Identification of Genes and miRNAs Associated with TAFI-Related Thrombosis: An in Silico Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of the TAFI Interactome and Associated miRNAs
2.2. FEA of the Predicted TAFI Interactors and Associated miRNAs
2.3. RNA Disease Analysis of the Predicted miRNAs
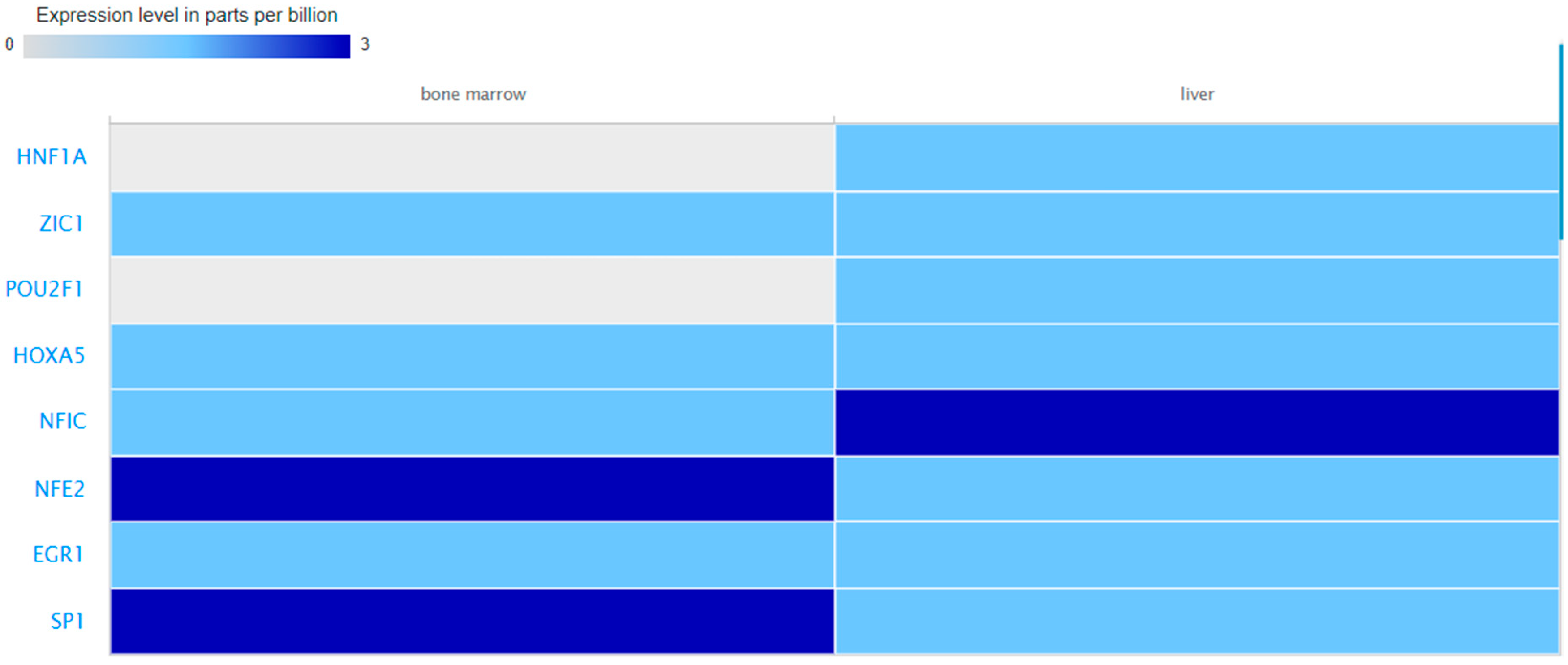
2.4. Tissue Expression Exploration of the Predicted miRNAs and TFs
3. Results
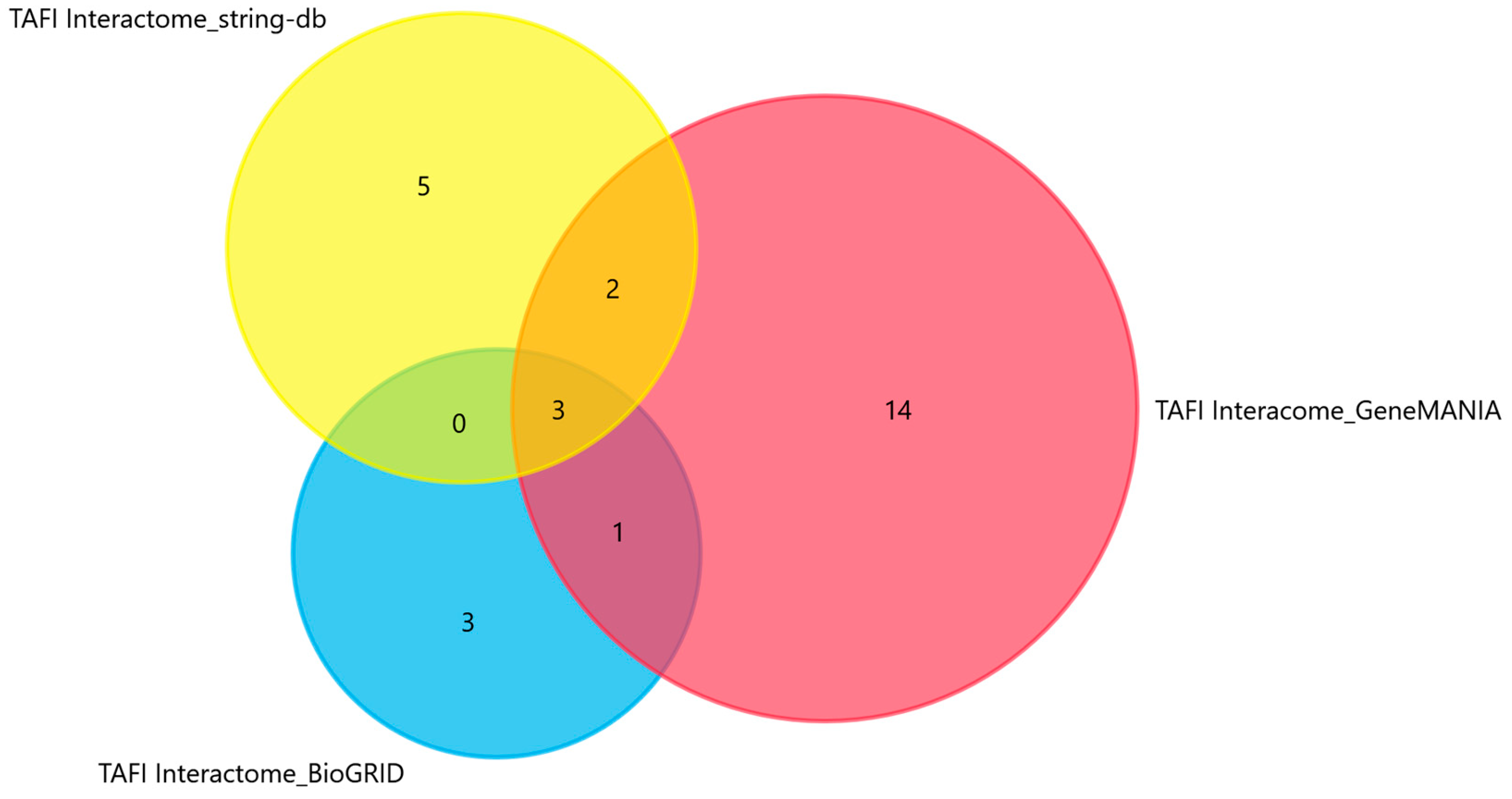
3.1. Predicted Interactors and Associated miRNAs of the CPB2 Gene Encoding the TAFI Proenzyme
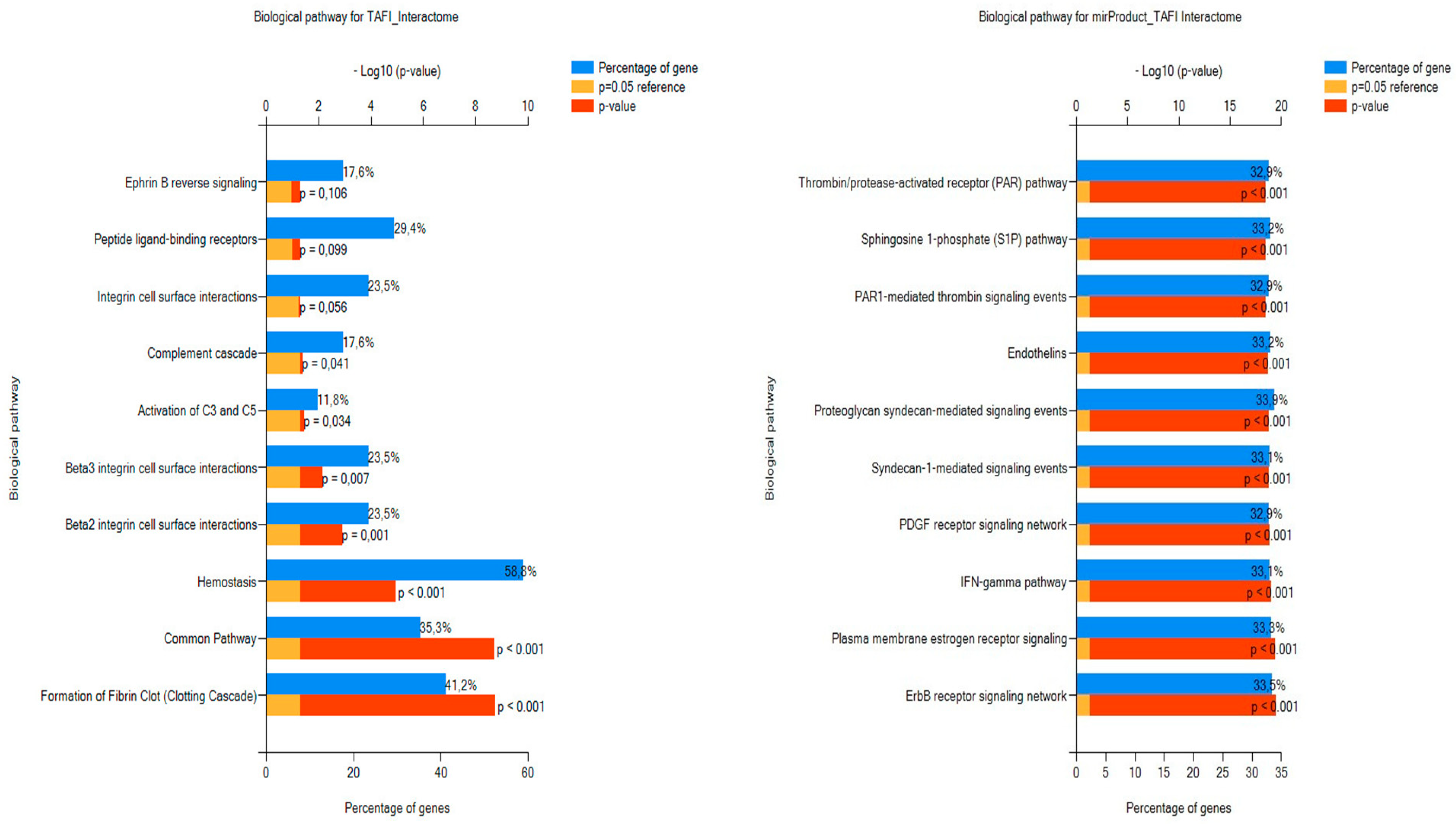
3.2. FEA Results for the Predicted TAFI Interactors and Associated miRNAs
3.2.1. DAVID FEA Results for the TAFI Interactome in Relation to Biological Processes and Tissue Expression
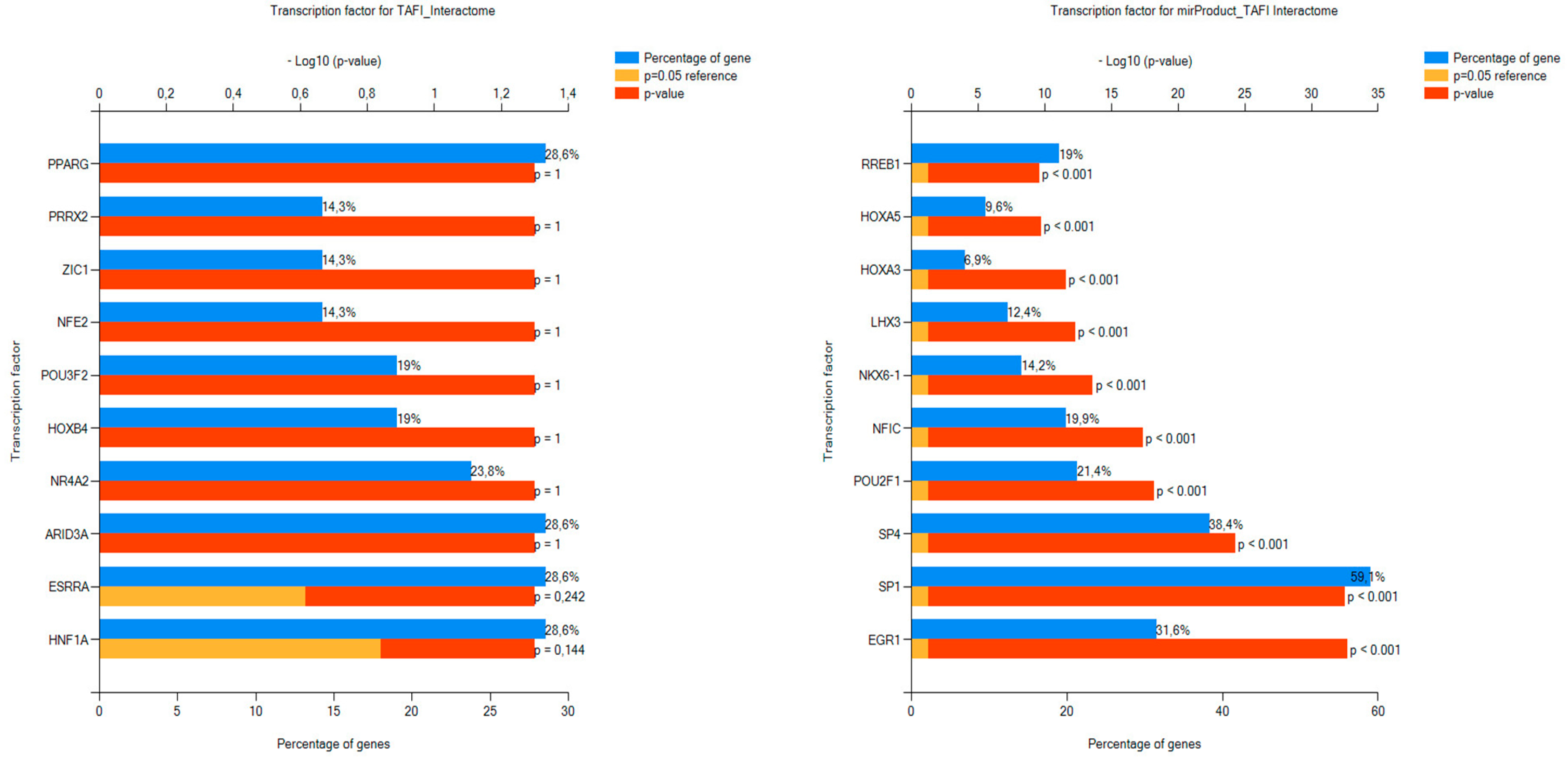
3.2.2. FunRich FEA Results for the TAFI Interactors and Associated miRNAs with Respect to BPs and TFs
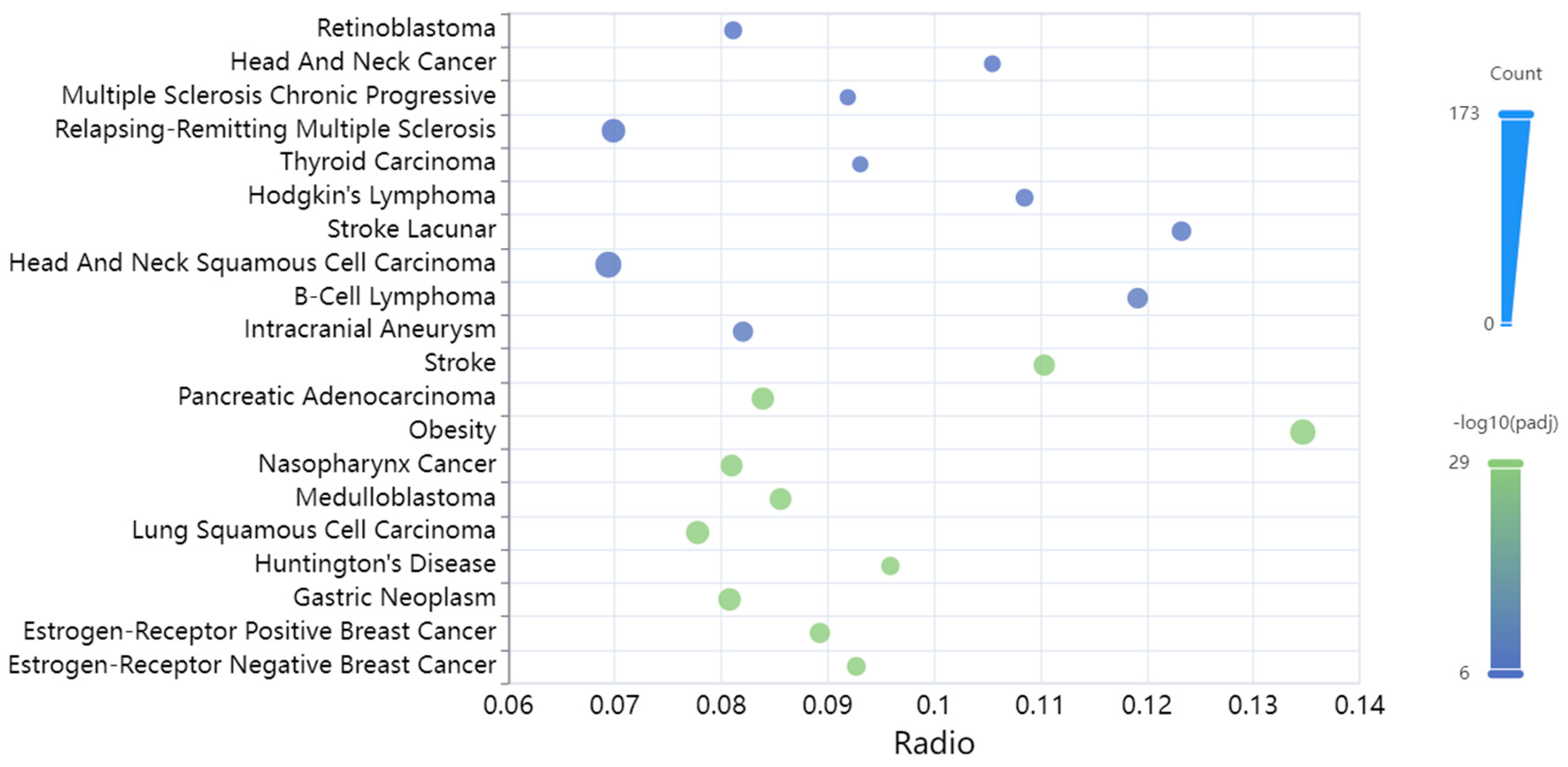
3.3. Diseases Associated with the miRNAs Targeting the TAFI Interactome
3.4. Expression Values of the Query miRNAs and TFs in Blood, Liver and Bone Marrow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furie, B. Pathogenesis of thrombosis. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 2009, 255–258. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Heit, J.A. 4—Thrombophilia: Clinical and Laboratory Assessment and Management. In Consultative Hemostasis and Thrombosis, 3rd ed.; Kitchens, C.S., Kessler, C.M., Konkle, B.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 205–239. ISBN 9781455722969. [Google Scholar]
- Eaton, D.L.; Malloy, B.E.; Tsai, S.P.; Henzel, W.; Drayna, D. Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma. J. Biol. Chem. 1991, 266, 21833–21838. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 3670. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Buijtenhuijs, P.; Marx, P.F.; Meijers, J.C.; Bouma, B.N. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood 2003, 101, 4844–4846. [Google Scholar] [CrossRef]
- Brailovsky, Y.; Lakhter, V.; Newman, J.; Allen, S.; Elkaryoni, A.; Desai, P.; Masic, D.; Bechara, C.F.; Bontekoe, E.; Hoppensteadt, D.; et al. Fibrinolytic Status and Risk of Death After Acute Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231162079. [Google Scholar] [CrossRef]
- Foley, J.H.; Kim, P.Y.; Mutch, N.J.; Gils, A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J. Thromb. Haemost. 2013, 11, 306–315. [Google Scholar] [CrossRef]
- Tregouet, D.A.; Schnabel, R.; Alessi, M.C.; Godefroy, T.; Declerck, P.J.; Nicaud, V.; Munzel, T.; Bickel, C.; Rupprecht, H.J.; Lubos, E.; et al. AtheroGene Investigators. Activated thrombin activatable fibrinolysis inhibitor levels are associated with the risk of cardiovascular death in patients with coronary artery disease: The AtheroGene study. J. Thromb. Haemost. 2009, 7, 49–57. [Google Scholar] [CrossRef]
- Fernandes, A.B.; Lima, L.M.; Sousa, M.O.; Toledo, V.D.P.; Kazmi, R.S.; Lwaleed, B.A.; Carvalho, M.D.G. Impaired fibrinolysis in angiographically documented coronary artery disease. Adv. Hematol. 2015, 2015, 214680. [Google Scholar] [CrossRef]
- Paola Cellai, A.; Antonucci, E.; Alessandrello Liotta, A.; Fedi, S.; Marcucci, R.; Falciani, M.; Giglioli, C.; Abbate, R.; Prisco, D. TAFI activity and antigen plasma levels are not increased in acute coronary artery disease patients admitted to a coronary care unit. Thromb. Res. 2006, 118, 495–500. [Google Scholar] [CrossRef]
- Schroeder, V.; Kucher, N.; Kohler, H.P. Role of thrombin activatable fibrinolysis inhibitor (TAFI) in patients with acute pulmonary embolism. J. Thromb. Haemost. 2003, 1, 492–493. [Google Scholar] [CrossRef]
- Yesudasan, S.; Averett, R.D. Recent advances in computational modeling of fibrin clot formation: A review. Comput. Biol. Chem. 2019, 83, 107148. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Fawzy, M.S.; Toraih, E.A. Data supporting the structural and functional characterization of Thrombin-Activatable Fibrinolysis Inhibitor in breast cancer. Data Brief. 2015, 5, 981–989. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Fonseka, P.; Pathan, M.; Chitti, S.V.; Kang, T.; Mathivanan, S. FunRich enables enrichment analysis of OMICs datasets. J. Mol. Biol. 2021, 433, 166747. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Garcia-Moreno, A.; López-Domínguez, R.; Villatoro-García, J.A.; Ramirez-Mena, A.; Aparicio-Puerta, E.; Hackenberg, M.; Pascual-Montano, A.; Carmona-Saez, P. Functional Enrichment Analysis of Regulatory Elements. Biomedicines 2022, 10, 590. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.; Hu, Y.; Ye, M.; Yao, L.; Wu, L.; Zhang, W.; Wang, M.; Deng, T.; Guo, F.; et al. RNADisease v4.0: An updated resource of RNA-associated diseases, providing RNA-disease analysis, enrichment and prediction. Nucleic Acids Res. 2023, 51, D1397–D1404. [Google Scholar] [CrossRef]
- Kavakiotis, I.; Alexiou, A.; Tastsoglou, S.; Vlachos, I.S.; Hatzigeorgiou, A.G. DIANA-miTED: A microRNA tissue expression database. Nucleic Acids Res. 2022, 50, D1055–D1061. [Google Scholar] [CrossRef]
- Moreno, P.; Fexova, S.; George, N.; Manning, J.R.; Miao, Z.; Mohammed, S.; Muñoz-Pomer, A.; Fullgrabe, A.; Bi, Y.; Bush, N.; et al. Expression Atlas update: Gene and protein expression in multiple species. Nucleic Acids Res. 2022, 50, D129–D140. [Google Scholar] [CrossRef]
- Digre, A.; Lindskog, C. The Human Protein Atlas-Spatial localization of the human proteome in health and disease. Protein Sci. 2021, 30, 218–233. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Rawish, E.; Sauter, M.; Sauter, R.; Nording, H.; Langer, H.F. Complement, inflammation and thrombosis. Br. J. Pharmacol. 2021, 178, 2892–2904. [Google Scholar] [CrossRef]
- Posma, J.J.; Grover, S.P.; Hisada, Y.; Owens, A.P., 3rd; Antoniak, S.; Spronk, H.M.; Mackman, N. Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 13–24. [Google Scholar] [CrossRef]
- Mahajan-Thakur, S.; Böhm, A.; Jedlitschky, G.; Schrör, K.; Rauch, B.H. Sphingosine-1-Phosphate and Its Receptors: A Mutual Link between Blood Coagulation and Inflammation. Mediat. Inflamm. 2015, 2015, 831059. [Google Scholar] [CrossRef]
- Chen, X.; Geng, X.; Jin, S.; Xu, J.; Guo, M.; Shen, D.; Ding, X.; Liu, H.; Xu, X. The Association of Syndecan-1, Hypercoagulable State and Thrombosis and in Patients with Nephrotic Syndrome. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211010256. [Google Scholar] [CrossRef]
- Alhabibi, A.M.; Eldewi, D.M.; Wahab, M.A.A.; Farouk, N.; El-Hagrasy, H.A.; Saleh, O.I. Platelet-derived growth factor-beta as a new marker of deep venous thrombosis. J. Res. Med. Sci. 2019, 24, 48. [Google Scholar]
- Bertin, F.R.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Henry, K.M.; Herman, K.D.; Thompson, A.A.; Isles, H.M.; Tulotta, C.; Sammut, D.; Rougeot, J.J.; Khoshaein, N.; Reese, A.E.; et al. Inhibition of ErbB kinase signalling promotes resolution of neutrophilic inflammation. Elife 2019, 8, e50990. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Y.; Zhang, J.; Dong, J.F.; Zhao, Z. Transcription factors in megakaryocytes and platelets. Front. Immunol. 2023, 14, 1140501. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Liu, Z. Impact of Neutrophil Extracellular Traps on Thrombosis Formation: New Findings and Future Perspective. Front. Cell. Infect. Microbiol. 2022, 12, 910908. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Ding, J.; You, Y.; Dong, Z.; Chehade, H.; Alvero, A.; Mor, Y.; Draghici, S.; Mor, G. Identification of key signaling pathways induced by SARS-CoV2 that underlie thrombosis and vascular injury in COVID-19 patients. J. Leukoc. Biol. 2021, 109, 35–47. [Google Scholar] [CrossRef]
- Danckwardt, S.; Trégouët, D.A.; Castoldi, E. Post-transcriptional control of hemostatic genes: Mechanisms and emerging therapeutic concepts in thrombo-inflammatory disorders. Cardiovasc. Res. 2023, 119, cvad046. [Google Scholar] [CrossRef]
- Plé, H.; Landry, P.; Benham, A.; Coarfa, C.; Gunaratne, P.H.; Provost, P. The repertoire and features of human platelet microRNAs. PLoS ONE 2012, 7, e50746. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Cereghini, S. Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996, 10, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Suwanichkul, A. HNF1 activates transcription of the human gene for insulin-like growth factor binding protein-1. DNA Cell Biol. 1993, 12, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Babajko, S.; Tronche, F.; Groyer, A. Liver-specific expression of human insulin-like growth factor binding protein 1: Functional role of transcription factor HNF1 in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A.; Rosenblatt, M.F.; Zucker-Franklin, D.; Jackson, C.W.; Hunt, P.; Saris, C.J.; Orkin, S.H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 1995, 81, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Jalapothu, D.; Boieri, M.; Crossland, R.E.; Shah, P.; Butt, I.A.; Norden, J.; Dressel, R.; Dickinson, A.M.; Inngjerdingen, M. Tissue-Specific Expression Patterns of MicroRNA during Acute Graft-versus-Host Disease in the Rat. Front. Immunol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Galmiche, A.; Rak, J.; Roumenina, L.T.; Saidak, Z. Coagulome and the tumor microenvironment: An actionable interplay. Trends Cancer. 2022, 8, 369–383. [Google Scholar] [CrossRef]
- Ortiz, A.F.H.; Suriano, E.S.; Eltawil, Y.; Sekhon, M.; Gebran, A.; Garland, M.; Cuenca, N.T.R.; Cadavid, T.; Almarie, B. Prevalence and risk factors of unruptured intracranial aneurysms in ischemic stroke patients—A global meta-analysis. Surg. Neurol. Int. 2023, 14, 222. [Google Scholar] [CrossRef]
- Chang, J.C. Stroke Classification: Critical Role of Unusually Large von Willebrand Factor Multimers and Tissue Factor on Clinical Phenotypes Based on Novel “Two-Path Unifying Theory” of Hemostasis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620913634. [Google Scholar] [CrossRef]
- Marta-Enguita, J.; Navarro-Oviedo, M.; Muñoz, R.; Olier-Arenas, J.; Zalba, G.; Lecumberri, R.; Mendioroz, M.; Paramo, J.A.; Roncal, C.; Orbe, J. Inside the Thrombus: Association of Hemostatic Parameters with Outcomes in Large Vessel Stroke Patients. Front. Neurol. 2021, 12, 599498. [Google Scholar] [CrossRef]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef]
- Desilles, J.P.; Di Meglio, L.; Delvoye, F.; Maïer, B.; Piotin, M.; Ho-Tin-Noé, B.; Mazighi, M. Composition and Organization of Acute Ischemic Stroke Thrombus: A Wealth of Information for Future Thrombolytic Strategies. Front. Neurol. 2022, 13, 870331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ajimu, K.; Yalikun, N.; Zheng, Y.; Xu, F. Potential Therapeutic Strategies for Intracranial Aneurysms Targeting Aneurysm Pathogenesis. Front. Neurosci. 2019, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, Y.; Liu, X.; Meng, X.; Li, Y. Cell-free microRNA-21: Biomarker for intracranial aneurysm rupture. Chin. Neurosurg. J. 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, J.; Liang, D.; Sun, H. Extracellular Vesicles and Their Associated miRNAs as Potential Biomarkers in Intracranial Aneurysm. Front. Mol. Biosci. 2022, 9, 785314. [Google Scholar] [CrossRef]
- Luo, M.; Du, M.; Shu, C.; Liu, S.; Li, J.; Zhang, L.; Li, X. The Function of microRNAs in Pulmonary Embolism: Review and Research Outlook. Front. Pharmacol. 2021, 12, 743945. [Google Scholar] [CrossRef]
- Sobrero, M.; Montecucco, F.; Carbone, F. Circulating MicroRNAs for Diagnosis of Acute Pulmonary Embolism: Still a Long Way to Go. Biomed. Res. Int. 2022, 2022, 4180215. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Gene Name as Retrieved by GeneCards |
|---|---|
| F2 | Coagulation Factor II, Thrombin |
| THBD | Thrombomodulin |
| PLG | Plasminogen |
| C5 | Complement C5 |
| C3 | Complement C3 |
| F2R | Coagulation Factor II Thrombin Receptor |
| SERPINC1 | Serpin Family C Member 1 |
| AGT | Angiotensinogen |
| UBE2A | Ubiquitin Conjugating Enzyme E2 A |
| FGA | Fibrinogen Alpha Chain |
| A2M | Alpha-2-Macroglobulin |
| ERO1LB | Endoplasmic Reticulum Oxidoreductase 1 Beta |
| FN1 | Fibronectin 1 |
| TINAG | Tubulointerstitial Nephritis Antigen |
| FGG | Fibrinogen Gamma Chain |
| FGB | Fibrinogen Beta Chain |
| PITRM1 | Pitrilysin Metallopeptidase 1 |
| CPA1 | Carboxypeptidase A1 |
| CPA6 | Carboxypeptidase A6 |
| CPA5 | Carboxypeptidase A5 |
| CPA4 | Carboxypeptidase A4 |
| CPB1 | Carboxypeptidase B1 |
| ACY1 | Aminoacylase 1 |
| CPA2 | Carboxypeptidase A2 |
| CPA3 | Carboxypeptidase A3 |
| MBL2 | Mannose Binding Lectin 2 |
| CP | Ceruloplasmin |
| CYP2C8 | Cytochrome P450 Family 2 Subfamily C Member 8 |
| miRNAs | RNA ID |
|---|---|
| hsa-miR-18a-5p | MIMAT0000072 |
| hsa-miR-142-3p | MIMAT0000434 |
| hsa-miR-18b-5p | MIMAT0001412 |
| hsa-miR-4735-3p | MIMAT0019861 |
| hsa-miR-96-5p | MIMAT0000095 |
| hsa-miR-1-3p | MIMAT0000416 |
| hsa-miR-206 | MIMAT0000462 |
| hsa-miR-613 | MIMAT0003281 |
| hsa-miR-1271-5p | MIMAT0005796 |
| hsa-miR-203a-3p | MIMAT0000264 |
| hsa-miR-224-5p | MIMAT0000281 |
| hsa-let-7a-5p | MIMAT0000062 |
| hsa-let-7b-5p | MIMAT0000063 |
| hsa-let-7c-5p | MIMAT0000064 |
| hsa-let-7d-5p | MIMAT0000065 |
| hsa-let-7e-5p | MIMAT0000066 |
| hsa-let-7f-5p | MIMAT0000067 |
| hsa-miR-98-5p | MIMAT0000096 |
| hsa-let-7g-5p | MIMAT0000414 |
| hsa-let-7i-5p | MIMAT0000415 |
| hsa-miR-4458 | MIMAT0018980 |
| hsa-miR-4500 | MIMAT0019036 |
| hsa-miR-183-5p | MIMAT0000261 |
| hsa-miR-212-5p | MIMAT0022695 |
| Term | Gene Count | p-Value | Benjamini |
|---|---|---|---|
| Hemostasis | 8 | 1.2E−13 | 1.5E−12 |
| Blood coagulation | 8 | 1.2E−13 | 1.5E−12 |
| Complement pathway | 3 | 9.1E−4 | 7.6E−3 |
| Innate immunity | 5 | 1.7E−3 | 1.1E−2 |
| Complement alternate pathway | 2 | 1.6E−2 | 7.9E−2 |
| Acute phase | 2 | 2.6E−2 | 1.0E−1 |
| Immunity | 5 | 2.9E−2 | 1.0E−1 |
| Term | Gene Count | p-Value | Benjamini |
|---|---|---|---|
| Plasma | 12 | 7.4E−14 | 4.1E−12 |
| Cerebrospinal fluid | 3 | 1.9E−3 | 5.4E−2 |
| Liver | 15 | 4.8E−3 | 8.4E−2 |
| Platelet | 5 | 6.0E−3 | 8.4E−2 |
| Serum | 2 | 2.9E−2 | 2.9E−1 |
| Bile | 2 | 3.1E−2 | 2.9E−1 |
| Urine | 2 | 4.0E−2 | 3.2E−1 |
| Milk | 2 | 4.6E−2 | 3.2E−1 |
| Blood | 4 | 8.6E−2 | 5.3E−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouka, E.; Zarogiannis, S.G.; Hatzoglou, C.; Gourgoulianis, K.I.; Malli, F. Identification of Genes and miRNAs Associated with TAFI-Related Thrombosis: An in Silico Study. Biomolecules 2023, 13, 1318. https://doi.org/10.3390/biom13091318
Rouka E, Zarogiannis SG, Hatzoglou C, Gourgoulianis KI, Malli F. Identification of Genes and miRNAs Associated with TAFI-Related Thrombosis: An in Silico Study. Biomolecules. 2023; 13(9):1318. https://doi.org/10.3390/biom13091318
Chicago/Turabian StyleRouka, Erasmia, Sotirios G. Zarogiannis, Chrissi Hatzoglou, Konstantinos I. Gourgoulianis, and Foteini Malli. 2023. "Identification of Genes and miRNAs Associated with TAFI-Related Thrombosis: An in Silico Study" Biomolecules 13, no. 9: 1318. https://doi.org/10.3390/biom13091318
APA StyleRouka, E., Zarogiannis, S. G., Hatzoglou, C., Gourgoulianis, K. I., & Malli, F. (2023). Identification of Genes and miRNAs Associated with TAFI-Related Thrombosis: An in Silico Study. Biomolecules, 13(9), 1318. https://doi.org/10.3390/biom13091318









