AMPKα1 Deficiency in Astrocytes from a Rat Model of ALS Is Associated with an Altered Metabolic Resilience
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Primary Cultures of Rat Cortical Astrocytes
2.3. Glucose Deprivation and Glutamate Exposure Protocols
2.4. Total RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.5. Western Blot
2.6. ATP Assay
2.7. MTT Assay
2.8. XF Real Time ATP Rate Assay
2.9. d-[3H]-Aspartate Uptake
2.10. Statistical Analyses
3. Results
3.1. AMPK Expression Is Altered in hSOD1G93A Primary Cultures of Astrocytes
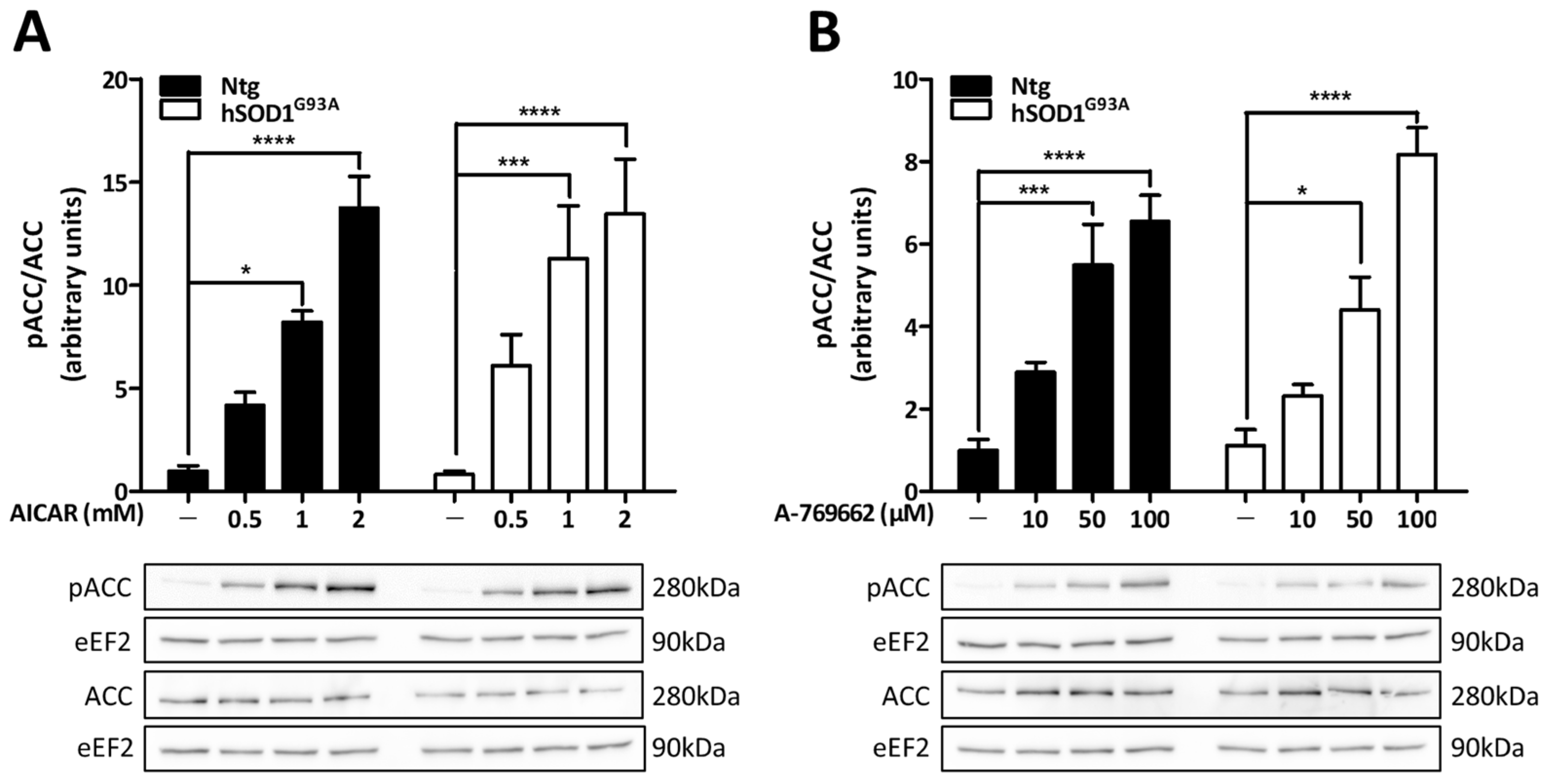
3.2. Pharmacological Activators Induce AMPK Activity in Both Ntg and hSOD1G93A Astrocytes
3.3. Altered AMPK Activity in hSOD1G93A Astrocytes
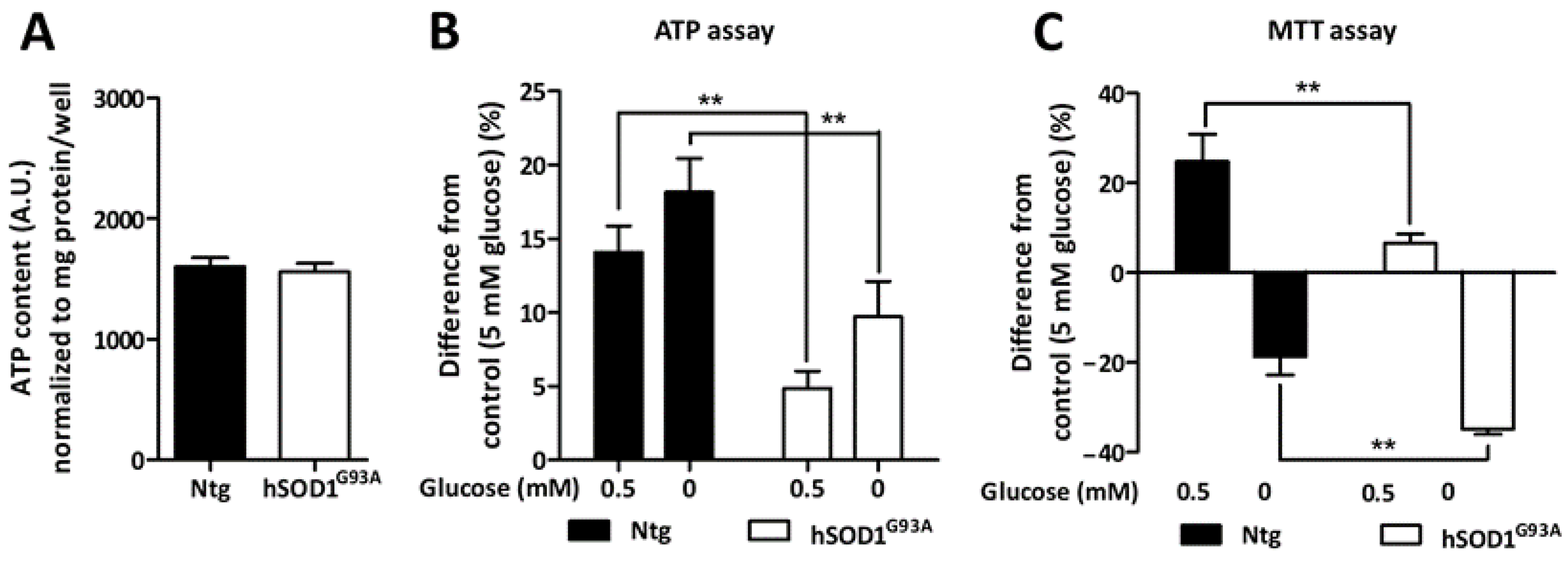
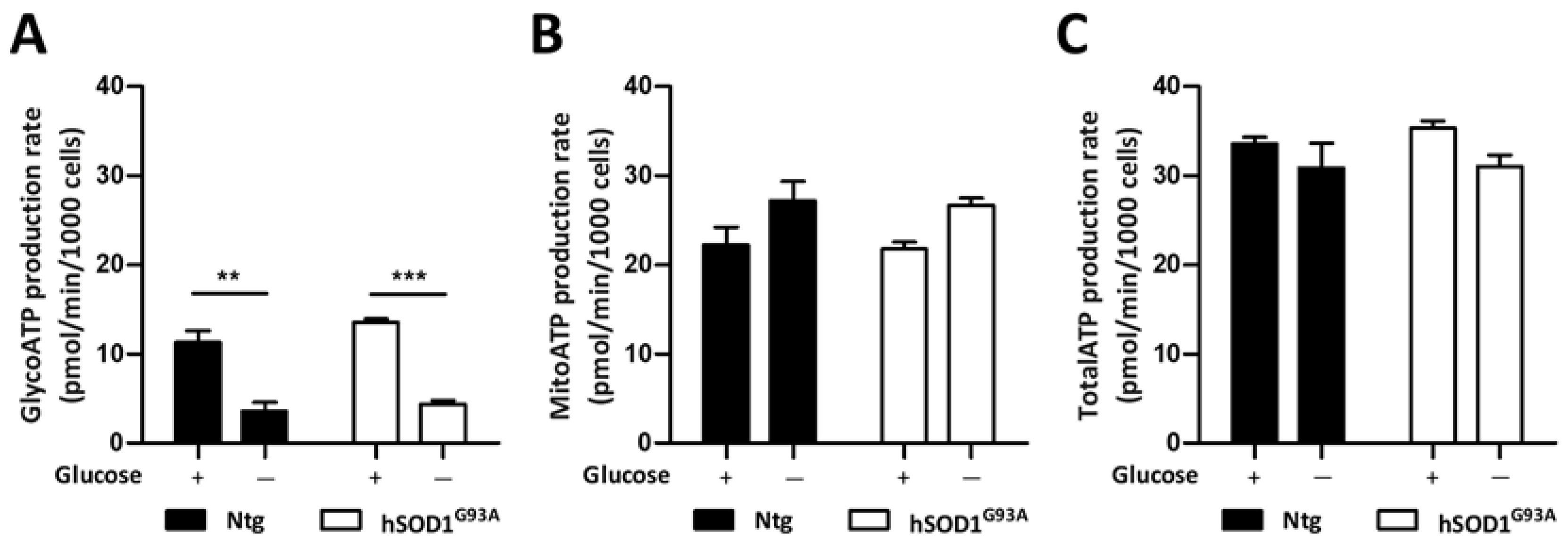
3.4. Metabolic Adaptation of Ntg and hSOD1G93A Astrocytes during Glucose Deprivation
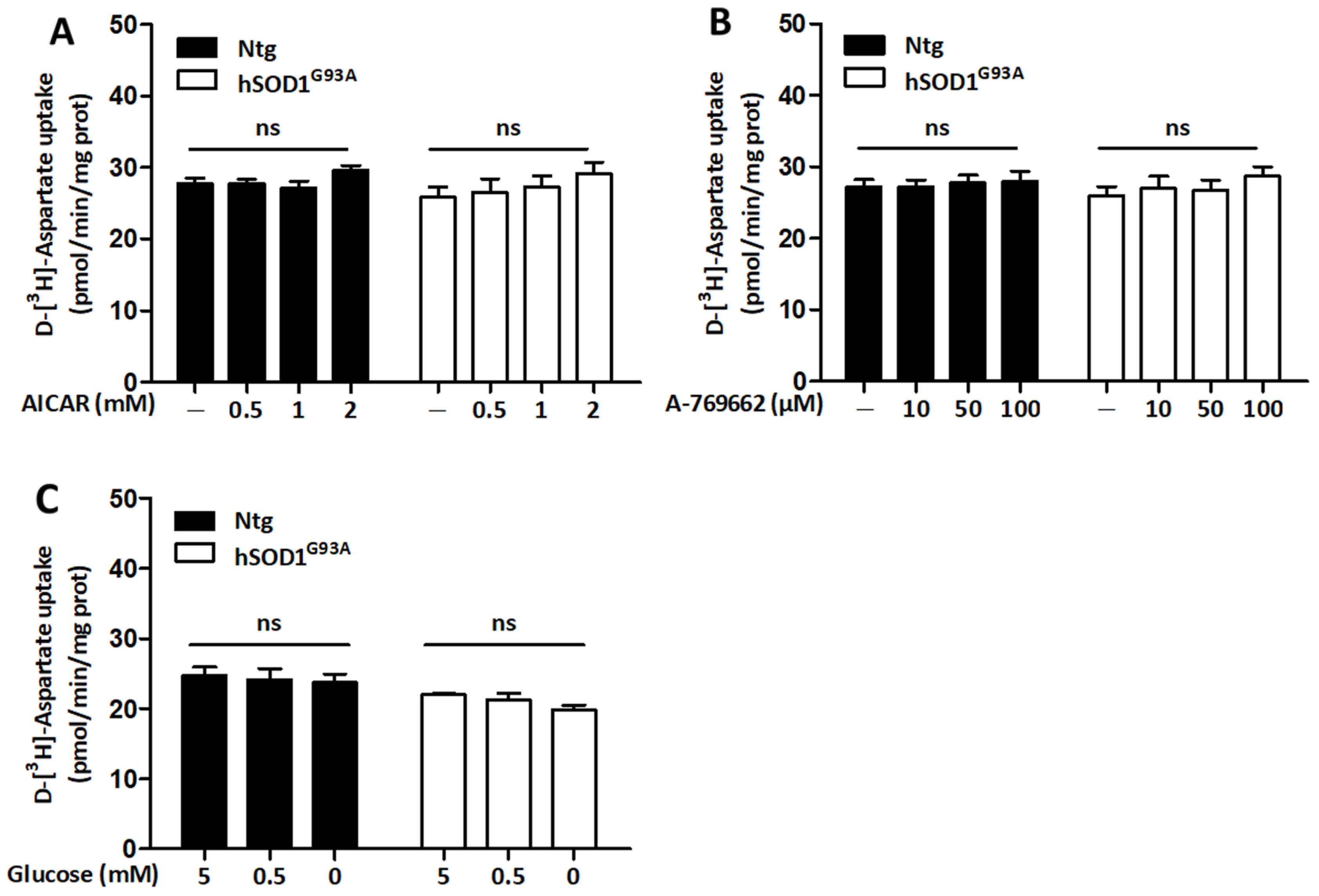
3.5. Regulation of Glutamate Uptake Does Not Rely on the Activity of AMPK in Both Ntg and hSOD1G93A Astrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandoorne, T.; De Bock, K.; Van Den Bosch, L. Energy Metabolism in ALS: An Underappreciated Opportunity? Acta Neuropathol. 2018, 135, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, K.; Youssef, M.; You, J.; Sung, H.-K.; Park, J. Evidence of Metabolic Dysfunction in Amyotrophic Lateral Sclerosis (ALS) Patients and Animal Models. Biomolecules 2023, 13, 863. [Google Scholar] [CrossRef] [PubMed]
- Bouteloup, C.; Desport, J.C.; Clavelou, P.; Guy, N.; Derumeaux-Burel, H.; Ferrier, A.; Couratier, P. Hypermetabolism in ALS Patients: An Early and Persistent Phenomenon. J. Neurol. 2009, 256, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Fayemendy, P.; Marin, B.; Labrunie, A.; Boirie, Y.; Walrand, S.; Achamrah, N.; Coëffier, M.; Preux, P.M.; Lautrette, G.; Desport, J.C.; et al. Hypermetabolism Is a Reality in Amyotrophic Lateral Sclerosis Compared to Healthy Subjects. J. Neurol. Sci. 2021, 420, 117257. [Google Scholar] [CrossRef] [PubMed]
- Lederer, C.W.; Torrisi, A.; Pantelidou, M.; Santama, N.; Cavallaro, S. Pathways and Genes Differentially Expressed in the Motor Cortex of Patients with Sporadic Amyotrophic Lateral Sclerosis. BMC Genomics 2007, 8, 26. [Google Scholar] [CrossRef]
- Browne, S.E.; Yang, L.; Dimauro, J.; Fuller, S.W.; Licata, S.C.; Beal, M.F. Bioenergetic Abnormalities in Discrete Cerebral Motor Pathways Presage Spinal Cord Pathology in the G93A SOD1 Mouse Model of ALS. Neurobiol. Dis. 2006, 22, 599–610. [Google Scholar] [CrossRef]
- Hor, J.H.; Santosa, M.M.; Lim, V.J.W.; Ho, B.X.; Taylor, A.; Khong, Z.J.; Ravits, J.; Fan, Y.; Liou, Y.C.; Soh, B.S.; et al. ALS Motor Neurons Exhibit Hallmark Metabolic Defects That Are Rescued by SIRT3 Activation. Cell Death Differ. 2021, 28, 1379–1397. [Google Scholar] [CrossRef]
- Lim, M.A.; Selak, M.A.; Xiang, Z.; Krainc, D.; Neve, R.L.; Kraemer, B.C.; Watts, J.L.; Kalb, R.G. Reduced Activity of AMP-Activated Protein Kinase Protects against Genetic Models of Motor Neuron Disease. J. Neurosci. 2012, 32, 1123–1141. [Google Scholar] [CrossRef]
- Perera, N.D.; Sheean, R.K.; Scott, J.W.; Kemp, B.E.; Horne, M.K.; Turner, B.J. Mutant TDP-43 Deregulates AMPK Activation by PP2A in ALS Models. PLoS ONE 2014, 9, e90499. [Google Scholar] [CrossRef]
- Coughlan, K.S.; Mitchem, M.R.; Hogg, M.C.; Prehn, J.H.M. “Preconditioning” with Latrepirdine, an Adenosine 5’-Monophosphate-Activated Protein Kinase Activator, Delays Amyotrophic Lateral Sclerosis Progression in SOD1G93A Mice. Neurobiol. Aging 2015, 36, 1140–1150. [Google Scholar] [CrossRef]
- Davies, S.P.; Hawley, S.A.; Woods, A.; Carling, D.; Haystead, T.A.J.; Hardie, D.G. Purification of the AMP-activated Protein Kinase on ATP-γ-Sepharose and Analysis of Its Subunit Structure. Eur. J. Biochem. 1994, 223, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Mitchelhill, K.I.; Stapleton, D.; Gao, G.; House, C.; Michell, B.; Katsis, F.; Witters, L.A.; Kemp, B.E. Mammalian AMP-Activated Protein Kinase Shares Structural and Functional Homology with the Catalytic Domain of Yeast Snf1 Protein Kinase. J. Biol. Chem. 1994, 269, 2361–2364. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Zhang, Y.Y.; Yan, S.F.; Neumann, D.; Schlattner, U.; Wang, Z.X.; Wu, J.W. AMP-Activated Protein Kinase Undergoes Nucleotide-Dependent Conformational Changes. Nat. Struct. Mol. Biol. 2012, 19, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xin, F.J.; Wang, J.; Hu, J.; Zhang, Y.Y.; Wan, S.; Cao, L.S.; Lu, C.; Li, P.; Yan, S.F.; et al. Conserved Regulatory Elements in AMPK. Nature 2013, 498, E8–E10. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP Is a True Physiological Regulator of Amp-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Domise, M.; Vingtdeux, V. Chapter 7 AMPK in Neurodegenerative Diseases. In AMP-Activated Protein Kinase; Springer: Berlin/Heidelberg, Germany, 2016; pp. 153–177. ISBN 9783319435893. [Google Scholar]
- Clement, A.M.; Nguyen, M.D.; Roberts, E.A.; Garcia, M.L.; Boillée, S.; Rule, M.; McMahon, A.P.; Doucette, W.; Siwek, D.; Ferrante, R.J.; et al. Wild-Type Nonneuronal Cells Extend Survival of SOD1 Mutant Motor Neurons in ALS Mice. Science 2003, 302, 113–117. [Google Scholar] [CrossRef]
- Boillée, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Terrie, L.; Baatsen, P.; Kerstens, A.; De Swert, L.; Janky, R.; Corthout, N.; Masrori, P.; Van Damme, P.; Hyttel, P.; et al. FUS-ALS HiPSC-Derived Astrocytes Impair Human Motor Units through Both Gain-of-Toxicity and Loss-of-Support Mechanisms. Mol. Neurodegener. 2023, 18, 1–26. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Van Den Bosch, L. Why Should We Care about Astrocytes in a Motor Neuron Disease? Front. Mol. Med. 2023, 3, 1047540. [Google Scholar] [CrossRef]
- Yamanaka, K.; Komine, O. The Multi-Dimensional Roles of Astrocytes in ALS. Neurosci. Res. 2018, 126, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Tsai, G.; Kuncl, R.W.; Clawson, L.; Cornblath, D.R.; Drachman, D.B.; Pestronk, A.; Stauch, B.L.; Coyle, J.T. Abnormal Excitatory Amino Acid Metabolism in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1990, 28, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Martin, L.J.; Kuncl, R.W. Decreased Glutamate Transort by the Brain and Spinal Cord in Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 1992, 326, 1464–1468. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Van Kammen, M.; Levey, A.I.; Martin, L.J.; Kuncl, R.W. Selective Loss of Glial Glutamate Transporter GLT-1 in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1995, 38, 73–84. [Google Scholar] [CrossRef]
- Howland, D.S.; Liu, J.; She, Y.; Goad, B.; Maragakis, N.J.; Kim, B.; Erickson, J.; Kulik, J.; DeVito, L.; Psaltis, G.; et al. Focal Loss of the Glutamate Transporter EAAT2 in a Transgenic Rat Model of SOD1 Mutant-Mediated Amyotrophic Lateral Sclerosis (ALS). Proc. Natl. Acad. Sci. USA 2002, 99, 1604–1609. [Google Scholar] [CrossRef]
- Lei, H.; Dirren, E.; Poitry-Yamate, C.; Schneider, B.L.; Gruetter, R.; Aebischer, P. Evolution of the Neurochemical Profiles in the G93A-SOD1 Mouse Model of Amyotrophic Lateral Sclerosis. J. Cereb. Blood Flow Metab. 2019, 39, 1283–1298. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The Role of Excitotoxicity in the Pathogenesis of Amyotrophic Lateral Sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Higginbottom, A.; Heath, P.R.; Barber, S.; Greenald, D.; Kirby, J.; Shaw, P.J. Dysregulation of Astrocyte-Motoneuron Cross-Talk in Mutant Superoxide Dismutase 1-Related Amyotrophic Lateral Sclerosis. Brain 2011, 134, 2627–2641. [Google Scholar] [CrossRef]
- Velebit, J.; Horvat, A.; Smolič, T.; Prpar Mihevc, S.; Rogelj, B.; Zorec, R.; Vardjan, N. Astrocytes with TDP-43 Inclusions Exhibit Reduced Noradrenergic CAMP and Ca2+ Signaling and Dysregulated Cell Metabolism. Sci. Rep. 2020, 10, 6003. [Google Scholar] [CrossRef]
- Allen, S.P.; Hall, B.; Woof, R.; Francis, L.; Gatto, N.; Shaw, A.C.; Myszczynska, M.; Hemingway, J.; Coldicott, I.; Willcock, A.; et al. C9orf72 Expansion within Astrocytes Reduces Metabolic Flexibility in Amyotrophic Lateral Sclerosis. Brain 2019, 142, 3771–3790. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, Q.; Gu, X.; Chen, Y.; Chen, X.; Cao, B.; Ou, R.; Shang, H. Decreased Glycogenolysis by MiR-338-3p Promotes Regional Glycogen Accumulation within the Spinal Cord of Amyotrophic Lateral Sclerosis Mice. Front. Mol. Neurosci. 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.K.; Torres, P.; Ayala, V.; Portero-Otin, M.; Pamplona, R.; Andrés-Benito, P.; Ferrer, I.; Guinovart, J.J.; Duran, J. Glycogen Accumulation Modulates Life Span in a Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurochem. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bonifacino, T.; Bartolucci, M.; Milanese, M.; Gallia, E.; Provenzano, F.; Cortese, K.; Panfoli, I.; Bonanno, G. Characterization of the Mitochondrial Aerobic Metabolism in the Pre- and Perisynaptic Districts of the SOD1 G93A Mouse Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2018, 55, 9220–9233. [Google Scholar] [CrossRef]
- Ravera, S.; Torazza, C.; Bonifacino, T.; Provenzano, F.; Rebosio, C.; Milanese, M.; Usai, C.; Panfoli, I.; Bonanno, G. Altered Glucose Catabolism in the Presynaptic and Perisynaptic Compartments of SOD1G93A Mouse Spinal Cord and Motor Cortex Indicates That Mitochondria Are the Site of Bioenergetic Imbalance in ALS. J. Neurochem. 2019, 151, 336–350. [Google Scholar] [CrossRef]
- Belo Do Nascimento, I.; Verfaillie, M.; Ates, G.; Beckers, P.; Joris, V.; Desmet, N.; Massie, A.; Hermans, E. AMPK Modulates the Metabolic Adaptation of C6 Glioma Cells in Glucose-Deprived Conditions without Affecting Glutamate Transport. Cells 2022, 11, 1800. [Google Scholar] [CrossRef]
- Dumont, A.O.; Hermans, E.; Goursaud, S. Differential Regulation of the Glutamate Transporter Variants GLT-1a and GLT-1b in the Cortex and Spinal Cord of Transgenic Rats Expressing HSOD1G93A. Neurochem. Int. 2013, 63, 61–68. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Mckenna, M.C.; Dienel, G.A.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A. Energy Metabolism of the Brain. Basic Neurochem. 2012, 200–231. [Google Scholar] [CrossRef]
- Turnley, A.M.; Stapleton, D.; Mann, R.J.; Witters, L.A.; Kemp, B.E.; Bartlett, P.F. Cellular Distribution and Developmental Expression of AMP-Activated Protein Kinase Isoforms in Mouse Central Nervous System. J. Neurochem. 1999, 72, 1707–1716. [Google Scholar] [CrossRef]
- Meares, G.P.; Qin, H.; Liu, Y.; Holdbrooks, A.T.; Benveniste, E.N. AMP-Activated Protein Kinase Restricts IFN-γ Signaling. J. Immunol. 2013, 190, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, B.; Kost, A.; Kasprowska, D.; Liber, S.; Machnik, G.; Wiaderkiewicz, R.; Labuzek, K. AMP-Activated Protein Kinase Is Involved in Induction of Protective Autophagy in Astrocytes Exposed to Oxygen-Glucose Deprivation. Cell Biol. Int. 2014, 38, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.C.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Norenberg, M.D. Primary Cultures of Astrocytes: Their Value in Understanding Astrocytes in Health and Disease. Physiol. Behav. 2012, 37, 2569–2588. [Google Scholar] [CrossRef]
- Muraleedharan, R.; Gawali, M.V.; Tiwari, D.; Sukumaran, A.; Oatman, N.; Anderson, J.; Nardini, D.; Bhuiyan, M.A.N.; Tkáč, I.; Ward, A.L.; et al. AMPK-Regulated Astrocytic Lactate Shuttle Plays a Non-Cell-Autonomous Role in Neuronal Survival. Cell Rep. 2020, 32, 108092. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.; Choi, K.; Dasgupta, B. Insight on Transcriptional Regulation of the Energy Sensing AMPK and Biosynthetic MTOR Pathway Genes. Front Cell Dev. Biol. 2020, 8, 671. [Google Scholar] [CrossRef]
- Marini, C.; Cossu, V.; Kumar, M.; Milanese, M.; Cortese, K.; Bruno, S.; Bellese, G.; Carta, S.; Zerbo, R.A.; Torazza, C.; et al. The Role of Endoplasmic Reticulum in the Differential Endurance against Redox Stress in Cortical and Spinal Astrocytes from the Newborn Sod1g93a Mouse Model of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1392. [Google Scholar] [CrossRef]
- Gomes, C.; Sequeira, C.; Barbosa, M.; Cunha, C.; Vaz, A.R.; Brites, D. Astrocyte Regional Diversity in ALS Includes Distinct Aberrant Phenotypes with Common and Causal Pathological Processes. Exp. Cell Res. 2020, 395, 112209. [Google Scholar] [CrossRef]
- Jelluma, N.; Yang, X.; Stokoe, D.; Evan, G.I.; Dansen, T.B.; Haas-Kogan, D.A. Glucose Withdrawal Induces Oxidative Stress Followed by Apoptosis in Glioblastoma Cells but Not in Normal Human Astrocytes. Mol. Cancer Res. 2006, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, C.S.; Li, M.; Wang, W.; Wang, Z.; Hawley, S.A.; Ma, T.; Feng, J.W.; Tian, X.; Qi, Q.; et al. Hierarchical Activation of Compartmentalized Pools of AMPK Depends on Severity of Nutrient or Energy Stress. Cell Res. 2019, 29, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.L.; Curtis, S.D.; Lyons, A.C.; Zhang, J.; Chen, M.; Mehta, S.; Shaw, R.J.; Zhang, J.; He, C.Y. Spatial Regulation of AMPK Signaling Revealed by a Sensitive Kinase Activity Reporter. Nat. Commun. 2022, 13, 3856. [Google Scholar] [CrossRef]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Yu, H.J.; Liu, Z.; Dong, J.; Mustard, K.J.; Hawley, S.A.; et al. Aging-Associated Reductions in AMP-Activated Protein Kinase Activity and Mitochondrial Biogenesis. Cell Metab. 2007, 79, 151–156. [Google Scholar] [CrossRef]
- Liu, F.; Benashski, S.E.; Persky, R.; Xu, Y.; Li, J.; Mccullough, L.D. Age-Related Changes in AMP-Activated Protein Kinase after Stroke. Age 2011, 34, 157–168. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-Activated Protein Kinase (AMPK) Controls the Aging Process via an Integrated Signaling Network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Winkler, U.; Seim, P.; Enzbrenner, Y.; Köhler, S.; Sicker, M.; Hirrlinger, J. Activity-Dependent Modulation of Intracellular ATP in Cultured Cortical Astrocytes. J. Neurosci. Res. 2017, 95, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, A.; Porras, O.H.; Barros, L.F. Glutamate Triggers Rapid Glucose Transport Stimulation in Astrocytes as Evidenced by Real-Time Confocal Microscopy. J. Neurosci. 2003, 23, 7337–7342. [Google Scholar] [CrossRef] [PubMed]
- Weisová, P.; Concannon, C.G.; Devocelle, M.; Prehn, J.H.M.; Ward, M.W. Regulation of Glucose Transporter 3 Surface Expression by the AMP-Activated Protein Kinase Mediates Tolerance to Glutamate Excitation in Neurons. J. Neurosci. 2009, 29, 2997–3008. [Google Scholar] [CrossRef]
- Voss, C.M.; Pajęcka, K.; Stridh, M.H.; Nissen, J.D.; Schousboe, A.; Waagepetersen, H.S. AMPK Activation Affects Glutamate Metabolism in Astrocytes. Neurochem. Res. 2015, 40, 2431–2442. [Google Scholar] [CrossRef]
- Maixner, D.W.; Yan, X.; Gao, M.; Yadav, R.; Weng, H.-R. Adenosine Monophosphate-Activated Protein Kinase Regulates Interleukin-1β Expression and Glial Glutamate Transporter Function in Rodents with Neuropathic Pain Dylan. Physiol. Behav. 2015, 92, 135–140. [Google Scholar] [CrossRef]
- Burlando, B.; Milanese, M.; Giordano, G.; Bonifacino, T.; Ravera, S.; Blanchini, F.; Bonanno, G. A Multistationary Loop Model of ALS Unveils Critical Molecular Interactions Involving Mitochondria and Glucose Metabolism. PLoS ONE 2020, 15, e0244234. [Google Scholar] [CrossRef]
- Granatiero, V.; Sayles, N.M.; Savino, A.M.; Konrad, C.; Kharas, M.G.; Kawamata, H.; Manfredi, G. Modulation of the IGF1R-MTOR Pathway Attenuates Motor Neuron Toxicity of Human ALS SOD1G93A Astrocytes. Autophagy 2021, 17, 4029–4042. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belo do Nascimento, I.; Ates, G.; Desmet, N.; Beckers, P.; Massie, A.; Hermans, E. AMPKα1 Deficiency in Astrocytes from a Rat Model of ALS Is Associated with an Altered Metabolic Resilience. Biomolecules 2023, 13, 1183. https://doi.org/10.3390/biom13081183
Belo do Nascimento I, Ates G, Desmet N, Beckers P, Massie A, Hermans E. AMPKα1 Deficiency in Astrocytes from a Rat Model of ALS Is Associated with an Altered Metabolic Resilience. Biomolecules. 2023; 13(8):1183. https://doi.org/10.3390/biom13081183
Chicago/Turabian StyleBelo do Nascimento, Inês, Gamze Ates, Nathalie Desmet, Pauline Beckers, Ann Massie, and Emmanuel Hermans. 2023. "AMPKα1 Deficiency in Astrocytes from a Rat Model of ALS Is Associated with an Altered Metabolic Resilience" Biomolecules 13, no. 8: 1183. https://doi.org/10.3390/biom13081183
APA StyleBelo do Nascimento, I., Ates, G., Desmet, N., Beckers, P., Massie, A., & Hermans, E. (2023). AMPKα1 Deficiency in Astrocytes from a Rat Model of ALS Is Associated with an Altered Metabolic Resilience. Biomolecules, 13(8), 1183. https://doi.org/10.3390/biom13081183






