R.D.; van Erp, C.; Habashi, J.P.; Soleimani, A.A.; Klein, E.C.; Lisi, M.T.; Gamradt, M.; ap Rhys, C.M.; Holm, T.M.; Loeys, B.L.; et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007, 13, 204–210. [Google Scholar] [CrossRef]
- Duboc, D.; Meune, C.; Pierre, B.; Wahbi, K.; Eymard, B.; Toutain, A.; Berard, C.; Vaksmann, G.; Weber, S.; Bécane, H.M. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am. Heart J. 2007, 154, 596–602. [Google Scholar] [CrossRef]
- Shah, M.N.A.; Yokota, T. Cardiac therapies for Duchenne muscular dystrophy. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231182934. [Google Scholar] [CrossRef]
- Dhargave, P.; Nalini, A.; Nagarathna, R.; Sendhilkumar, R.; James, T.T.; Raju, T.R.; Sathyaprabha, T.N. Effect of Yoga and Physiotherapy on Pulmonary Functions in Children with Duchenne Muscular Dystrophy—A Comparative Study. Int. J. Yoga 2021, 14, 133–140. [Google Scholar]
- Harjpal, P.; Kovela, R.K.; Raipure, A.; Dandale, C.; Qureshi, M.I. The Refinement of Home Exercise Program for Children and Adolescents with Muscular Dystrophy in the Present COVID-19 Pandemic Scenario: A Scoping Review. Cureus 2022, 14, e29344. [Google Scholar] [CrossRef]
- Saad, F.A. Novel insights into the complex architecture of osteoporosis molecular genetics. Ann. N. Y. Acad. Sci. 2020, 1462, 37–52. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Krieg, A.M. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017, 27, 1–3. [Google Scholar] [CrossRef]
- Verhaart, I.E.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar]
- Abreu, N.J.; Waldrop, M.A. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr. Pulmonol. 2021, 56, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar]
- Mizobe, Y.; Miyatake, S.; Takizawa, H.; Hara, Y.; Yokota, T.; Nakamura, A.; Takeda, S.; Aoki, Y. In vivo Evaluation of single-exon and multiexon skipping in mdx52 mice. Methods Mol. Biol. 2018, 1828, 275–292. [Google Scholar]
- Ousterout, D.G.; Kabadi, A.M.; Thakore, P.I.; Majoros, W.H.; Reddy, T.E.; Gersbach, C.A. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 2015, 6, 6244. [Google Scholar] [PubMed]
- Chemello, F.; Chai, A.C.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Atmanli, A.; Mireault, A.A.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 2021, 7, eabg4910. [Google Scholar] [CrossRef]
- Ferreiro, V.; Giliberto, F.; Muñiz, G.M.; Francipane, L.; Marzese, D.M.; Mampel, A.; Roqué, M.; Frechtel, G.D.; Szijan, I. Asymptomatic Becker muscular dystrophy in a family with a multiexon deletion. Muscle Nerve 2009, 39, 239–243. [Google Scholar] [CrossRef]
- Ashby, D.W.; Williams, G.E.; Smith, O.E. Bone dystrophy in association with muscular dystrophy (myopathy). Br. Med. J. 1951, 1, 1486–1488. [Google Scholar] [CrossRef]
- Walton, J.N.; Warrick, C.K. Osseous changes in myopathy. Br. J. Radiol. 1954, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Wang, C.; Di Marco, M.; Horrocks, I.; Abu-Arafeh, I.; Baxter, A.; Cordeiro, N.; McLellan, L.; McWilliam, K.; Naismith, K.; et al. Fractures and bone health monitoring in boys with Duchenne muscular dystrophy managed within the Scottish Muscle Network. Neuromuscul. Disord. 2019, 29, 59–66. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Schwartz, B.D.; Mengle-Gaw, L.J.; Smith, E.C.; Castro, D.; Mah, J.K.; McDonald, C.M.; Kuntz, N.L.; Finkel, R.S.; Guglieri, M.; et al. Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology 2019, 93, e1312–e1323. [Google Scholar] [CrossRef]
- Griggs, R.C.; Mendell, J.R.; Brooke, M.H.; Fenichel, G.M.; Miller, J.P.; Province, M.; Moxley, R.T., 3rd; Huntzinger, D.; Vaughn, A.; Cohen, M.; et al. Clinical investigation in Duchenne dystrophy: V. Use of creatine kinase and pyruvate kinase in carrier detection. Muscle Nerve 1985, 8, 60–67. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Zhang, H.; Das, A.; Basu, M.; Malin, J.; Cao, K.; Hannenhalli, S. Comprehensive map of age-associated splicing changes across human tissues and their contributions to age-associated diseases. Sci. Rep. 2018, 8, 10929. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Millington, L.; Mahabeer, I.; van Ruiten, H. Duchenne muscular dystrophy. BMJ 2020, 368, l7012. [Google Scholar] [CrossRef] [PubMed]
- Saillour, Y.; Cossée, M.; Leturcq, F.; Vasson, A.; Beugnet, C.; Poirier, K.; Commere, V.; Sublemontier, S.; Viel, M.; Letourneur, F.; et al. Detection of exonic copy-number changes using a highly efficient oligonucleotide-based comparative genomic hybridization-array method. Hum. Mutat. 2008, 29, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Miike, T.; Iwashita, H.; Uyama, E.; Yoshioka, K.; Sugino, S.; Ando, M. PCR and immunoblot analyses of dystrophin in Becker muscular dystrophy. J. Neurol. Sci. 1994, 124, 225–229. [Google Scholar] [CrossRef]
- Niebrój-Dobosz, I.; Lukasiuk, M. Immunoblot analysis of sarcoplasmic calcium binding proteins in Duchenne muscular dystrophy. J. Neurol. 1995, 242, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.; Gabriel, G.; Drousiotou, A.; Meznanic-Petrusa, M.; Middleton, L. Dystrophinopathy presenting as congenital muscular dystrophy. Neuromuscul. Disord. 1994, 4, 387–392. [Google Scholar] [CrossRef]
- Atehortúa, S.C.; Lugo, L.H.; Ceballos, M.; Orozco, E.; Castro, P.A.; Arango, J.C.; Mateus, H.E. Cost-Effectiveness Analysis of Diagnosis of Duchenne/Becker Muscular Dystrophy in Colombia. Value Health Reg. Issues 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Dowling, P.; Zweyer, M.; Raucamp, M.; Henry, M.; Meleady, P.; Swandulla, D.; Ohlendieck, K. Proteomic and cell biological profiling of the renal phenotype of the mdx-4cv mouse model of Duchenne muscular dystrophy. Eur. J. Cell Biol. 2020, 99, 151059. [Google Scholar] [CrossRef]
- Nigro, V.; Savarese, M. Next-generation sequencing approaches for the diagnosis of skeletal muscle disorders. Curr. Opin. Neurol. 2016, 29, 621–627. [Google Scholar] [CrossRef]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
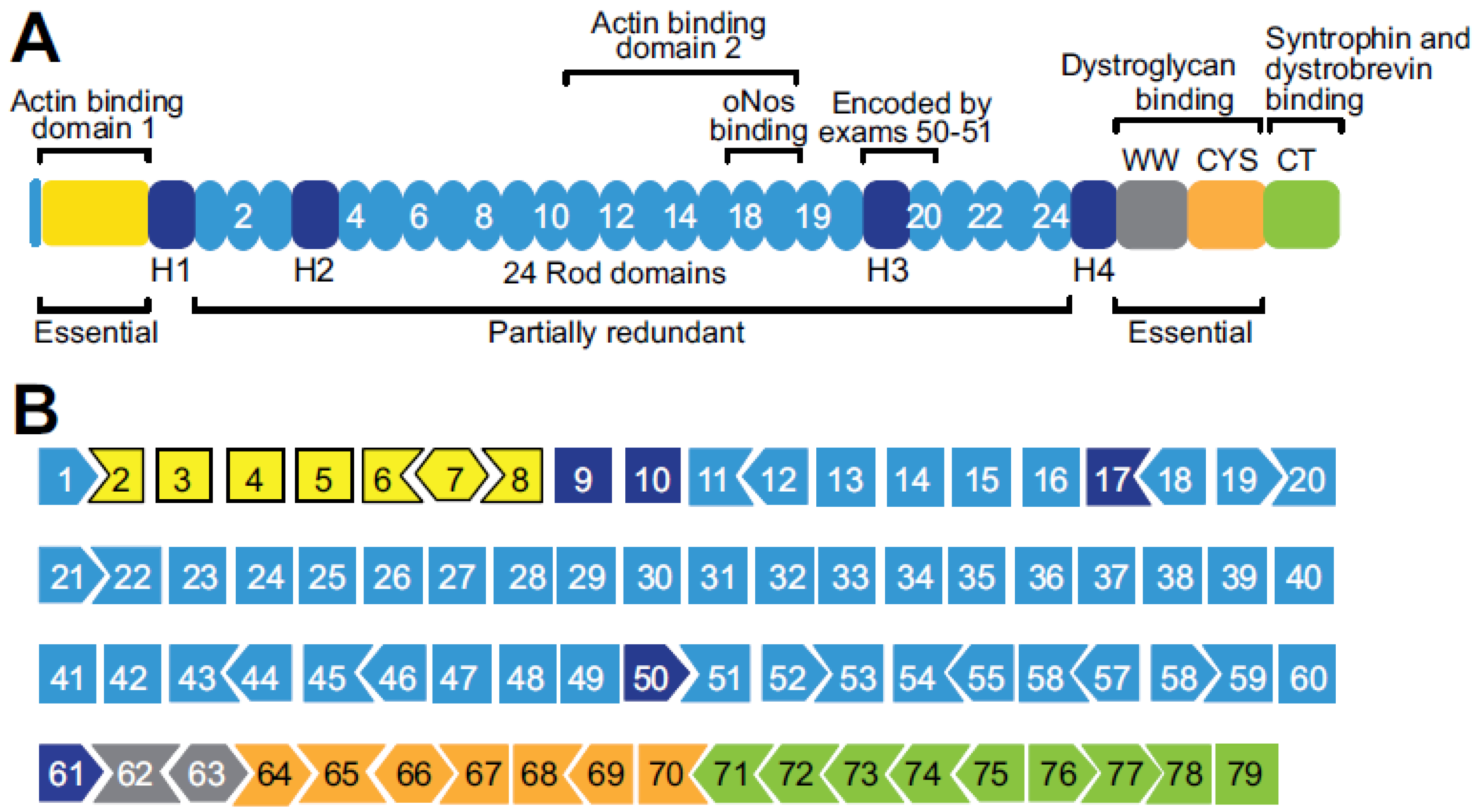
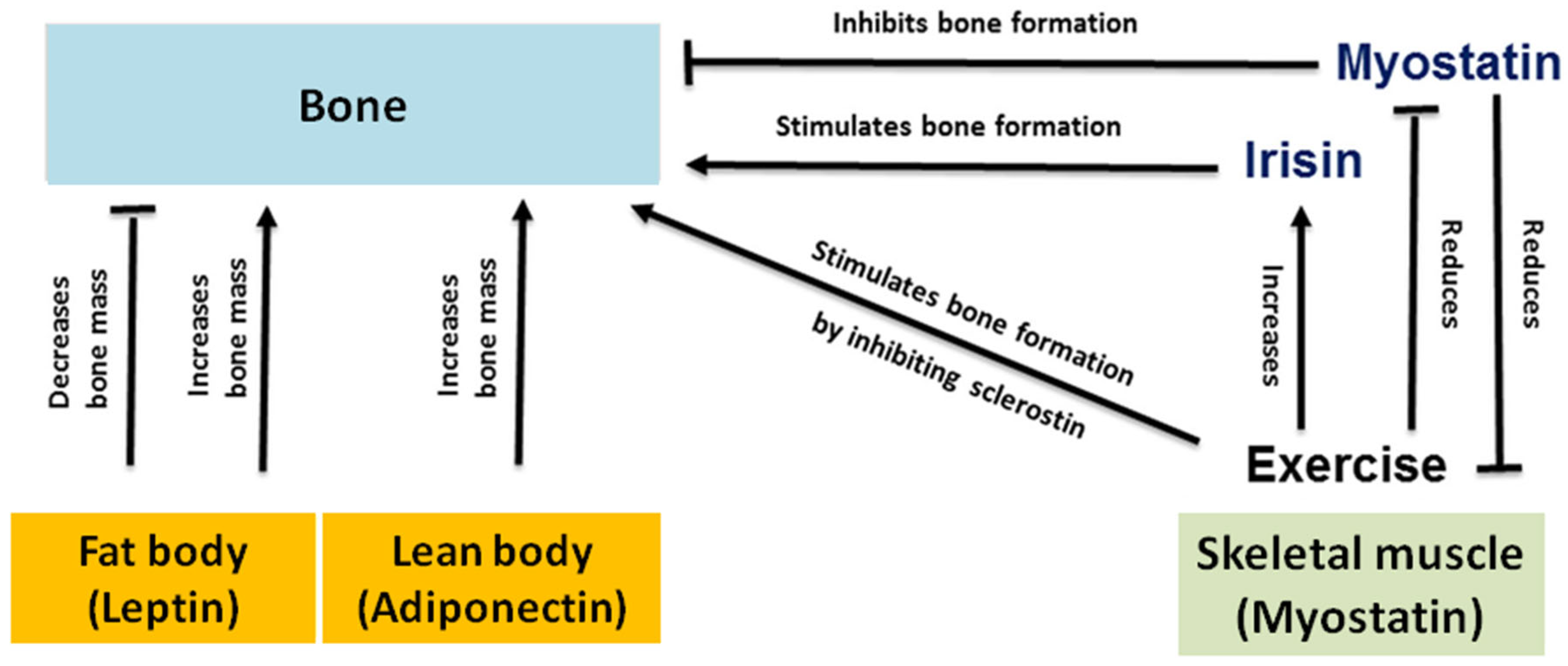
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, F.A.; Siciliano, G.; Angelini, C. Advances in Dystrophinopathy Diagnosis and Therapy. Biomolecules 2023, 13, 1319. https://doi.org/10.3390/biom13091319
Saad FA, Siciliano G, Angelini C. Advances in Dystrophinopathy Diagnosis and Therapy. Biomolecules. 2023; 13(9):1319. https://doi.org/10.3390/biom13091319
Chicago/Turabian StyleSaad, Fawzy A., Gabriele Siciliano, and Corrado Angelini. 2023. "Advances in Dystrophinopathy Diagnosis and Therapy" Biomolecules 13, no. 9: 1319. https://doi.org/10.3390/biom13091319
APA StyleSaad, F. A., Siciliano, G., & Angelini, C. (2023). Advances in Dystrophinopathy Diagnosis and Therapy. Biomolecules, 13(9), 1319. https://doi.org/10.3390/biom13091319





