The Potential of Fasting-Mimicking Diet as a Preventive and Curative Strategy for Alzheimer’s Disease
Abstract
1. Introduction
2. Nutrition, Metabolism, and Longevity: The Potential Role of Caloric Restriction
3. Fasting-Mimicking Diet: What Evidence for Health Status
4. Alzheimer’s Disease and Dementia: The Metabolic Pathway
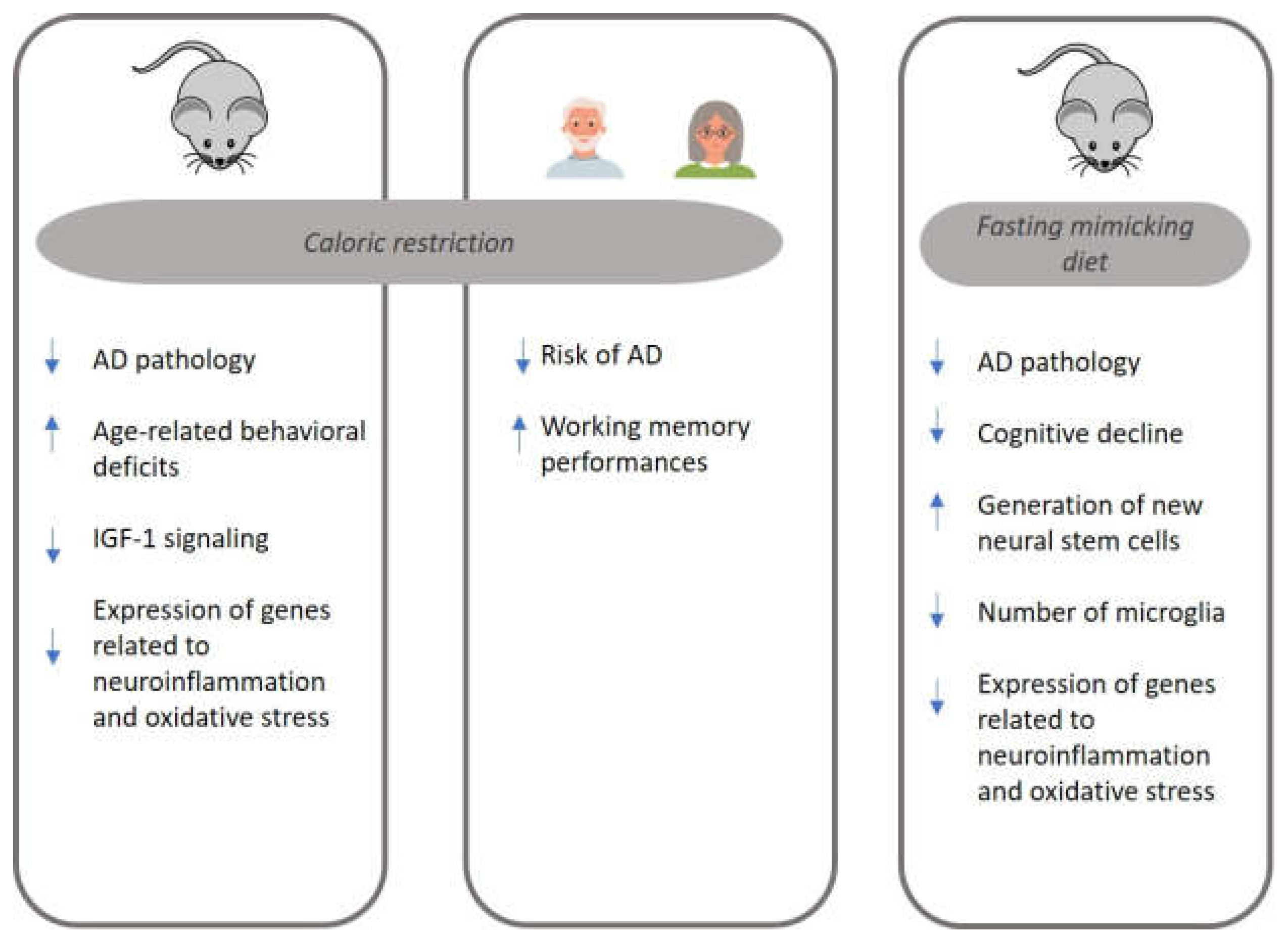
5. Alzheimer’s Disease: From Caloric Restriction to Fasting-Mimicking Diets
6. Summary and Final Remarks
Key Points
- The Fasting Mimicking Diet (FMD) is a program that aims to mimic the effects of fasting while still allowing some food intake.
- The FMD involves consuming a low-calorie, low-protein, and low-carbohydrate diet for 4–7 days.
- Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by the accumulation of amyloid plaques and neurofibrillary tangles in the brain, leading to cognitive decline.
- Animal studies have suggested that fasting can reduce the levels of amyloid beta in the brain, a key component of amyloid plaques.
- Limited human studies have found that the FMD may improve cognitive function in patients with mild cognitive impairment, a precursor to AD.
- While the FMD holds promise for improving cognitive function in AD, its effectiveness and safety require further investigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 19 May 2023).
- Strac, D.S.; Konjevod, M.; Sagud, M.; Perkovic, M.N.; Erjavec, G.N.; Vuic, B.; Simic, G.; Vukic, V.; Mimica, N.; Pivac, N. Personalizing the Care and Treatment of Alzheimer’s Disease: An Overview. Pharmgenomics Pers. Med. 2021, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, R.; Reddy, P.H. Amyloid Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s Disease Neurons. J. Alzheimers Dis. 2017, 57, 975. [Google Scholar] [CrossRef]
- Reitz, C.; Rogaeva, E.; Beecham, G.W. Late-Onset vs Nonmendelian Early-Onset Alzheimer Disease. Neurol. Genet. 2020, 6, e512. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.D.; McGuire, L.C.; Patel, R.; Baumgart, M.; Lamb, R.; Jeffers, E.M.; Olivari, B.S.; Croft, J.B.; Thomas, C.W.; Hacker, K. Modifiable Risk Factors for Alzheimer Disease and Related Dementias Among Adults Aged ≥45 Years—United States, 2019. Morb. Mortal. Wkly. Rep. 2022, 71, 680. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain Lifestyle Intervention Benefits a Large Elderly Population at Risk for Cognitive Decline and Dementia Regardless of Baseline Characteristics: The FINGER Trial. Alzheimer’s Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Demetrius, L.A.; Driver, J. Alzheimer’s as a Metabolic Disease. Biogerontology 2013, 14, 641–649. [Google Scholar] [CrossRef]
- Van Den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Van De Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Dierkes, J.; Ramel, A.; Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Söderlund, F.; Bärebring, L.; Åkesson, A. Quality of dietary fat and risk of Alzheimer’s disease and dementia in adults aged ≥50 years: A systematic review. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Longo, V. Dietary Restriction with and without Caloric Restriction for Healthy Aging. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, C.; D’adamo, S.; Stefanelli, C.; Flamigni, F.; Cetrullo, S. Nutrients and Pathways That Regulate Health Span and Life Span. Geriatrics 2020, 5, 95. [Google Scholar] [CrossRef]
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A.P. Effects of Calorie Restriction on Health Span and Insulin Resistance: Classic Calorie Restriction Diet vs. Ketosis-Inducing Diet. Nutrients 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Fanti, M.; Mishra, A.; Longo, V.D.; Brandhorst, S. Time-Restricted Eating, Intermittent Fasting, and Fasting-Mimicking Diets in Weight Loss. Curr. Obes. Rep. 2021, 10, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Blaževitš, O.; Di Tano, M.; Longo, V.D. Fasting and Fasting Mimicking Diets in Cancer Prevention and Therapy. Trends Cancer 2023, 9, 212–222. [Google Scholar] [CrossRef]
- Allman-Farinelli, M.; Boljevac, B.; Vuong, T.; Hekler, E. Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review. Nutrients 2023, 15, 1756. [Google Scholar] [CrossRef]
- Vucic, V.; Ristic-Medic, D.; Arsic, A.; Petrovic, S.; Paunovic, M.; Vasiljevic, N.; Ilich, J.Z. Nutrition and Physical Activity as Modulators of Osteosarcopenic Adiposity: A Scoping Review and Recommendations for Future Research. Nutrients 2023, 15, 1619. [Google Scholar] [CrossRef]
- Grande de França, N.A.; Rolland, Y.; Guyonnet, S.; Souto Barreto, P. de The Role of Dietary Strategies in the Modulation of Hallmarks of Aging. Ageing Res. Rev. 2023, 87, 101908. [Google Scholar] [CrossRef]
- Morris, A.L.; Mohiuddin, S.S. Biochemistry, Nutrients; StatPearls: St. Petersburg, FL, USA,, 2022. [Google Scholar]
- Viña, J.; Borras, C.; Abdelaziz, K.M.; Garcia-Valles, R.; Gomez-Cabrera, M.C. The Free Radical Theory of Aging Revisited: The Cell Signaling Disruption Theory of Aging. Antioxid. Redox Signal. 2013, 19, 779. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; De Cabo, R.; Bernier, M.; Sollott, S.J.; Fabbri, E.; Navas, P.; Ferrucci, L. Reconsidering the Role of Mitochondria in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1334–1342. [Google Scholar] [CrossRef]
- Romano, A.; Greco, E.; Vendemiale, G.; Serviddio, G. Bioenergetics and Mitochondrial Dysfunction in Aging: Recent Insights for a Therapeutical Approach. Curr. Pharm. Des. 2014, 20, 2978–2992. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative Stress Response Elicited by Mitochondrial Dysfunction: Implication in the Pathophysiology of Aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Mitchell, S.E. Caloric Restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.L.; Martin, C.K.; Redman, L.M. Calorie Restriction for Enhanced Longevity: The Role of Novel Dietary Strategies in the Present Obesogenic Environment. Ageing Res. Rev. 2020, 64, 101038. [Google Scholar] [CrossRef]
- Speakman, J.R. Why Does Caloric Restriction Increase Life and Healthspan? The ‘Clean Cupboards’ Hypothesis. Natl. Sci. Rev. 2020, 7, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043. [Google Scholar] [CrossRef]
- Ungvari, Z.; Parrado-Fernandez, C.; Csiszar, A.; De Cabo, R. Mechanisms Underlying Caloric Restriction and Life Span Regulation: Implications for Vascular Aging. Circ. Res. 2008, 102, 519. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105. [Google Scholar] [CrossRef]
- Il’yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E. Effects of 2 Years of Caloric Restriction on Oxidative Status Assessed by Urinary F2-Isoprostanes: The CALERIE 2 Randomized Clinical Trial. Aging Cell. 2018, 17, e12719. [Google Scholar] [CrossRef]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-Term Calorie Restriction Is Highly Effective in Reducing the Risk for Atherosclerosis in Humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S. Aging, Adiposity, and Calorie Restriction. JAMA 2007, 297, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Calorie Restriction and Cardiometabolic Health. Eur. J. Prev. Cardiol. 2008, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E. Effect of Calorie Restriction with or without Exercise on Insulin Sensitivity, Beta-Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E. Calorie Restriction Increases Muscle Mitochondrial Biogenesis in Healthy Humans. PLoS Med. 2007, 4, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Carmona-Gutierrez, D.; Mueller, M.I.; Madeo, F. The Ups and Downs of Caloric Restriction and Fasting: From Molecular Effects to Clinical Application. EMBO Mol. Med. 2022, 14, e14418. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef]
- Leong, I. Sustained Caloric Restriction in Health. Nat. Rev. Endocrinol. 2018, 14, 322. [Google Scholar] [CrossRef]
- Abbott, A. Reduced-Calorie Diet Shows Signs of Slowing Ageing in People. Nature 2018, 555, 570–571. [Google Scholar] [CrossRef]
- Dubé, J.J.; Amati, F.; Toledo, F.G.S.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of Weight Loss and Exercise on Insulin Resistance, and Intramyocellular Triacylglycerol, Diacylglycerol and Ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef]
- Willcox, B.J.; Willcox, D.C.; Suzuki, M. Demographic, Phenotypic, and Genetic Characteristics of Centenarians in Okinawa and Japan: Part 1—Centenarians in Okinawa. Mech. Ageing Dev. 2017, 165, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Leeuwenburgh, C. Fasting or Caloric Restriction for Healthy Aging. Exp. Gerontol. 2013, 48, 1003. [Google Scholar] [CrossRef]
- Mishra, A.; Longo, V.D. Fasting and Fasting Mimicking Diets in Obesity and Cardiometabolic Disease Prevention and Treatment. Phys. Med. Rehabil. Clin. N. Am. 2022, 33, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-Mimicking Diet and Markers/Risk Factors for Aging, Diabetes, Cancer, and Cardiovascular Disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Han, R.; Zhao, J.; Wang, S.; Huang, M.; Wang, Y.; Chen, Y. Intermittent Administration of a Fasting-Mimicking Diet Intervenes in Diabetes Progression, Restores β Cells and Reconstructs Gut Microbiota in Mice. Nutr. Metab. 2018, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Longo, V.D. Protein Quantity and Source, Fasting-Mimicking Diets, and Longevity. Adv. Nutr. 2019, 10, S340–S350. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet That Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Mecocci, P.; Baroni, M.; Senin, U.; Boccardi, V. Brain Aging and Late-Onset Alzheimer’s Disease: A Matter of Increased Amyloid or Reduced Energy? J. Alzheimers Dis. 2018, 64, S397–S404. [Google Scholar] [CrossRef]
- Boccardi, V.; Comanducci, C.; Baroni, M.; Mecocci, P. Of Energy and Entropy: The Ineluctable Impact of Aging in Old Age Dementia. Int. J. Mol. Sci. 2017, 18, 2672. [Google Scholar] [CrossRef]
- Vlassenko, A.G.; Vaishnavi, S.N.; Couture, L.; Sacco, D.; Shannon, B.J.; Mach, R.H.; Morris, J.C.; Raichle, M.E.; Mintun, M.A. Spatial Correlation between Brain Aerobic Glycolysis and Amyloid-β (Aβ) Deposition. Proc. Natl. Acad. Sci. USA 2010, 107, 17763–17767. [Google Scholar] [CrossRef] [PubMed]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Adam, M.; Brickman. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Vanzulli, I.; Papanikolaou, M.; De-La-Rocha, I.C.; Pieropan, F.; Rivera, A.D.; Gomez-Nicola, D.; Verkhratsky, A.; Rodríguez, J.J.; Butt, A.M. Disruption of Oligodendrocyte Progenitor Cells Is an Early Sign of Pathology in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2020, 94, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Chacon-De-La-Rocha, I.; Fryatt, G.; Rivera, A.D.; Verkhratsky, A.; Raineteau, O.; Gomez-Nicola, D.; Butt, A.M. Accelerated Dystrophy and Decay of Oligodendrocyte Precursor Cells in the APP/PS1 Model of Alzheimer’s-like Pathology. Front. Cell. Neurosci. 2020, 14, 575082. [Google Scholar] [CrossRef]
- Butt, A.M.; De La Rocha, I.C.; Rivera, A. Oligodendroglial Cells in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 325–333. [Google Scholar] [CrossRef]
- Afsar, A.; Chacon Castro, M.D.C.; Soladogun, A.S.; Zhang, L. Recent Development in the Understanding of Molecular and Cellular Mechanisms Underlying the Etiopathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 7258. [Google Scholar] [CrossRef]
- Rivera, A.D.; Chacon-De-La-Rocha, I.; Pieropan, F.; Papanikolau, M.; Azim, K.; Butt, A.M. Keeping the Ageing Brain Wired: A Role for Purine Signalling in Regulating Cellular Metabolism in Oligodendrocyte Progenitors. Pflug. Arch. 2021, 473, 775–783. [Google Scholar] [CrossRef]
- Butt, A.M.; Papanikolaou, M.; Rivera, A. Physiology of Oligodendroglia. Adv. Exp. Med. Biol. 2019, 1175, 117–128. [Google Scholar] [CrossRef]
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte Dysfunction in Alzheimer Disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef]
- Rubio-Araiz, A.; Finucane, O.M.; Keogh, S.; Lynch, M.A. Anti-TLR2 Antibody Triggers Oxidative Phosphorylation in Microglia and Increases Phagocytosis of β-Amyloid. J. Neuroinflammation 2018, 15, 247. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimers Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Self, W.; Kerman, B.E.; Santoni, G.; Navalpur Shanmugam, N.K.; Abdullah, L.; Golden, L.R.; Fonteh, A.N.; Harrington, M.G.; Gräff, J.; et al. Nutritional Metabolism and Cerebral Bioenergetics in Alzheimer’s Disease and Related Dementias. Alzheimers Dement. 2022, 19, 1041–1066. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R.E. Caloric Restriction: Beneficial Effects on Brain Aging and Alzheimer’s Disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.V.; Gordon, M.N.; Connor, K.E.; Good, R.A.; Engelman, R.W.; Mason, J.; Morgan, D.G.; Morgan, T.E.; Finch, C.E. Caloric Restriction Attenuates Abeta-Deposition in Alzheimer Transgenic Models. Neurobiol. Aging 2005, 26, 995–1000. [Google Scholar] [CrossRef]
- Mouton, P.R.; Chachich, M.E.; Quigley, C.; Spangler, E.; Ingram, D.K. Caloric Restriction Attenuates Amyloid Deposition in Middle-Aged APP/ PS1 Mice. Neurosci. Lett. 2009, 464, 184. [Google Scholar] [CrossRef]
- Halagappa, V.K.M.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent Fasting and Caloric Restriction Ameliorate Age-Related Behavioral Deficits in the Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef]
- Parrella, E.; Maxim, T.; Maialetti, F.; Zhang, L.; Wan, J.; Wei, M.; Cohen, P.; Fontana, L.; Longo, V.D. Protein Restriction Cycles Reduce IGF-1 and Phosphorylated Tau, and Improve Behavioral Performance in an Alzheimer’s Disease Mouse Model. Aging Cell 2013, 12, 257–268. [Google Scholar] [CrossRef]
- Yu, Q.; Zou, L.; Kong, Z.; Yang, L. Cognitive Impact of Calorie Restriction: A Narrative Review. J. Am. Med. Dir. Assoc. 2020, 21, 1394–1401. [Google Scholar] [CrossRef]
- Maharajan, N.; Vijayakumar, K.; Jang, C.H.; Cho, G.W. Caloric Restriction Maintains Stem Cells through Niche and Regulates Stem Cell Aging. J. Mol. Med. 2020, 98, 25–37. [Google Scholar] [CrossRef]
- Wadie, C.M.; Ali, R.H.; Mohamed, A.E.H.A.; Labib, J.M.W.; Sabaa, A.R.; Awad, H.E.A.; Abou-Bakr, D.A. A Comparative Study of Acetyl-l-carnitine and Caloric Restriction Impact on Hippocampal Autophagy, Apoptosis, Neurogenesis, and Astroglial Function in AlCl3-Induced Alzheimer’s in Rats. Can. J. Physiol. Pharmacol. 2023, 101, 244–257. [Google Scholar] [CrossRef]
- Lü, W.; Yu, T.; Kuang, W. Effects of Dietary Restriction on Cognitive Function: A Systematic Review and Meta-Analysis. Nutr. Neurosci. 2023, 26, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Q.; Yu, L.L.; Qi, G.Y.; Mi, Y.S.; Wu, W.Q.; Lee, Y.K.; Zhai, Q.X.; Tian, F.W.; Chen, W. Can Dietary Patterns Prevent Cognitive Impairment and Reduce Alzheimer’s Disease Risk: Exploring the Underlying Mechanisms of Effects. Neurosci. Biobehav. Rev. 2022, 135, 104556. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L.; Tan, L. Nutrition and the Risk of Alzheimer’s Disease. Biomed. Res. Int. 2013, 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimers Dement. 2015, 11, 1007. [Google Scholar] [CrossRef]
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Flöel, A. Caloric Restriction Improves Memory in Elderly Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255–1260. [Google Scholar] [CrossRef]
- Leclerc, E.; Trevizol, A.P.; Grigolon, R.B.; Subramaniapillai, M.; McIntyre, R.S.; Brietzke, E.; Mansur, R.B. The Effect of Caloric Restriction on Working Memory in Healthy Non-Obese Adults. CNS Spectr. 2020, 25, 2–8. [Google Scholar] [CrossRef]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent Fasting Enhanced the Cognitive Function in Older Adults with Mild Cognitive Impairment by Inducing Biochemical and Metabolic Changes: A 3-Year Progressive Study. Nutrients 2020, 12, 2644. [Google Scholar] [CrossRef]
- Rangan, P.; Lobo, F.; Parrella, E.; Rochette, N.; Morselli, M.; Stephen, T.L.; Cremonini, A.L.; Tagliafico, L.; Persia, A.; Caffa, I.; et al. Fasting-Mimicking Diet Cycles Reduce Neuroinflammation to Attenuate Cognitive Decline in Alzheimer’s Models. Cell Rep. 2022, 40, 111417. [Google Scholar] [CrossRef]
- Fay-Watt, V.; O’Connor, S.; Roshan, D.; Romeo, A.C.; Longo, V.D.; Sullivan, F.J. The Impact of a Fasting Mimicking Diet on the Metabolic Health of a Prospective Cohort of Patients with Prostate Cancer: A Pilot Implementation Study. Prostate Cancer Prostatic Dis. 2022, 26, 317–322. [Google Scholar] [CrossRef]
| Caloric restriction (CR) | Throughout the entire duration of the dietary intervention, participants have successfully implemented a reduction in caloric intake by 20–30% below the average, ensuring they maintain adequate nutrition and avoid any risk of malnutrition. |
| Intermittent fasting (IF) | Alternating periods of fasting and eating. IF includes:
|
| Restriction of specific macronutrients | Glucose and carbohydrate restriction; Protein restriction; amino acid restriction; Micronutrient restriction. |
| Ketogenic diets (KD) | A high-fat, low-carbohydrate dietary approach that aims to induce a state of ketosis in the body. The typical macronutrient distribution involves consuming a very low amount of carbohydrates (generally less than 50 g per day or 5–10% of total calories), a moderate amount of protein, and a high proportion of dietary fat (70–75% of total calories). |
| Fasting mimicking diet (FMD) | A dietary protocol designed to mimic the effects of a prolonged fast providing some nutrient intake. The main components of an FMD typically include consuming plant-based foods such as vegetables, nuts, and seeds and healthy fats like olive oil. The macronutrient distribution is calculated to provide around 40–50% of normal calorie intake on the first day and around 10–20% for the following days of the fasting period (4–7 days every 15–365 days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccardi, V.; Pigliautile, M.; Guazzarini, A.G.; Mecocci, P. The Potential of Fasting-Mimicking Diet as a Preventive and Curative Strategy for Alzheimer’s Disease. Biomolecules 2023, 13, 1133. https://doi.org/10.3390/biom13071133
Boccardi V, Pigliautile M, Guazzarini AG, Mecocci P. The Potential of Fasting-Mimicking Diet as a Preventive and Curative Strategy for Alzheimer’s Disease. Biomolecules. 2023; 13(7):1133. https://doi.org/10.3390/biom13071133
Chicago/Turabian StyleBoccardi, Virginia, Martina Pigliautile, Anna Giulia Guazzarini, and Patrizia Mecocci. 2023. "The Potential of Fasting-Mimicking Diet as a Preventive and Curative Strategy for Alzheimer’s Disease" Biomolecules 13, no. 7: 1133. https://doi.org/10.3390/biom13071133
APA StyleBoccardi, V., Pigliautile, M., Guazzarini, A. G., & Mecocci, P. (2023). The Potential of Fasting-Mimicking Diet as a Preventive and Curative Strategy for Alzheimer’s Disease. Biomolecules, 13(7), 1133. https://doi.org/10.3390/biom13071133








