LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies and Other Reagents
2.3. Tissue Immunofluorescence Staining of ICAM-1
2.4. Flow Cytometric Analysis of LFA-1 Expression on Peripheral Tissue Lymphocytes
2.5. In Vivo Lymphocyte Migration Assays
2.6. Analysis of Lymphocyte Subsets in LNs
2.7. Data Analysis
3. Results
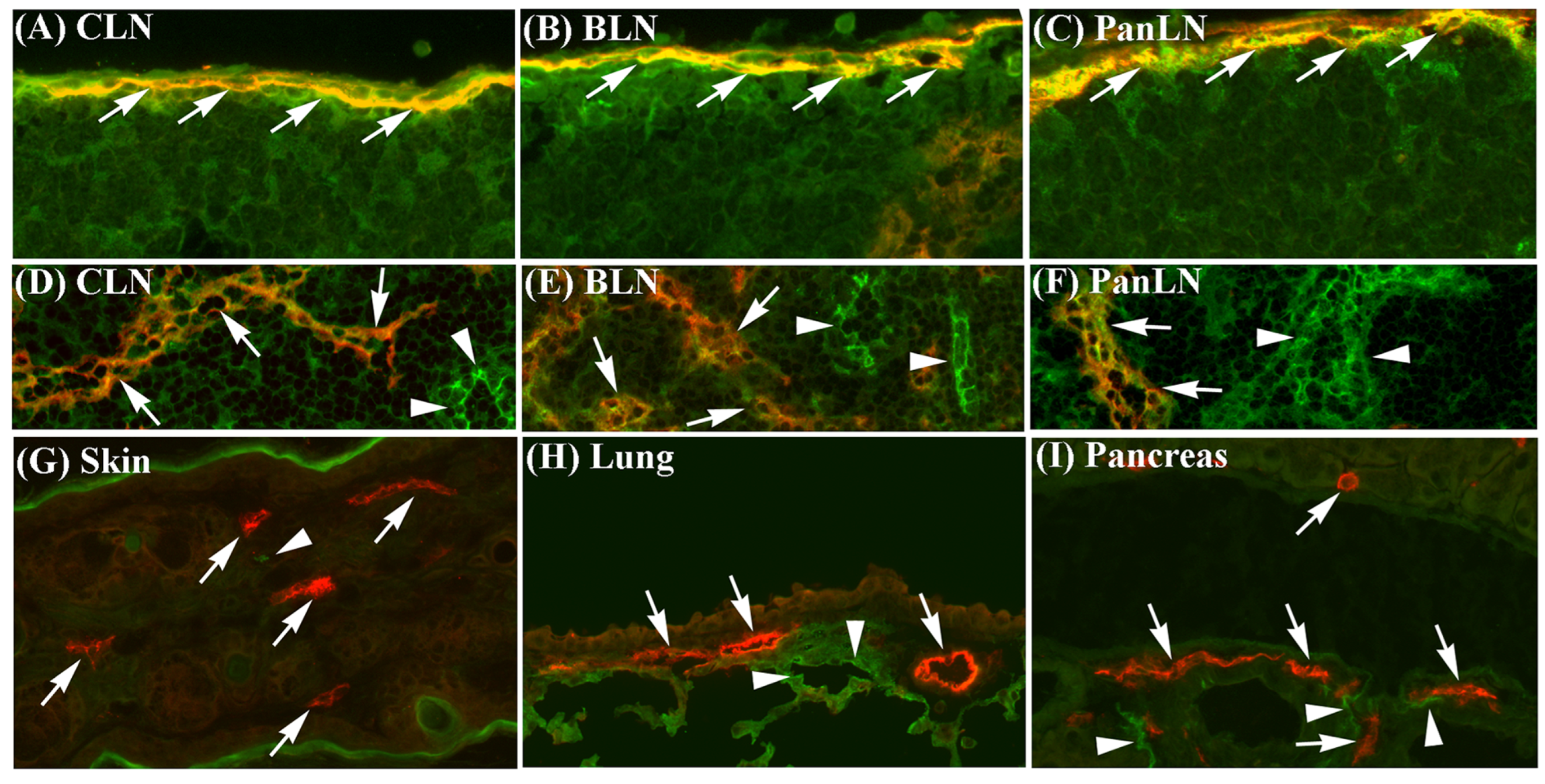
3.1. ICAM-1 Is Expressed on Sinus Endothelia in LNs but Not on Lymphatic Vessel Endothelia in Peripheral Tissues
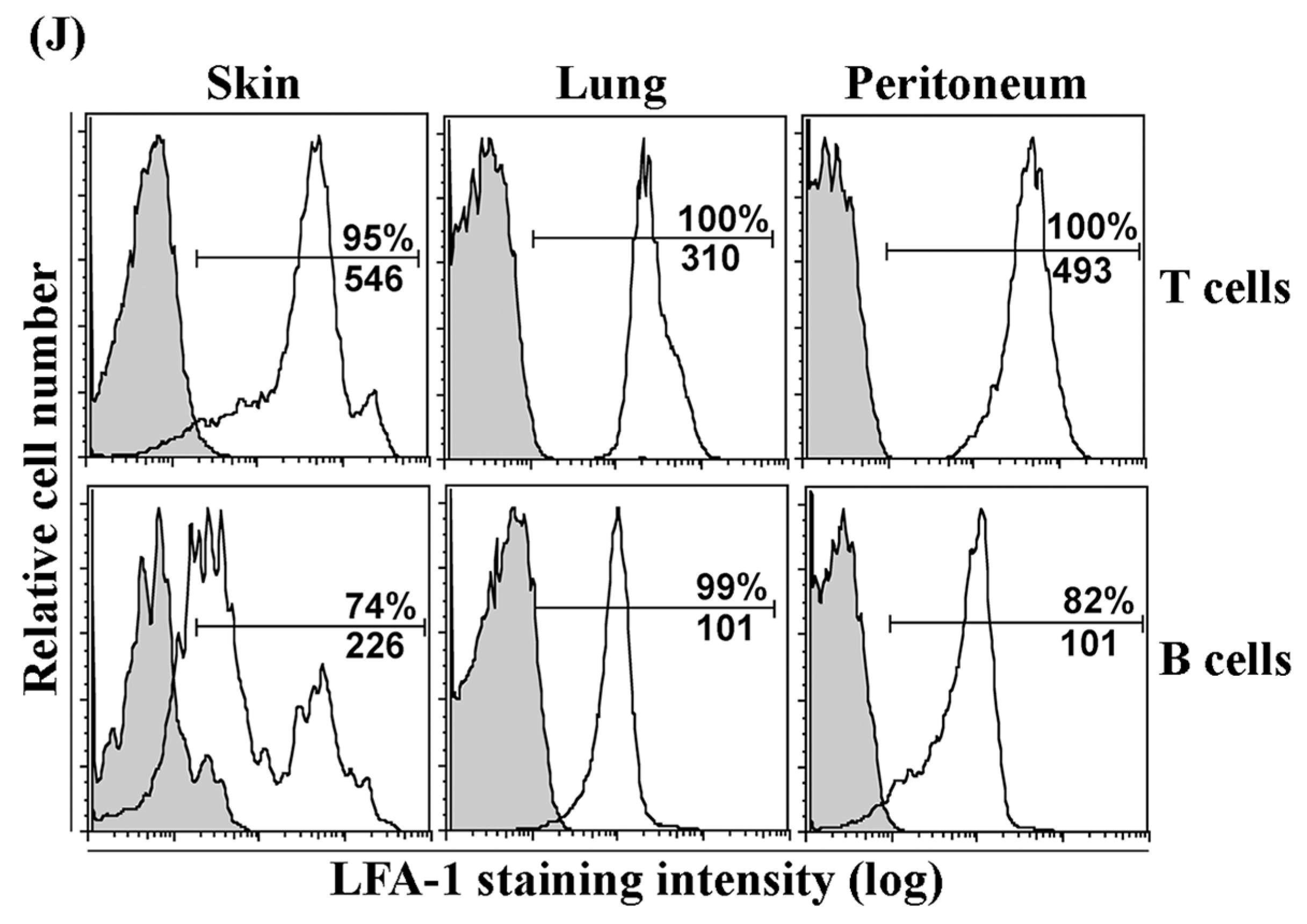
3.2. LFA-1 Is Expressed on Peripheral Tissue Lymphocytes
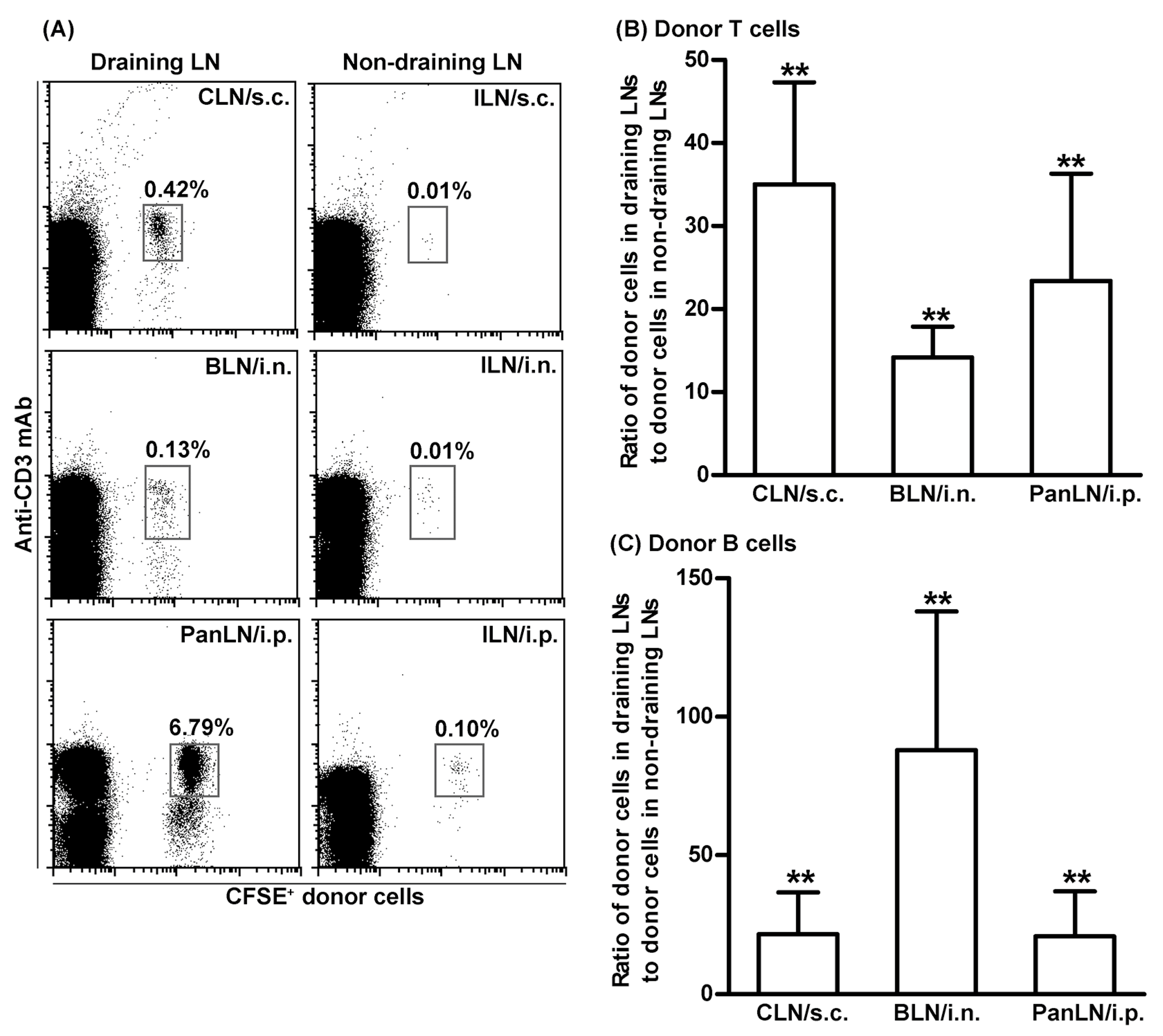
3.3. T Cells Migrate More Efficiently Than B Cells from Peripheral Tissues into Draining LNs
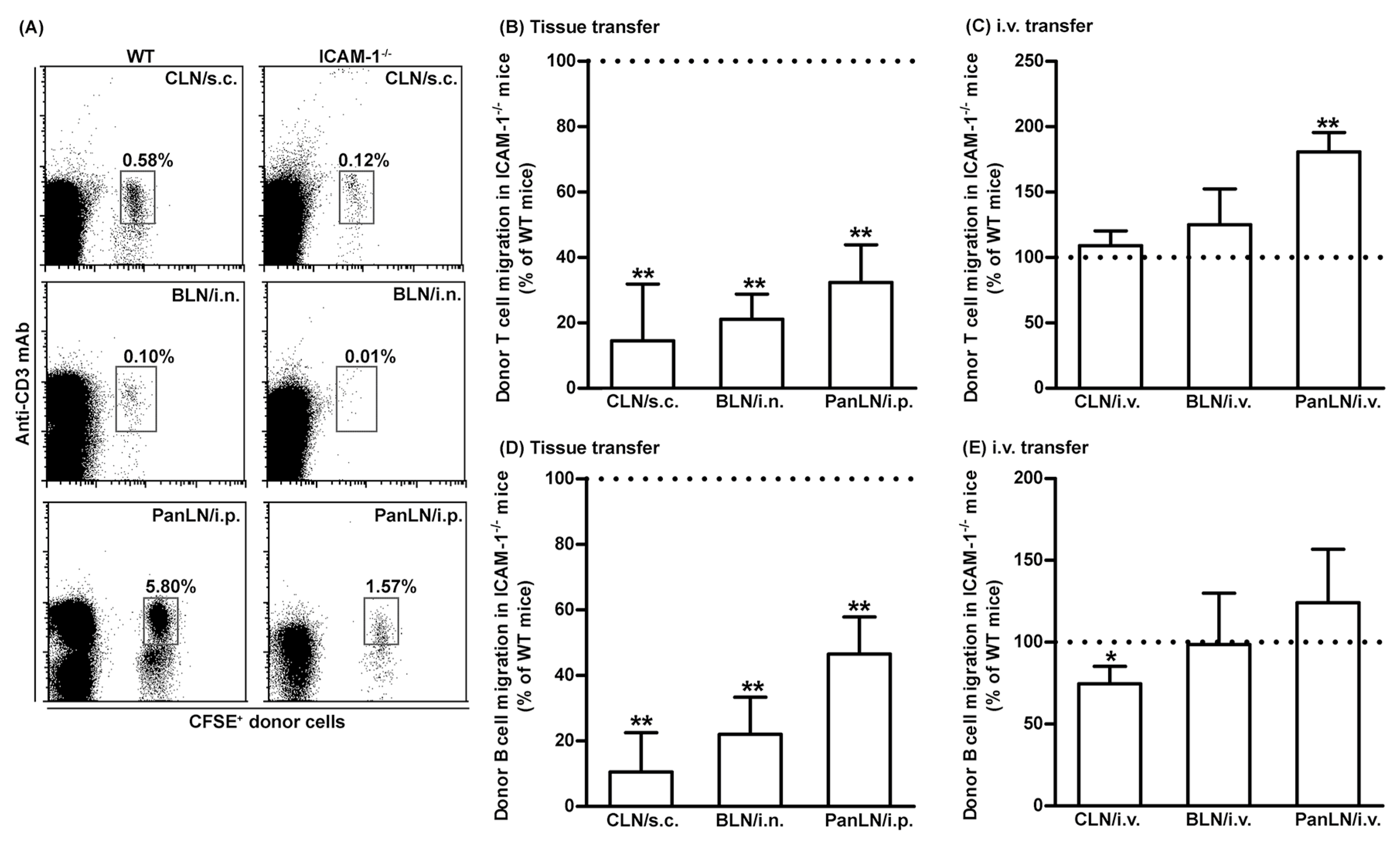
3.4. ICAM-1 Deficiency in Host Tissues Impairs the Migration of Lymphocytes from Peripheral Tissues into Draining LNs, but Not from Blood Vessels into LNs
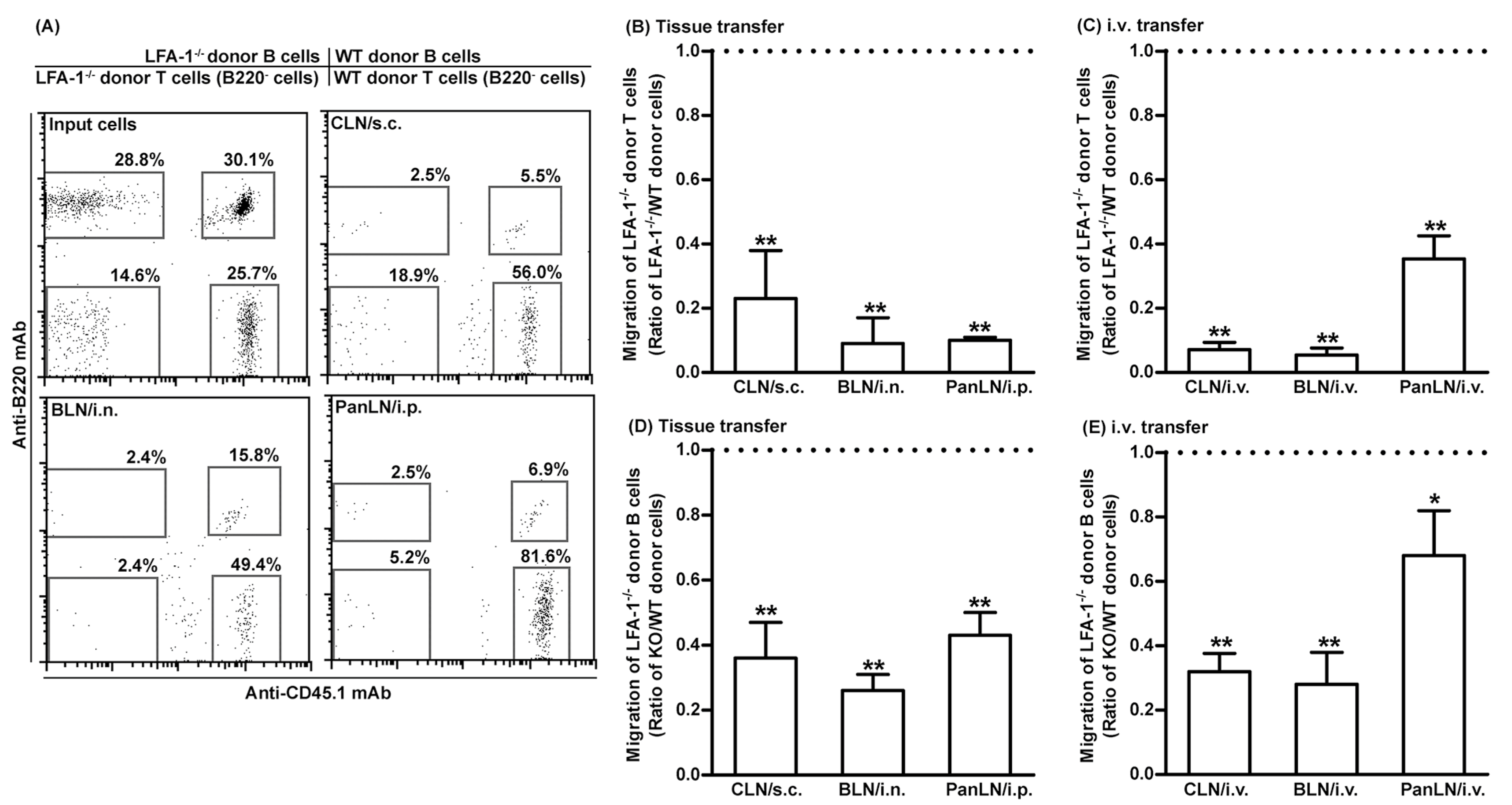
3.5. LFA-1-Deficient Lymphocytes Migrate Poorly from Peripheral Tissues into Draining LNs
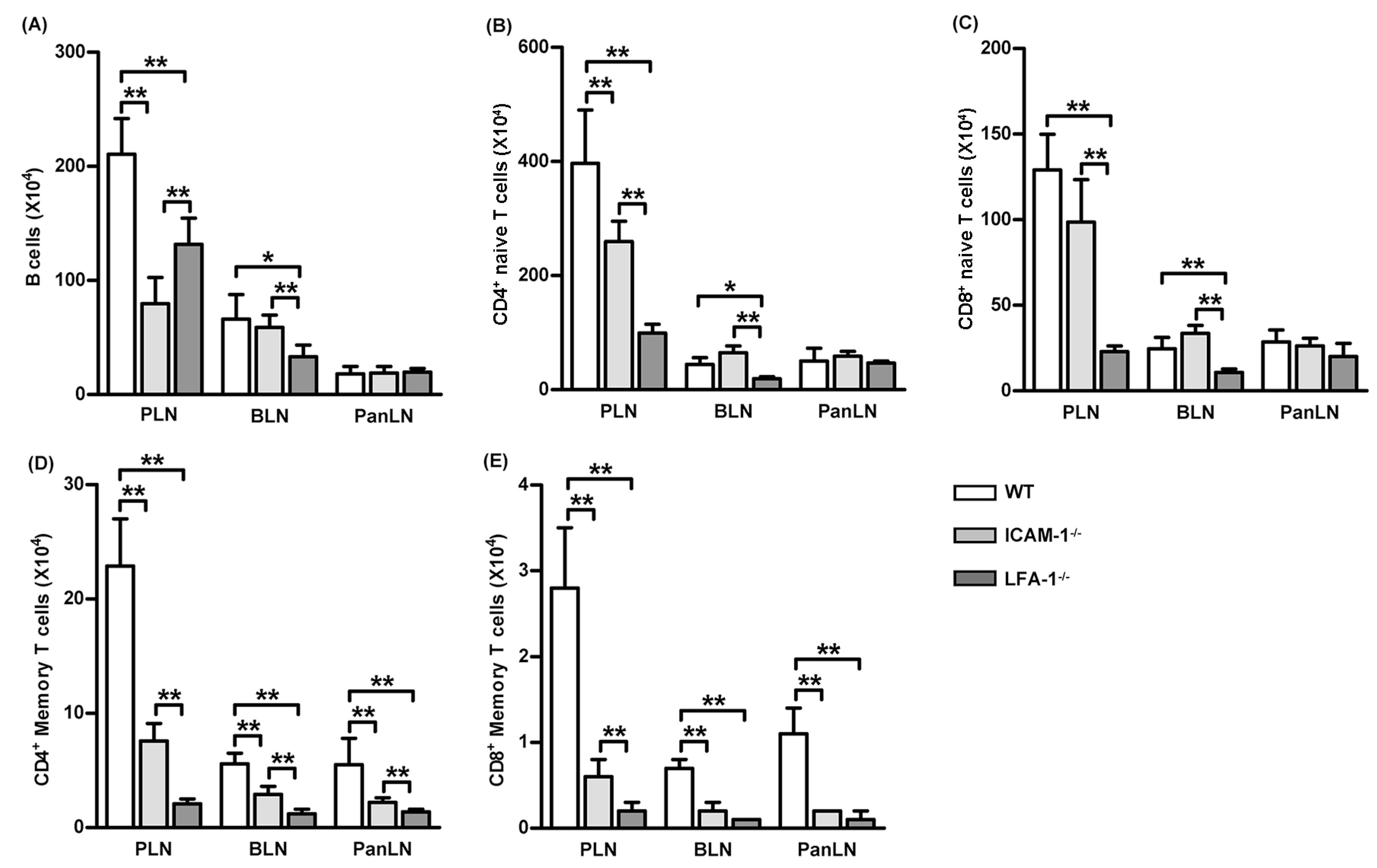
3.6. Lack of the LFA-1/ICAM-1 Adhesion Pathway Reduces the Number of Memory T Cells in LNs
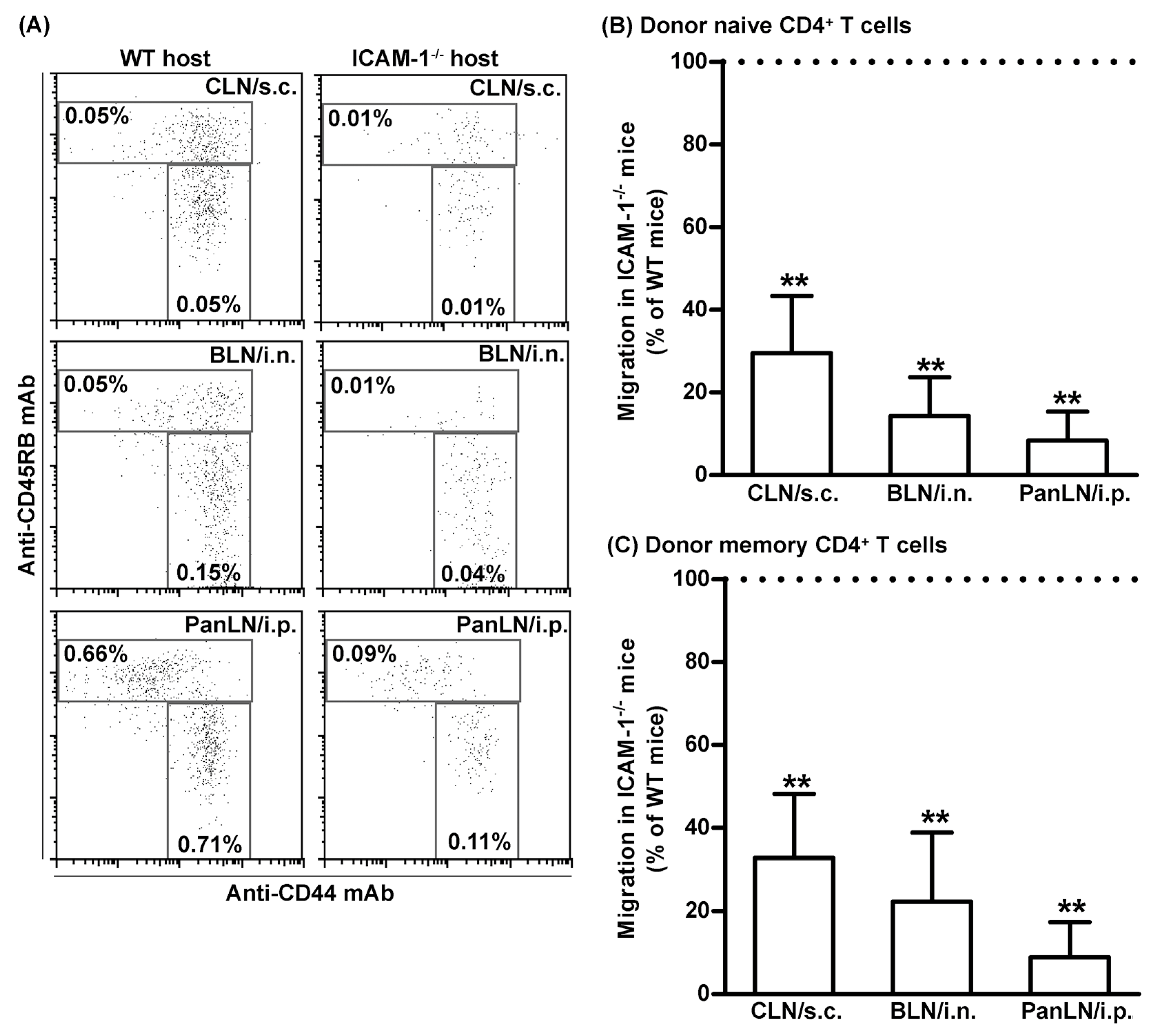
3.7. ICAM-1 Deficiency in Host Tissues Impairs the Migration of Both Naive and Memory CD4+ T Cells from Peripheral Tissues into Draining LNs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| BLN | Bronchial LN |
| CFSE | Carboxyfluorescein succinimidyl ester |
| CLN | Cervical LN |
| HEV | High endothelial venule |
| ICAM-1 | Intercellular adhesion molecule-1 |
| LFA-1 | Leukocyte function-associated antigen-1 |
| LN | Lymph node |
| LYVE-1 | Lymphatic vessel endothelial receptor-1 |
| mAb | Monoclonal antibody |
| PanLN | Pancreatic LN |
| PLN | Peripheral LN |
| WT | Wild type |
References
- Campbell, D.J.; Kim, C.H.; Butcher, E.C. Chemokines in the systemic organization of immunity. Immunol. Rev. 2003, 195, 58–71. [Google Scholar] [CrossRef]
- Bajénoff, M.; Egen, J.G.; Qi, H.; Huang, A.Y.; Castellino, F.; Germain, R.N. Highways, byways and breadcrumbs: Directing lymphocyte traffic in the lymph node. Trends Immunol. 2007, 28, 346–352. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- von Andrian, U.H.; Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003, 3, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995, 57, 827–872. [Google Scholar] [CrossRef] [PubMed]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef]
- Mackay, C.R.; Kimpton, W.G.; Brandon, M.R.; Cahill, R.N. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med. 1988, 167, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R.; Marston, W.L.; Dudler, L. Naive and memory t cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 1990, 171, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.G.; Morris, B. The origin of the cells in the efferent lymph from a single lymph node. J. Exp. Med. 1965, 121, 901–910. [Google Scholar] [CrossRef]
- Reinhardt, R.L.; Khoruts, A.; Merica, R.; Zell, T.; Jenkins, M.K. Visualizing the generation of memory cd4 t cells in the whole body. Nature 2001, 410, 101–105. [Google Scholar] [CrossRef]
- Masopust, D.; Vezys, V.; Marzo, A.L.; Lefrançois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001, 291, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Cose, S.; Brammer, C.; Khanna, K.M.; Masopust, D.; Lefrançois, L. Evidence that a significant number of naive t cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 2006, 36, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Luettig, B.; Pape, L.; Bode, U.; Bell, E.B.; Sparshott, S.M.; Wagner, S.; Westermann, J. Naive and memory t lymphocytes migrate in comparable numbers through normal rat liver: Activated t cells accumulate in the periportal field. J. Immunol. 1999, 163, 4300–4307. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J. The physiology of lymphocyte migration through the single lymph node in vivo. Semin. Immunol. 1999, 11, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wagner, N.; Pham, L.N.; Magno, V.; Shan, Z.; Butcher, E.C.; Michie, S.A. Lymphocyte homing to bronchus-associated lymphoid tissue (balt) is mediated by l-selectin/pnad, alpha4beta1 integrin/vcam-1, and lfa-1 adhesion pathways. J. Exp. Med. 2003, 197, 1255–1267. [Google Scholar] [CrossRef]
- Nakano, H.; Mori, S.; Yonekawa, H.; Nariuchi, H.; Matsuzawa, A.; Kakiuchi, T. A novel mutant gene involved in t-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood 1998, 91, 2886–2895. [Google Scholar] [CrossRef]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. Ccr7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef]
- Gunn, M.D.; Kyuwa, S.; Tam, C.; Kakiuchi, T.; Matsuzawa, A.; Williams, L.T.; Nakano, H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999, 189, 451–460. [Google Scholar] [CrossRef]
- Stein, J.V.; Rot, A.; Luo, Y.; Narasimhaswamy, M.; Nakano, H.; Gunn, M.D.; Matsuzawa, A.; Quackenbush, E.J.; Dorf, M.E.; von Andrian, U.H. The cc chemokine thymus-derived chemotactic agent 4 (tca-4, secondary lymphoid tissue chemokine, 6ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling t lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000, 191, 61–76. [Google Scholar] [CrossRef]
- Warnock, R.A.; Askari, S.; Butcher, E.C.; von Andrian, U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998, 187, 205–216. [Google Scholar] [CrossRef]
- Warnock, R.A.; Campbell, J.J.; Dorf, M.E.; Matsuzawa, A.; McEvoy, L.M.; Butcher, E.C. The role of chemokines in the microenvironmental control of t versus b cell arrest in peyer’s patch high endothelial venules. J. Exp. Med. 2000, 191, 77–88. [Google Scholar] [CrossRef]
- Xu, B.; Cook, R.E.; Michie, S.A. α4β7 integrin/madcam-1 adhesion pathway is crucial for b cell migration into pancreatic lymph nodes in nonobese diabetic mice. J. Autoimmun. 2010, 35, 124–129. [Google Scholar] [CrossRef]
- Salmi, M.; Koskinen, K.; Henttinen, T.; Elima, K.; Jalkanen, S. Clever-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood 2004, 104, 3849–3857. [Google Scholar] [CrossRef]
- Irjala, H.; Elima, K.; Johansson, E.-L.; Merinen, M.; Kontula, K.; Alanen, K.; Grenman, R.; Salmi, M.; Jalkanen, S. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur. J. Immunol. 2003, 33, 815–824. [Google Scholar] [CrossRef]
- Irjala, H.; Johansson, E.-L.; Grenman, R.; Alanen, K.; Salmi, M.; Jalkanen, S. Mannose receptor is a novel ligand for l-selectin and mediates lymphocyte binding to lymphatic endothelium. J. Exp. Med. 2001, 194, 1033–1042. [Google Scholar] [CrossRef]
- Karikoski, M.; Irjala, H.; Maksimow, M.; Miiluniemi, M.; Granfors, K.; Hernesniemi, S.; Elima, K.; Moldenhauer, G.; Schledzewski, K.; Kzhyshkowska, J.; et al. Clever-1/stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. Eur. J. Immunol. 2009, 39, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Debes, G.F.; Arnold, C.N.; Young, A.J.; Krautwald, S.; Lipp, M.; Hay, J.B.; Butcher, E.C. Chemokine receptor ccr7 required for t lymphocyte exit from peripheral tissues. Nat. Immunol. 2005, 6, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Thomas, S.Y.; Luster, A.D. Chemokine receptor ccr7 guides t cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005, 6, 895–901. [Google Scholar] [CrossRef]
- Brown, M.N.; Fintushel, S.R.; Lee, M.H.; Jennrich, S.; Geherin, S.A.; Hay, J.B.; Butcher, E.C.; Debes, G.F. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: Changing requirements during the course of inflammation. J. Immunol. 2010, 185, 4873–4882. [Google Scholar] [CrossRef] [PubMed]
- Höpken, U.E.; Winter, S.; Achtman, A.H.; Krüger, K.; Lipp, M. Ccr7 regulates lymphocyte egress and recirculation through body cavities. J. Leukoc. Biol. 2010, 87, 671–682. [Google Scholar] [CrossRef]
- Berlin-Rufenach, C.; Otto, F.; Mathies, M.; Westermann, J.; Owen, M.J.; Hamann, A.; Hogg, N. Lymphocyte migration in lymphocyte function-associated antigen (lfa)-1-deficient mice. J. Exp. Med. 1999, 189, 1467–1478. [Google Scholar] [CrossRef]
- Hamann, A.; Jablonski-Westrich, D.; Duijvestijn, A.; Butcher, E.C.; Baisch, H.; Harder, R.; Thiele, H.G. Evidence for an accessory role of lfa-1 in lymphocyte-high endothelium interaction during homing. J. Immunol. 1988, 140, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.J.; Breier, G.; Butcher, E.C.; Schulz, M.; Engelhardt, B. Icam-1, vcam-1, and madcam-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 1996, 148, 1819–1838. [Google Scholar]
- Lehmann, J.C.U.; Jablonski-Westrich, D.; Haubold, U.; Gutierrez-Ramos, J.-C.; Springer, T.; Hamann, A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (icam-1) and icam-2 in lymphocyte trafficking. J. Immunol. 2003, 171, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Stanley, P.; Jones, K.; Svensson, L.; McDowall, A.; Hogg, N. The role of the integrin lfa-1 in t-lymphocyte migration. Immunol. Rev. 2007, 218, 135–146. [Google Scholar] [CrossRef]
- Xu, H.; Guan, H.; Zu, G.; Bullard, D.; Hanson, J.; Slater, M.; Elmets, C.A. The role of icam-1 molecule in the migration of langerhans cells in the skin and regional lymph node. Eur. J. Immunol. 2001, 31, 3085–3093. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.-H.; Guo, Y.-J.; Sy, M.-S.; Bigby, M. In vivo treatment with anti-icam-1 and anti-lfa-1 antibodies inhibits contact sensitization-induced migration of epidermal langerhans cells to regional lymph nodes. Cell Immunol. 1994, 158, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef]
- Lehmann, C.; Wilkening, A.; Leiber, D.; Markus, A.; Krug, N.; Pabst, R.; Tschernig, T. Lymphocytes in the bronchoalveolar space reenter the lung tissue by means of the alveolar epithelium, migrate to regional lymph nodes, and subsequently rejoin the systemic immune system. Anat. Rec. 2001, 264, 229–236. [Google Scholar] [CrossRef]
- Turley, S.J.; Lee, J.-W.; Dutton-Swain, N.; Mathis, D.; Benoist, C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc. Natl. Acad. Sci. USA 2005, 102, 17729–17733. [Google Scholar] [CrossRef]
- Makgoba, M.W.; Sanders, M.E.; Luce GE, G.; Dustint, M.L.; Springer, T.A.; Clark, E.A.; Mannoni, P.; Shaw, S. Icam-1 a ligand for lfa-1-dependent adhesion of b, t and myeloid cells. Nature 1988, 331, 86–88. [Google Scholar] [CrossRef]
- Steffen, B.J.; Butcher, E.C.; Engelhardt, B. Evidence for involvement of icam-1 and vcam-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the sjl/j mouse. Am. J. Pathol. 1994, 145, 189–201. [Google Scholar]
- Sironi, M.; Conti, A.; Bernasconi, S.; Fra, A.M.; Pasqualini, F.; Nebuloni, M.; Lauri, E.; De Bortoli, M.; Mantovani, A.; Dejana, E.; et al. Generation and characterization of a mouse lymphatic endothelial cell line. Cell Tissue Res. 2006, 325, 91–100. [Google Scholar] [CrossRef]
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Ebata, N.; Sawa, Y.; Ashikaga, Y.; Yamaoka, Y.; Suzuki, M.; Yoshida, S. Lymphatic endothelium of the human tongue expresses multiple leukocyte adhesion molecules. Tissue Cell 1999, 31, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Reiss, Y.; Hoch, G.; Deutsch, U.; Engelhardt, B. T cell interaction with icam-1-deficient endothelium in vitro: Essential role for icam-1 and icam-2 in transendothelial migration of t cells. Eur. J. Immunol. 1998, 28, 3086–3099. [Google Scholar] [CrossRef]
- Lyck, R.; Reiss, Y.; Gerwin, N.; Greenwood, J.; Adamson, P.; Engelhardt, B. T-cell interaction with icam-1/icam-2 double-deficient brain endothelium in vitro: The cytoplasmic tail of endothelial icam-1 is necessary for transendothelial migration of t cells. Blood 2003, 102, 3675–3683. [Google Scholar] [CrossRef]
- Boscacci, R.T.; Pfeiffer, F.; Gollmer, K.; Sevilla, A.I.C.; Martin, A.M.; Soriano, S.F.; Natale, D.; Henrickson, S.; von Andrian, U.H.; Fukui, Y.; et al. Comprehensive analysis of lymph node stroma-expressed ig superfamily members reveals redundant and non-redundant roles for icam-1, icam-2, and vcam-1 in lymphocyte homing. Blood 2010, 116, 915–925. [Google Scholar] [CrossRef]
- Sligh, J.E., Jr.; Ballantyne, C.M.; Rich, S.S.; Hawkins, H.K.; Smith, C.W.; Bradley, A.; Beaudet, A.L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1993, 90, 8529–8533. [Google Scholar] [CrossRef]
- Xu, H.; A Gonzalo, J.; Pierre, Y.S.; Williams, I.R.; Kupper, T.S.; Cotran, R.S.; A Springer, T.; Gutierrez-Ramos, J.C. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J. Exp. Med. 1994, 180, 95–109. [Google Scholar] [CrossRef]
- Schmits, R.; Kündig, T.M.; Baker, D.M.; Shumaker, G.; Simard, J.J.; Duncan, G.; Wakeham, A.; Shahinian, A.; van der Heiden, A.; Bachmann, M.F.; et al. Lfa-1-deficient mice show normal ctl responses to virus but fail to reject immunogenic tumor. J. Exp. Med. 1996, 183, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Ruddell, A.; Mezquita, P.; Brandvold, K.A.; Farr, A.; Iritani, B.M. B lymphocyte-specific c-myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am. J. Pathol. 2003, 163, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Kirschbaum, S.B.; Paulovich, A.; Pauli, B.U.; Zhang, H.; Alexander, J.S.; Farr, A.G.; Ruddell, A. Lymphatic endothelial murine chloride channel calcium-activated 1 is a ligand for leukocyte lfa-1 and mac-1. J. Immunol. 2010, 185, 5769–5777. [Google Scholar] [CrossRef]
- Marttila-Ichihara, F.; Turja, R.; Miiluniemi, M.; Karikoski, M.; Maksimow, M.; Niemelä, J.; Martinez-Pomares, L.; Salmi, M.; Jalkanen, S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood 2008, 112, 64–72. [Google Scholar] [CrossRef]
- Ledgerwood, L.G.; Lal, G.; Zhang, N.; Garin, A.; Esses, S.J.; Ginhoux, F.; Merad, M.; Peche, H.; A Lira, S.; Ding, Y.; et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue t lymphocytes into afferent lymphatics. Nat. Immunol. 2008, 9, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on s1p receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.-J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef]
- Tomura, M.; Honda, T.; Tanizaki, H.; Otsuka, A.; Egawa, G.; Tokura, Y.; Waldmann, H.; Hori, S.; Cyster, J.G.; Watanabe, T.; et al. Activated regulatory t cells are the major t cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Investig. 2010, 120, 883–893. [Google Scholar] [CrossRef]
- Pflicke, H.; Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009, 206, 2925–2935. [Google Scholar] [CrossRef]
- Teijeira, A.; Hunter, M.C.; Russo, E.; Proulx, S.T.; Frei, T.; Debes, G.F.; Coles, M.; Melero, I.; Detmar, M.; Rouzaut, A.; et al. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by icam-1/lfa-1 interactions. Cell Rep. 2017, 18, 857–865. [Google Scholar] [CrossRef]
- Gorlino, C.V.; Ranocchia, R.P.; Harman, M.F.; García, I.A.; Crespo, M.I.; Morón, G.; Maletto, B.A.; Pistoresi-Palencia, M.C. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J. Immunol. 2014, 193, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Xiong, Y.; Li, L.; Saxena, V.; Smith, K.D.; Hippen, K.L.; Paluskievicz, C.; Willsonshirkey, M.; Blazar, B.R.; Abdi, R.; et al. Regulatory t cells condition lymphatic endothelia for enhanced transendothelial migration. Cell Rep. 2020, 30, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Fazil, M.H.U.T.; Prasannan, P.; Wong, B.H.S.; Kottaiswamy, A.; Salim, N.S.B.M.; Sze, S.K.; Verma, N.K. Gsk3β interacts with crmp2 and notch1 and controls t-cell motility. Front. Immunol. 2021, 12, 680071. [Google Scholar] [CrossRef] [PubMed]
- Martens, R.; Permanyer, M.; Werth, K.; Yu, K.; Braun, A.; Halle, O.; Halle, S.; Patzer, G.E.; Bošnjak, B.; Kiefer, F.; et al. Efficient homing of t cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat. Commun. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Chauveau, A.; Pirgova, G.; Cheng, H.-W.; De Martin, A.; Zhou, F.Y.; Wideman, S.; Rittscher, J.; Ludewig, B.; Arnon, T.I. Visualization of t cell migration in the spleen reveals a network of perivascular pathways that guide entry into t zones. Immunity 2020, 52, 794–807. [Google Scholar] [CrossRef]
- Noto, K.; Kato, K.; Okumura, K.; Yagita, H. Identification and functional characterization of mouse cd29 with a mab. Int. Immunol. 1995, 7, 835–842. [Google Scholar] [CrossRef]
- DeNucci, C.C.; Pagán, A.J.; Mitchell, J.S.; Shimizu, Y. Control of α4β7 integrin expression and cd4 t cell homing by the β1 integrin subunit. J. Immunol. 2010, 184, 2458–2467. [Google Scholar] [CrossRef]
- Miyazaki, T.; Taketomi, Y.; Higashi, T.; Ohtaki, H.; Takaki, T.; Ohnishi, K.; Hosonuma, M.; Kono, N.; Akasu, R.; Haraguchi, S.; et al. Hypercholesterolemic dysregulation of calpain in lymphatic endothelial cells interferes with regulatory t-cell stability and trafficking. Arter. Thromb. Vasc. Biol. 2023, 43, e66–e82. [Google Scholar] [CrossRef]
- Arasa, J.; Collado-Diaz, V.; Kritikos, I.; Medina-Sanchez, J.D.; Friess, M.C.; Sigmund, E.C.; Schineis, P.; Hunter, M.C.; Tacconi, C.; Paterson, N.; et al. Upregulation of vcam-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J. Exp. Med. 2021, 218, e20201413. [Google Scholar] [CrossRef]
- Piao, W.; Xiong, Y.; Famulski, K.; Brinkman, C.C.; Li, L.; Toney, N.; Wagner, C.; Saxena, V.; Simon, T.; Bromberg, J.S. Regulation of t cell afferent lymphatic migration by targeting ltβr-mediated non-classical nfκb signaling. Nat. Commun. 2018, 9, 3020. [Google Scholar] [CrossRef]
- Brinkman, C.C.; Iwami, D.; Hritzo, M.K.; Xiong, Y.; Ahmad, S.; Simon, T.; Hippen, K.L.; Blazar, B.R.; Bromberg, J.S. Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration. Nat. Commun. 2016, 7, 12021. [Google Scholar] [CrossRef] [PubMed]
- Rigby, D.A.; Ferguson, D.J.P.; A Johnson, L.; Jackson, D.G. Neutrophils rapidly transit inflamed lymphatic vessel endothelium via integrin-dependent proteolysis and lipoxin-induced junctional retraction. J. Leukoc. Biol. 2015, 98, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.; Franks, S.; Cromer, W.; Wells, S.; Bienkowska, M.; Jennings, M.; Ruddell, A.; Ando, T.; Wang, Y.; Gu, Y.; et al. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat. Res. Biol. 2010, 8, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Tsuruga, E.; Iwasawa, K.; Ishikawa, H.; Yoshida, S. Leukocyte adhesion molecule and chemokine production through lipoteichoic acid recognition by toll-like receptor 2 in cultured human lymphatic endothelium. Cell Tissue Res. 2008, 333, 237–252. [Google Scholar] [CrossRef]
- Ando, T.; Jordan, P.; Joh, T.; Wang, Y.; Jennings, M.; Houghton, J.; Alexander, J.; Ji, R.-C.; Jordan-Williams, K.L.; Ruddell, A.; et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: Sv-lec. Lymphat. Res. Biol. 2005, 3, 105–115. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xu, Z.; Gunderson, R.C.; Xu, B.; Michie, S.A. LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels. Biomolecules 2023, 13, 1194. https://doi.org/10.3390/biom13081194
Guo J, Xu Z, Gunderson RC, Xu B, Michie SA. LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels. Biomolecules. 2023; 13(8):1194. https://doi.org/10.3390/biom13081194
Chicago/Turabian StyleGuo, Jia, Zeyu Xu, Rachel C. Gunderson, Baohui Xu, and Sara A. Michie. 2023. "LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels" Biomolecules 13, no. 8: 1194. https://doi.org/10.3390/biom13081194
APA StyleGuo, J., Xu, Z., Gunderson, R. C., Xu, B., & Michie, S. A. (2023). LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels. Biomolecules, 13(8), 1194. https://doi.org/10.3390/biom13081194





