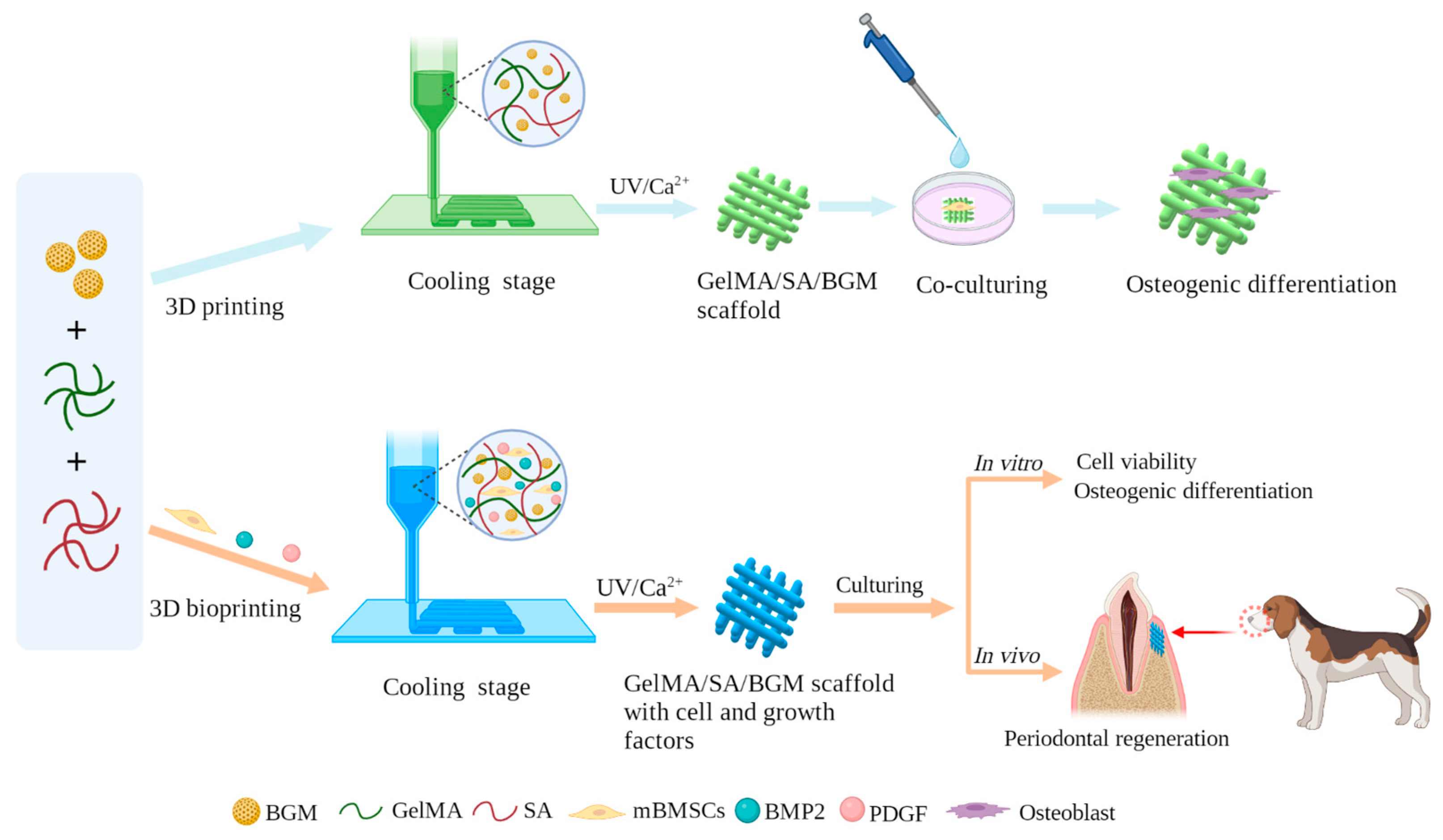
1. Introduction
Periodontal disease often results in defects in both bone tissue and soft tissue of gingiva [
1,
2,
3,
4,
5]. However, current clinical treatments are unable to effectively repair both types of tissue simultaneously [
6,
7]. In recent years, the application of 3D printing technology in tissue engineering has provided the possibility for accurate adjustment of the spatial structure and pore structure of scaffolds [
8,
9,
10]. In addition, 3D bioprinting technology has gradually become a research hotspot in the field of regenerative medicine because it can realize the layer-by-layer printing of biological inks containing biological materials, bioactive factors and cells, which provides a novel method for the personalized repair of tissue defects [
11,
12,
13], especially for periodontal tissue defects.
The application of biomaterials in the treatment of periodontal tissue defects has been widely studied and has achieved good clinical achievements. For example, Muhammad S Shaikh et al. carried out meta-analyses regarding the clinical outcomes of nano-hydroxyapatite or bovine-derived hydroxyapatite/cell-binding peptide complexes for regeneration of periodontal defects; the results showed that the above two biomaterials showed better therapeutic effects than open-flap debridement in the treatment of periodontal intrabony defects [
14,
15]. However, this class of inorganic biomaterials is mostly granular and cannot be easily shaped to scaffolds for irregular bone defects. Hydrogels are considered as ideal scaffold materials for tissue repair due to their microstructures and physicochemical properties similar to the natural extracellular matrix (ECM), which is not only conducive to cell attachment, migration and proliferation [
16,
17], but also can encapsulate living cells and maintain cell phenotypes and induce differentiation [
18]. Moreover, the cell-laden hydrogels are suitable as bio-inks for 3D bioprinting to construct personalized scaffolds and show great potential in diverse biomedical applications [
19,
20], particularly in periodontal tissue regeneration and repair. While a range of materials and techniques have been employed to design and construct cell-laden hydrogels for this purpose, there remains room for improvement in terms of in vitro bioactivity and the ability to regenerate both soft and hard periodontal tissue.
Gelatin methacryloyl (GelMA) is a kind of modified gelatin with photosensitive functional groups; it has attracted increasing interest in the preparation of 3D-printed bone tissue engineering scaffolds and cell delivery in recent years [
21,
22,
23]. However, due to the poor mechanical properties and temperature sensitivity, it is difficult to directly print biological scaffolds by extrusion-based 3D printing, which restricts its use in bone tissue engineering. Blending of other biomaterials (sodium alginate, SA) capable of forming hydrogels has been used to improve the printability of bio-inks, the biological and mechanical properties of which can be custom-tailored according to different requirements [
24,
25,
26]. Additionally, inorganic bioactive components (hydroxyapatite, nano clay, bioactive glass, etc.) are usually introduced to organic matrices to further enhance the biological properties of bio-inks [
27,
28]. Due to its excellent in vitro bioactivity, osteogenic activity and soft tissue repair ability, bioactive glass microspheres (BGMs) have attracted extensive attention in the field of tissue regeneration and have been used as bioactive fillers incorporated into hydrogel matrixes to improve their ability to repair bone or skin soft tissue [
29]. The addition of BGM into GelMA hydrogels could significantly improve their physicochemical and biological properties [
28]. Although GelMA, BGM and SA are promising materials for hydrogel scaffolds, their suitability as bio-inks for the 3D bioprinting of cell- and growth factor-loaded hydrogel scaffolds for periodontal tissue regeneration has yet to be determined.
Therefore, in this study, we aimed to develop a novel composite hydrogel with good bioactivity and biological properties, which can be fabricated into porous scaffolds by 3D printing and can be used as a bio-ink for cell bioprinting to prepare personalized restorative scaffolds for regeneration of periodontal soft and hard tissue. GelMA, SA and BGM were employed to prepare a composite hydrogel and fabricated porous scaffolds by 3D printing. The physicochemical properties and in vitro biological performance of the 3D-printed scaffolds were investigated. After that, we applied the above-mentioned hydrogel as a bio-ink to living cells and growth factors for the fabrication of a scaffold for periodontal tissue. Mouse bone marrow mesenchymal stem cells (mBMSCs) were used as model cells wrapped in the 3D-bioprinted hydrogel scaffolds because of the multidirectional differentiation potential of stem cells. To further improve the periodontal tissue repair ability of the cell-loaded scaffolds, BMP-2 and PDGF were added to the hydrogel for promoting the simultaneous repair of bone tissue and gingival soft tissue. The survivability and differentiation capability of the cells in the scaffolds as well as the capability of in vivo periodontal tissue regeneration were studied. There are few reports regarding the construction of bifunctional cell-laden hydrogel scaffolds in combination with BMP2 and PDGF for periodontal tissue repair. This research will present a good option for 3D bioprinting of bioactive scaffolds for simultaneous repair of periodontal bone and soft tissue. The fabrication and characterization of 3D-printed GelMA/SA/BGM scaffolds and 3D-bioprinted GelMA/SA/BGM scaffolds with cell and growth-factor scaffolds are illustrated in
Figure 1.
2. Materials and Methods
2.1. Materials
Anhydrous ethanol (AE), HCl, K2HPO4·3H2O, MgCl2·6H2O, Na2SO4 and NaCl were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Dodecylamine (DDA), Methacrylic anhydride (MA), Photoinitiator 2959 (PI-2959), Gelatin (G), sodium alginate (SA), CaCl2, Tetraethyl orthosilicate (TEOS), Triethyl phosphate (TEP) and Ca(NO3)2·4H2O (CN) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Collagenase type II, Tris, β-glycerophosphate sodium, Ascorbic acid, Dexamethasone and Cetylpyridinium chloride (CPC) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM), Penicillin–Streptomycin Solution (100 U/mL streptomycin, 100 U/mL penicillin), fetal bovine serum (FBS), Phosphate buffer (PBS) and Trypsin-EDTA (0.25%) were purchased from Gibco (Thermo Fisher, Waltham, MA, USA). C57BL/6 mouse bone marrow mesenchymal stem cells (mBMSCs) were purchased from Cyagen Biosciences Inc. (Guangzhou, China). Triton X-100 was purchased from Beijing Dingguo Changsheng Biotechnology (Beijing, China). Cell Counting Kit-8 was purchased from Dojindo (Kumamoto Ken, Japan). BCIP/NBT Alkaline Phosphatase Color Development Kit was purchased from Beyotime Biotechnology (Shanghai, China). Alkaline Phosphatase testing kit (AKP testing kit) was purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (Nanjing, China). Live-Death Staining Kit, BCA Protein Assay Kit and Alizarin Red staining solution (1%, pH 4.2) were purchased from Bestbio (Shanghai, China). RNase-free water was purchased from Leagene Biotechnology (Beijing, China). TRIzol™ Reagent was purchased from Invitrogen (Carlsbad, CA, USA). Prime ScriptTM RT Master Mix (Perfect Real Time) and TB GreenTM Premix Ex Taq™ II (Tli RNaseh Plus) were purchased from Takara (Kyoto, Japan).
2.2. Synthesis of BGM
The BGM was synthesized according to the previous literature [
30]. The molar composition of BGM was 60% SiO
2, 36% CaO and 4% P
2O
5. Briefly, 3 g of DDA was dissolved in a mixed solution of AE (80 mL) and deionized water (25 mL) at 40 °C. Then, 10.9 mL of TEOS and 1.1 mL triethyl phosphate (TEP) were added successively at a rate of 0.5 mL/min, and each was stirred for 0.5 h. After that, 6.87 g of CN was added to the above solution, stirred for 3 h and aged for 24 h at room temperature. The white precipitates collected by centrifugation were washed alternately with AE and distilled water 2 to 3 times. The precipitates were dried in a freeze-dryer for 48 h and calcined at 600 °C for 3 h. The morphology and microstructure of BGM were characterized by scanning electron microscopy (SEM, DSM 982-Gemini, Zeiss, Oberkochen, Germany) and X-ray diffraction (XRD, X’pert PRO, PANalytical, Almelo, The Netherlands), respectively. The chemical composition was determined by energy dispersive spectroscopy (EDS).
2.3. Synthesis of GelMA
GelMA was prepared in accordance with a previous study [
31]. Briefly, 10 g of G was fully dissolved into 100 mL of phosphate buffer saline (PBS) at 60 °C, and 8 mL of MA was slowly added at a rate of 0.5 mL·min
−1 under vigorous stirring. After 3 h of reaction, the mixture solution was dialyzed against distilled water to remove unreacted components through dialysis membranes (12–14 kDa molecular weight cut-off) at 40 °C for 7 days. Finally, the dialyzed solution was centrifuged and freeze-dried, and the lyophilized GelMA was stored at −20 °C for future use.
2.4. Preparation of GelMA/SA and GelMA/SA/BGM 3D-Printed Scaffolds
Before printing, the inks were first prepared. The lyophilized GelMA (15% w/v), BGM (5% w/v) and PI 2959 (0.5% w/v) were dissolved in deionized water to obtain a homogenous ink. A certain amount of SA (10% w/v) solution was added to the above ink to improve its printability. The ink was transferred to a printing barrel with a nozzle (400 μm inner diameter) that was fixed to a 3D printer (3D-bio printer, Bio-Architect ®WS, Regenovo, Hangzhou, China). The distance between the grids was 1 mm. The temperature of the ink was set at 20 °C, and the printing platform was adjusted to 5 °C to make the extruded strands solidify and obtain an initial intensity. The GelMA/SA/BGM scaffold was printed as designed by extrusion, layer by layer. After printing, the scaffold was transferred to a 4% CaCl2 solution, cross-linked for 5 min, irradiated for 30 s under 365 nm UV light, washed with deionized water three times, frozen at −80 °C overnight, and finally freeze-dried for 48 h. Pure GelMA/SA scaffold without BGM was also prepared with the same parameters.
2.5. Characterization of 3D-Printed Scaffolds
2.5.1. SEM Observation
The surface microstructure was characterized by scanning electron microscopy (SEM, S-3400N, Hitachi, Tokyo, Japan) at an accelerating voltage of 15 kV. Before observation, the lyophilized samples were sputter-coated with gold. The surface elemental composition was detected by an energy dispersive X-ray spectrometer (EDS) equipped on the SEM.
2.5.2. Swelling Test
To measure the equilibrium swelling ratio, samples were completely lyophilized, and the dry weight was recorded (Wd). Then, the samples were soaked in PBS at 37 °C for 24 h. The swollen samples were weighed (Ws) after gently wiping the surface using filter paper. The swelling ratio was calculated as (Ws − Wd)/Wd × 100%.
2.5.3. In Vitro Degradation Test
The degradation properties of composite hydrogel scaffolds were evaluated using collagenase solution. The lyophilized samples were weighed (W0) and immersed in collagenase II solution at a concentration of 2.5 U/mL at 37 °C. The samples were collected at specified time points (6, 12, 24, and 48 h), washed with deionized water, and then completely lyophilized. The weight of the dried samples after degradation was measured as Wd. The degradation ratio was calculated with the following equation: (Wd/W0) × 100%.
2.5.4. Mechanical Properties
The compression properties of composite hydrogel scaffolds were measured by a universal testing machine (Instron 336, Norwood, MA, USA). The samples used in the compression test were prepared in a cylinder, 10 mm in diameter and 2 mm in height. The stress–strain curve was recorded at a compression rate of 0.5 mm. The compression modulus was calculated using the slope of the linear deformation stage (10–20%) of the stress–strain curve.
2.5.5. In Vitro Bioactivity
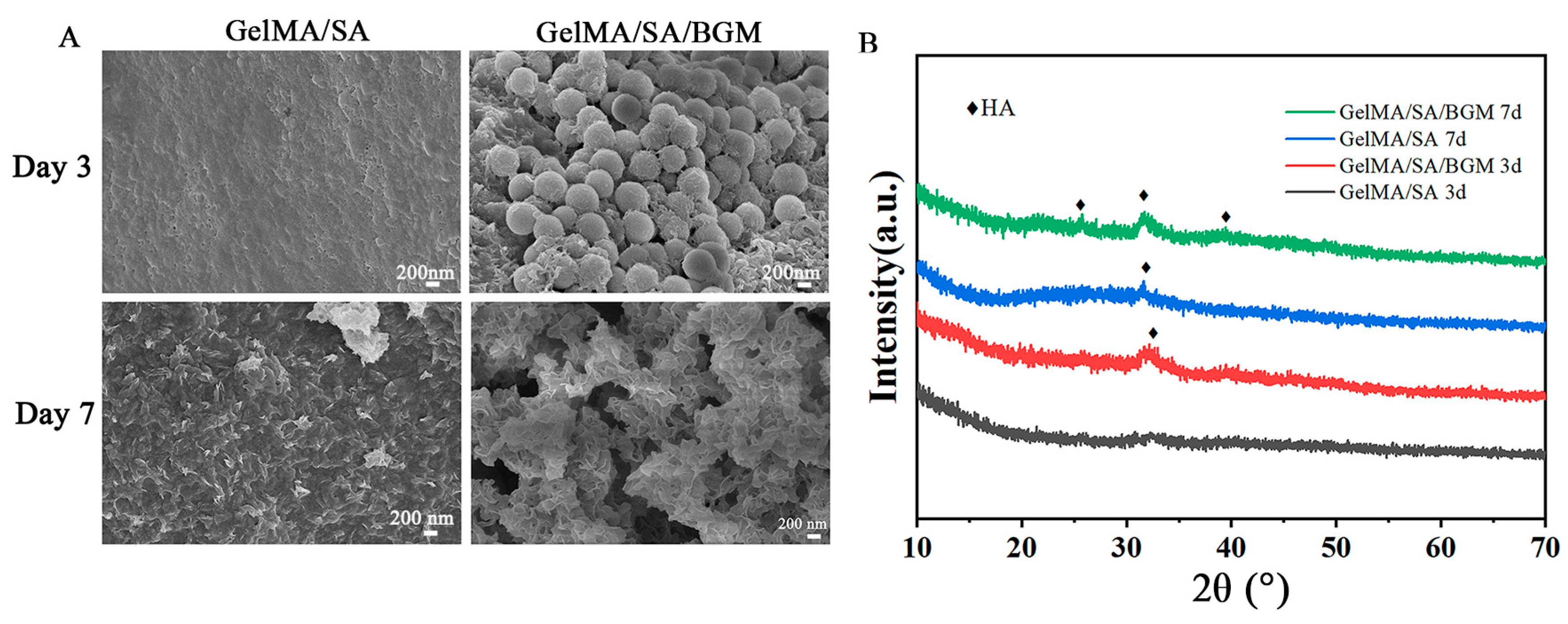
The in vitro bioactivity of composite hydrogel scaffolds was evaluated by inducing apatite formation on the scaffold surface. All samples (10 mm × 10 mm × 5 mm) were immersed in 50 mL of 2 × simulated body fluid (SBF) at 37 °C. After 3 and 7 days of immersion, the samples were collected, freeze-dried, and observed by SEM and XRD.
2.6. In Vitro Biocompatibility
2.6.1. Cell Viability and Morphology
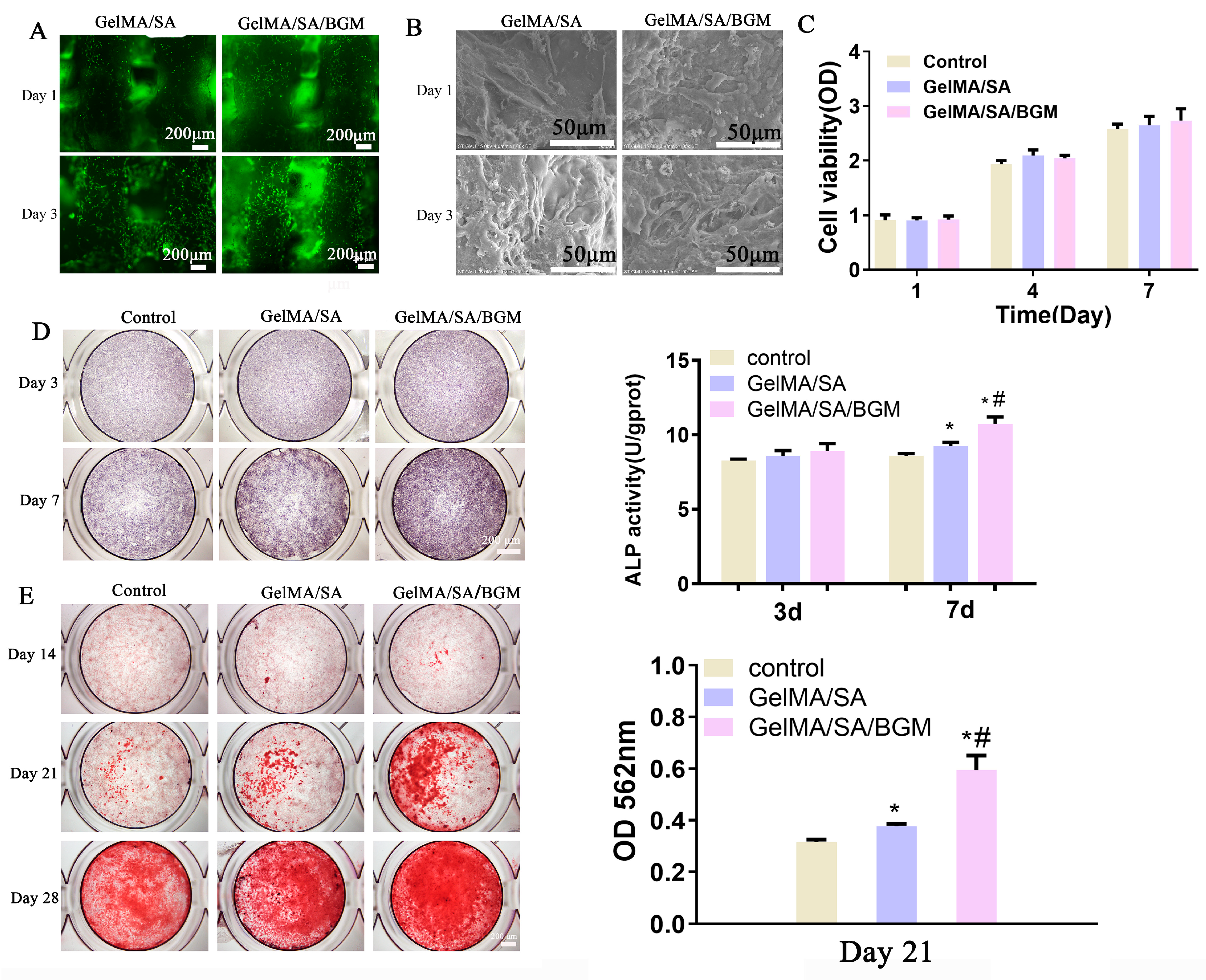
Before cell experiments, the scaffolds were treated under UV irradiation and 75% ethanol immersion for sterilization, washed three times with PBS, placed in 12-well plates, and incubated with DMEM overnight. After the scaffolds were incubated in the cell incubator for 30 min, the cell suspension (1 mL, 1 × 106 cells/mL) was added on each scaffold and incubated at 37 °C in 5% CO2. After 3 d and 7 d of culture, the cell viability on the scaffolds was evaluated using a Live-Death Staining Kit (Bestbio, Shanghai, China). The cell-laden scaffolds were rinsed with PBS three times and incubated with a 2 μM Calcein AM solution at room temperature for 30 min. Then, the scaffolds were thoroughly rinsed with PBS twice and incubated with 4 μM EthD-1 for 2 min. The living cells stained by Calcein AM and dead cells stained by EthD-1 were visualized using a fluorescence microscope (DMI3000B, Leica, Weztlar, Germany). SEM (S-3400N, Hitachi, Tokyo, Japan) was used to observe the cell morphology on the scaffolds after 4% paraformaldehyde fixation, gradient alcohol dehydration, and freeze-drying.
2.6.2. Cell Proliferation
The scaffold extracts were used for cell proliferation and osteogenesis assays. Briefly, the sterilized GelMA/SA and GelMA/SA/BGM scaffolds were soaked in DMEM at a mass/volume ratio of 5 mg/mL to prepare the extracts. After 24 h of incubation, the supernatant was collected by centrifugation and sterilized using a 0.22 μm filter (Millipore). mBMSs were seeded into 96-well plates at a density of 1.5 × 103 cells/well. After 24 h incubation, the culture medium was replaced with different extracts and incubated for 1, 3 and 7 days. The medium was changed every two days, and the DMEM without scaffolds immersion was used as the control group. At every time point, the culture medium was removed, and 200 μL of fresh medium containing 10% (v/v) CCK-8 solution was added and incubated for 2 h at 37 °C. A total of 100 μL of the medium was transferred to a new 96-well plate, and the absorbance was measured at 450 nm by a microplate spectrophotometer (Multiskan FC, Thermo, Waltham, MA, USA). Each group had three parallel samples.
2.7. The Osteogenic Activity of Composite Hydrogel Scaffolds
2.7.1. Alkaline Phosphatase (ALP) Activity
mBMSCs were seeded in 48-well plates at a density of 1 × 104 cells/well. After 24 h, the medium was replaced with GelMA/SA and GelMA/SA/BGM extracts containing osteogenic medium (10 nM dexamethasone, 10 mM β-glycerolphosphate and 50 ng/mL ascorbic acid). The osteogenic medium with extracts was used as the control group. The culture medium was changed every two days. At day 3 and 7, ALP staining was performed using a BCIP/NBT kit according to the manufacturer’s instruction. In addition, quantitative analysis of ALP was also detected by AKT assay kit cells according to the manufacturer’s instructions. The absorbance value was measured at 405 nm using 4-nitrophenol as the standard. The intracellular total protein content was determined by the BCA kit. Finally, the ALP activity was normalized to the total protein content. Each group had three parallel samples.
2.7.2. Alizarin Red Staining and Accumulated Calcium Assay
mBMSCs were seeded in 48-well plates at a density of 1 × 104 cells/well. After 24 h, the medium was replaced with GelMA/SA and GelMA/SA/BGM extracts containing osteogenic medium (10 nM dexamethasone, 10 mM β-glycerolphosphate and 50 ng/mL ascorbic acid). The osteogenic medium with extracts was used as the control group. The culture medium was changed every two days. After culturing for 14, 21 and 28 days, cells were rinsed with PBS, fixed, and incubated with 40 mM alizarin red S (ARS) (Sigma-Aldrich, Saint Louis, MO, USA) solution for 30 min to stain mineralized nodules. The stained nodules were observed using a stereoscopic microscope (EZ4HD, Leica, Weztlar, Germany). The quantitative analysis of ARS staining at day 21 was performed by adding 10% w/v cetylpyridinium chloride (Sigma-Aldrich, Saint Louis, MO, USA), and the absorbance of each well was detected at 562 nm.
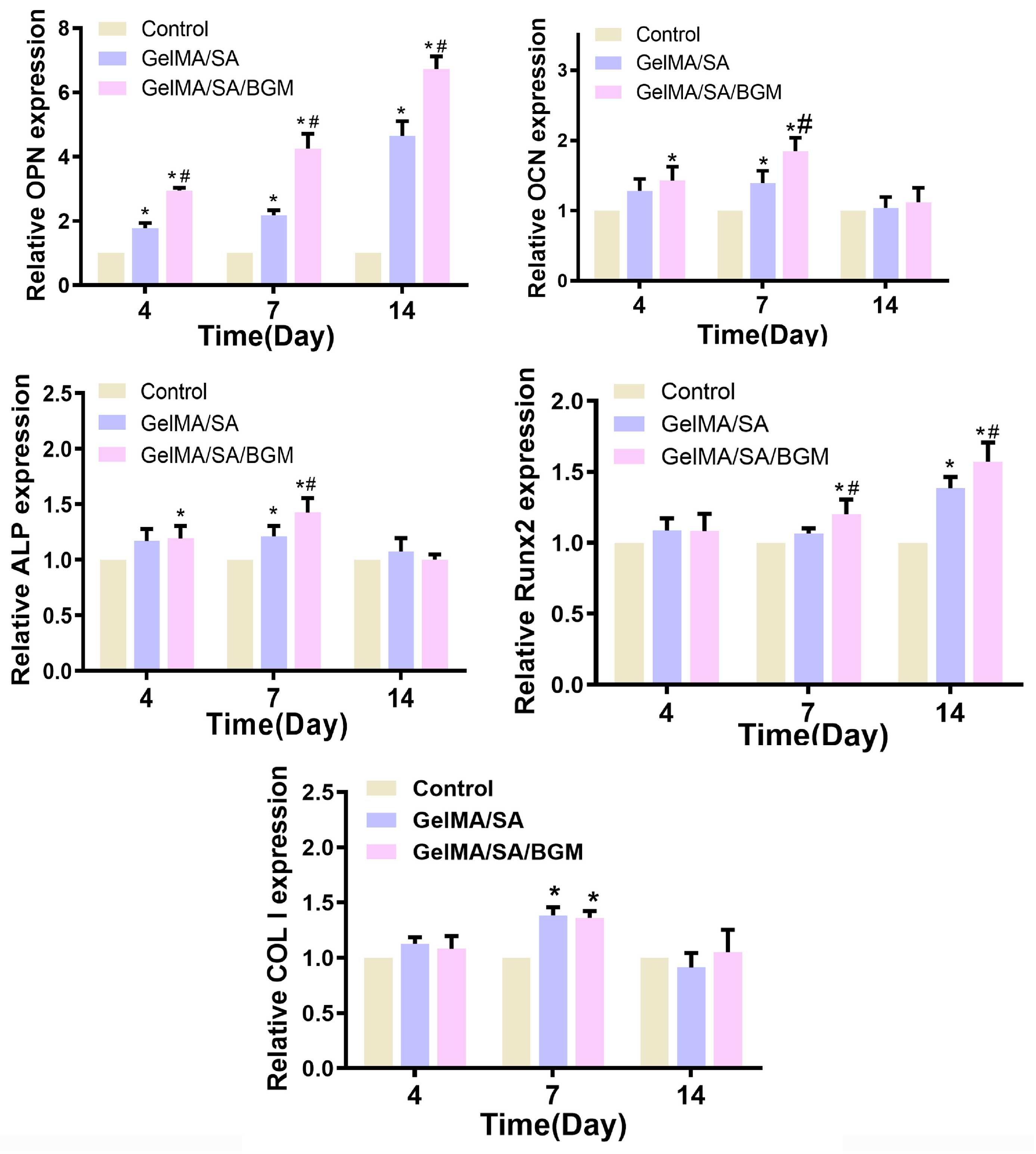
2.7.3. Osteogenesis-Related Gene Expression
mBMSCs were seeded in 6-well plates at a density of 1 × 10
5 cells/well. After 24 h, the medium was replaced with GelMA/SA and GelMA/SA/BGM extracts containing osteogenic medium (10 nM dexamethasone, 10 mM β-glycerolphosphate and 50 ng/mL ascorbic acid). The osteogenic medium with extracts was used as the control group. The culture medium was changed every two days. After 3, 7 and 14 days, the expression of osteogenesis-related genes in mBMSCs, including alkaline phosphatase (ALP), aosteopontin (OPN), osteocalcin (OCN), runt-related transcription factor 2 (Runx2) and collagen typeⅠ(ColⅠ), were analyzed by real-time quantitative polymerase (RT-qPCR). Typically, total RNA was extracted using a TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) and according to the manufacturer’s instructions. The isolated RNA was reverse-transcribed into cDNA using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Kyoto, Japan). Afterwards, the expression of osteogenesis-related genes was measured on a Bio-Rad MyiQ single color RT-qPCR system using a SYBR green PCR kit (Takara, Kyoto, Japan). The relative expression levels of target genes were calculated by the 2
−ΔΔCT formula and normalized to that of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each group had three parallel samples. The primers used for RT-qPCR are listed in
Table 1.
2.8. 3D Bioprinting of Cell-Laden Scaffolds
The inks prepared above were further used to prepare bio-inks by mixing with the cell suspension. The cell density in bio-inks was 1 × 107 cells/mL. In order to improve the biological activity of the scaffold, BMP-2 and PDGF were added into the bio-inks with a final concentration of 5 ug/mL and 100 ng/mL, respectively. Before printing, the working area was first sterilized by UV light irradiation. A nozzle with an inner diameter of 400 μm was used, and the bioprinting process was performed after adjusting the appropriate printing parameters. After printing, the scaffold was transferred to a 4% CaCl2 solution, cross-linked for 5 min, and then irradiated for 30 s under 365 nm UV light. Then, the scaffolds were washed with PBS containing 2% penicillin–streptomycin three times and finally replaced with fresh culture medium. Three groups of cell-laden scaffolds were prepared, and according to the different growth factors in the bio-inks, they were labeled as follows: mBMSCs group, mBMSCs/BMP2 group and mBMSCs/PDGF group.
2.9. Cell Viability, Proliferation and Differentiation
The cell-laden scaffolds were incubated in culture medium, and the medium was changed every two days. The scaffolds used for cell viability assay were printed in a dimension of 10 mm × 10 mm with two layers. After culturing for 1 and 3 days, cell viability in the cell-laden scaffolds was assessed using a Live-Death Staining Kit according to the manufacturer’s instructions. The live/dead cells were observed by a confocal microscope (Leica, TCSSP8, Germany). At 1, 3 and 5 days, cell proliferation in the cell-laden scaffolds (10 mm × 10 mm, 4 layers) was assessed by CCK8. The absorbance values were measured at 450 nm, with three parallel samples.
For ALP activity assay, the cell-laden scaffolds (10 mm × 10 mm, 4 layers) were printed. After culturing for 3, 7 and 10 days, the scaffolds were crushed evenly; then, 200 μL of 1% Triton X-100 was added to each well and lysed at 4 °C for 30 min. After centrifugation, the supernatant was taken for ALP quantification, and the total protein amount was calculated according to the methods in
Section 2.6.1.
For RT-qPCR analysis, the cell-laden scaffolds (10 mm × 10 mm, 10 layers) were printed and cultured in a 12-well plate. At days 7 and 14, the scaffolds were crushed, and the total RNA was extracted using the TRIzol method. The expressions of osteogenesis-related genes (OPN, CCN and Runx2) and the key gene (Fibronectin, FN) linked to soft tissue repair were analyzed by RT-qPCR. The steps of reverse transcription and PCR reaction are as specified in
Section 2.7.3.
2.10. In Vivo Experiment
2.10.1. Surgical Procedure
Three adult male beagles (average weight = 12–19 kg, 12–15 months) were used for the in vivo experiments. All animal experimental protocols were approved by the Animal Research Committee of Guangzhou Longgui Xingke animal farm (XK19011018, date: 3 December 2019) and were in compliance with the instructions of Institutional Animal Care and Use Committee (IACUC) guidelines.
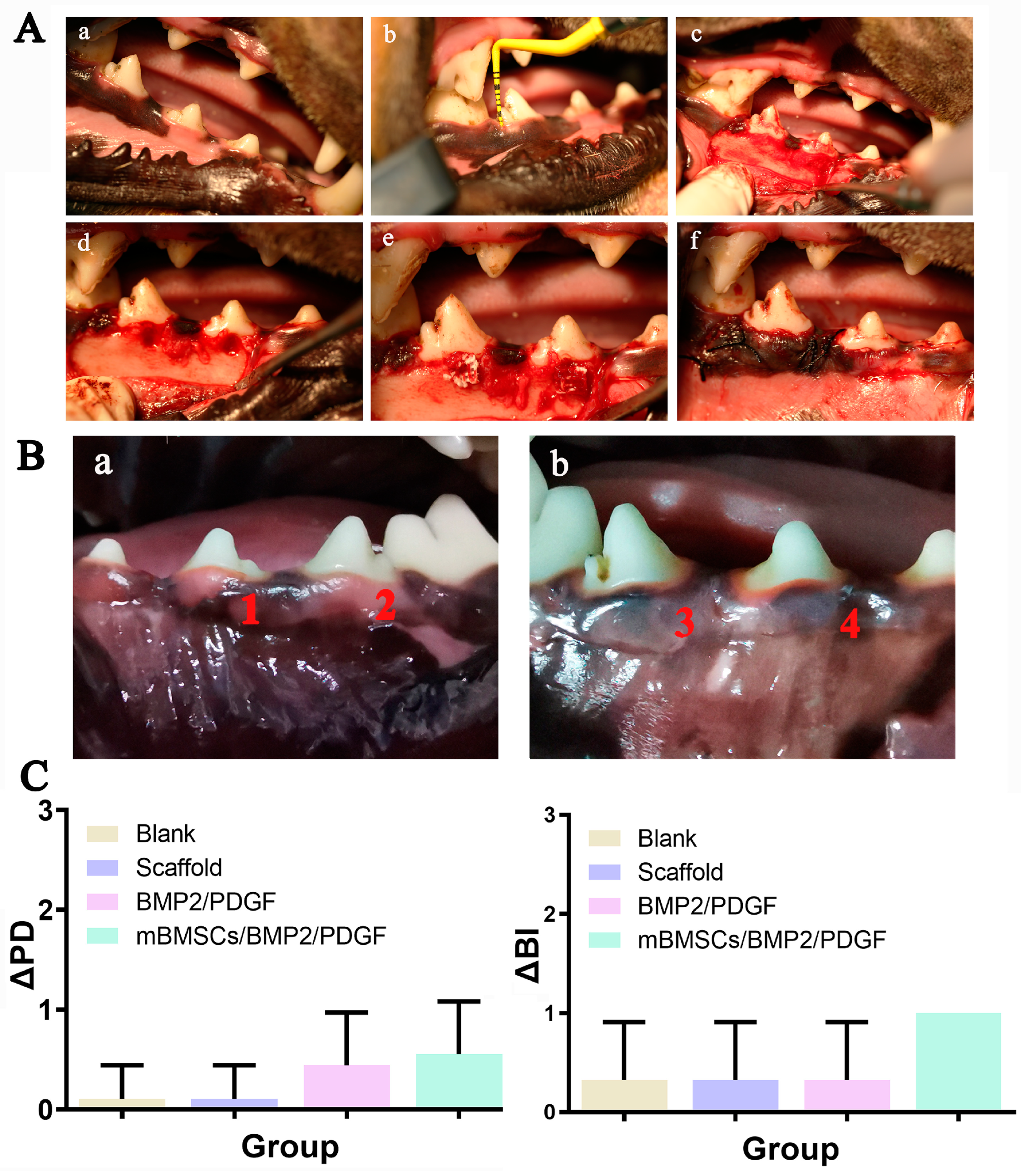
The periodontal defect model was established as follows: general anesthesia was administered to beagle dogs with pentobarbital at a dose of 30 mg/kg, and local infiltration anesthesia of the operative area was achieved with atticaine containing 1:100,000 norepinephrine. After successful anesthesia, periodontal evaluation, including probing depth (PD) and bleeding index (BI), was performed first. Then, in a lateral position, an opener was placed, and a flap procedure was performed in the mandibular premolar region of the beagle dogs. A buccal periodontal defect of 5 mm × 5 mm (width × depth) was made on 1/3 of the root of the third and fourth mandibular premolars on both sides with a dental drill. The scaffolds were divided into four groups: (1) non-implanted scaffold (blank group), (2) GelMA/SA/BGM scaffold (scaffold group), (3) GelMA/SA/BGM scaffold containing BMP2 and PDGF (BMP2/PDGF group), (4) cell-laden GelMA/SA/BGM scaffold containing BMP2 and PDGF (mBMSCs/BMP2/PDGF group); they were then appropriately sized and placed at the defect sites. The wound was alternately washed with normal saline and gentamicin and then reset and sutured. Penicillin (4 × 104 IU/kg) was injected intramuscularly for 3 days to prevent infection.
2.10.2. Micro-CT Assessment of Osteogenic Behavior around Implants
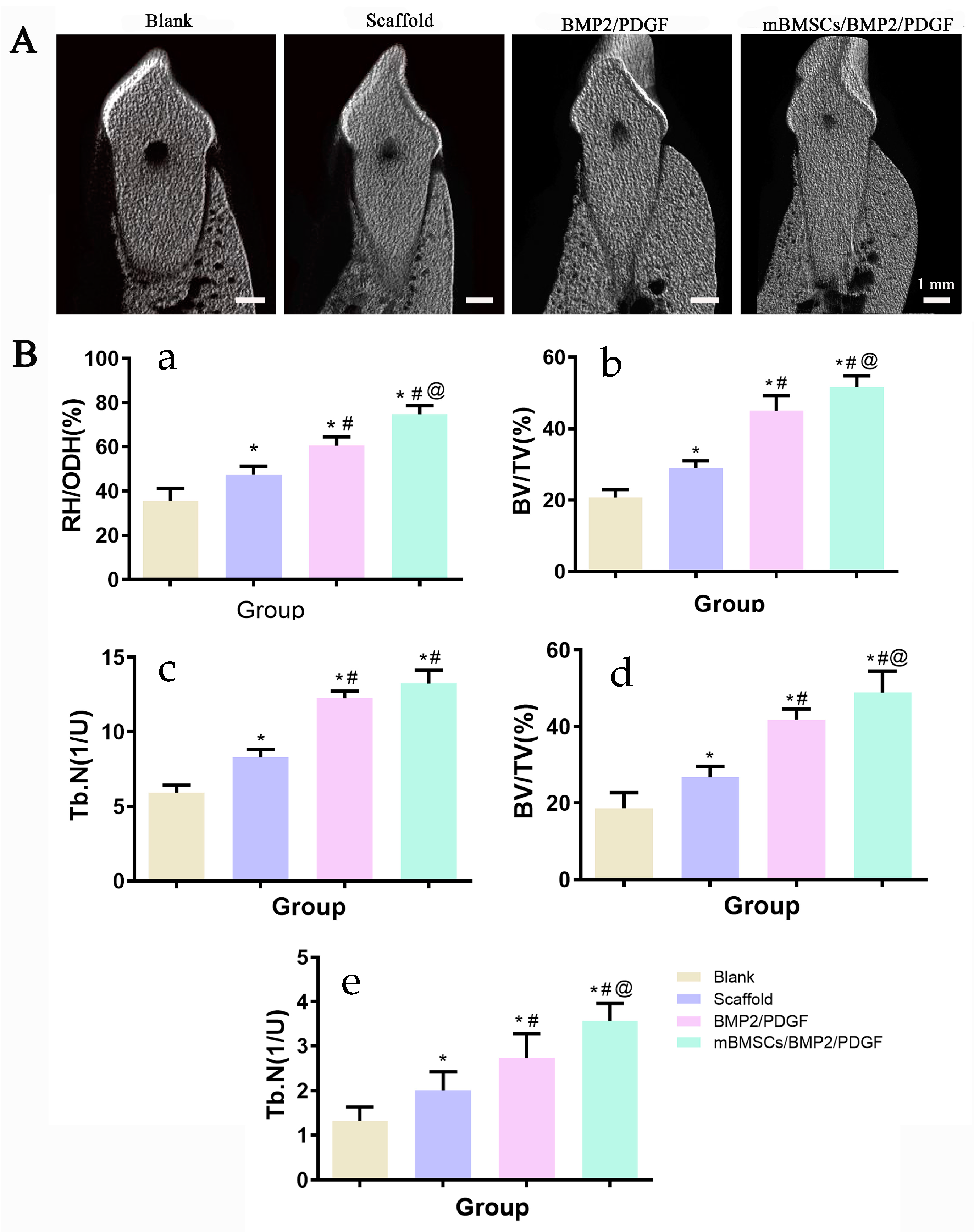
Eight weeks after the operation, periodontal evaluation was performed, including periodontal probing depth (PD) and gingival bleeding index (BI). Then, the beagles were executed with pentobarbital overdose. Bilateral mandibular samples (premolar to first molar area) were collected and fixed in 4% paraformaldehyde at room temperature for 48 h. Samples were fixed on the camera and scanned one by one with Micro-CT (SKYSCAN1172, Bruker, Karlsruhe, Germany). The rate of newborn bone height (Regeneration height per original defect height, RH/ODH), number of the new bone trabecular mass (trabecular number, Tb.N), and proportion of newborn bone volume to total volume (bone volume per total volume, BV/TV) were calculated and analyzed by NRecon and CTAn software (Bruker, Karlsruhe, Germany).
2.10.3. Histological Evaluation and Histomorphometric Measurement
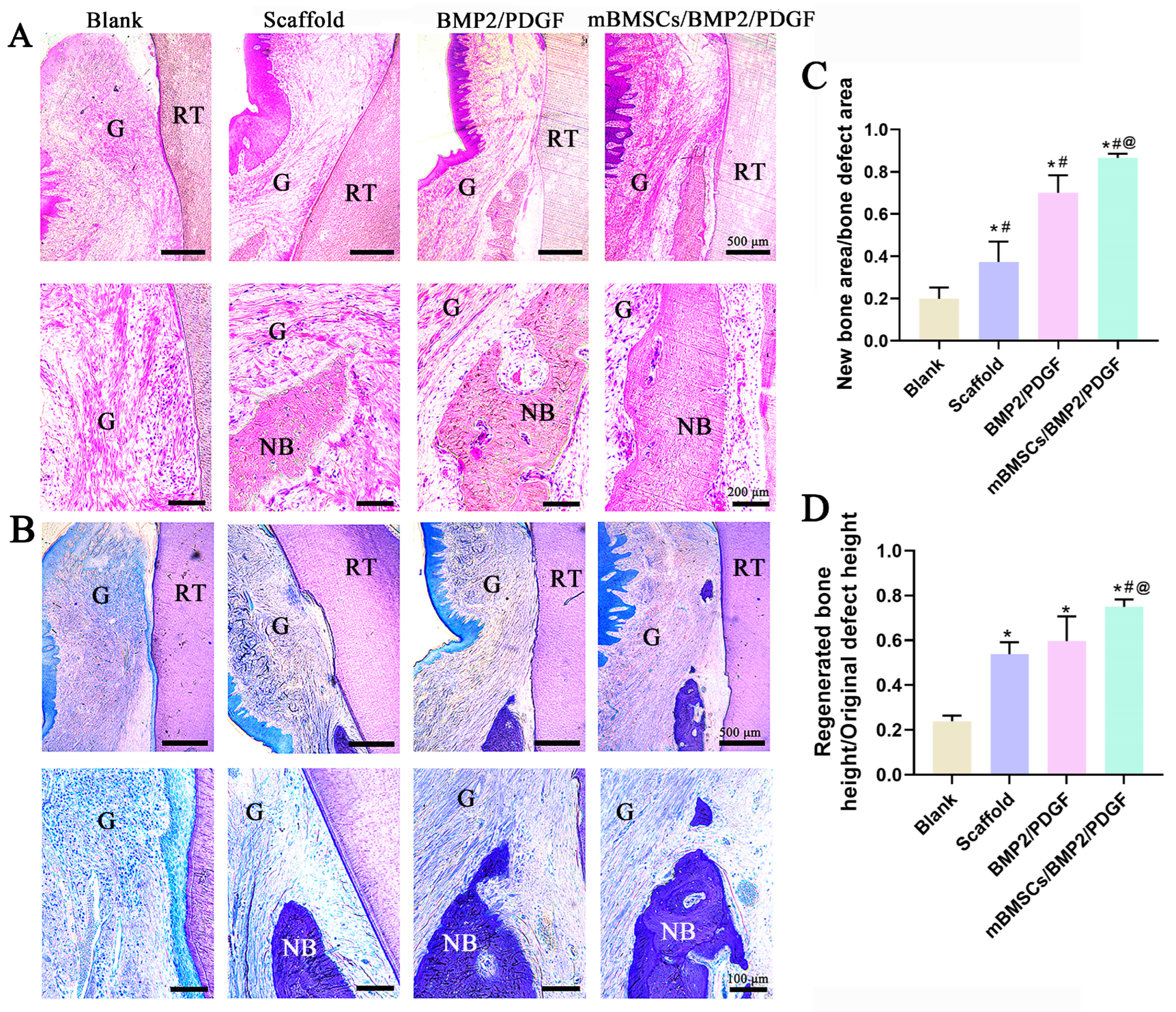
The samples were removed from the fixation solution, washed overnight with tap water, then dehydrated with gradient ethanol, followed by embedding with photocurable resin (T7200 resin embedding agent). After the resin curing was completed, the tissue sections were cut by a hard tissue slicer in the direction of the buccal tongue; the thickness of the sections was 100 μm. After polishing, HE staining and toluidine blue staining were performed according to the manufacturer’s instructions. Finally, the gingiva and new bone regeneration in the periodontal defect area were observed using a microscope (Olympus DP74, Tokyo, Japan). The histomorphological analysis was performed using a modified method, as described in the previous literature [
32,
33]. Specifically, the ratio of new bone height and new bone area to initial bone defect height and bone defect area were examined. Height measurements were initiated from the bottom of the bone defect, serving as the boundary, and were executed using image J 1.53e software. The quantification of the original bone defect area and regenerated bone area was accomplished by cropping various regions of the micrograph. The total height and area of the newly generated bone in each photomicrograph were determined by the total color pixels of each image. These measurements were subsequently divided by the total area of bone defect or total height pixels to derive the fraction.
2.11. Statistical Analysis
All data were expressed as means ± standard deviations. Statistical analysis was realized using SPSS version 16.0 (Statistical Package, Chicago, USA). One-way ANOVA was performed to discern the differences between groups. A value of p < 0.05 was considered to be statistically significant.
4. Discussion
The ideal periodontal repair should involve restoring both soft and hard periodontal tissues. Although tissue engineering techniques exhibit potential in regenerating periodontal tissues, the lack of appropriate scaffold materials has hindered the attainment of this objective. Nevertheless, recent advancements in 3D printing technology have facilitated the production of scaffolds with complex structures. This technique not only enables personalized restorations but also simulates the microenvironment of tissues and organs, providing an optimal environment for cell growth and extracellular matrix formation [
34,
35,
36]. With the development of 3D printing technology and biomaterials, 3D bioprinting of cell-laden composite hydrogel scaffolds has become a new direction for periodontal tissue regeneration. This novel biomanufacturing technology enables the precise construction of cells and cell encapsulation materials [
37]. To achieve successful 3D bioprinting, bio-inks aimed at preserving the cellular phenotype and function are required.
GelMA, a hydrogel material synthesized through chemical polymerization of gelatin and MA, exhibits a structure similar to natural ECM and possesses favorable biocompatibility and biodegradability [
38]. SA is a commonly employed hydrogel material in bioprinting due to its high viscosity and relatively simple cross-linking mechanism [
39,
40]. However, the surface structure of SA is not conducive to cell adhesion, necessitating the incorporation of other biomolecules to enhance cell adhesion [
10]. Although the composite hydrogel consisting of GelMA and SA exhibits favorable biocompatibility and printability, its application in bone tissue engineering remains restricted due to the absence of biological activity. To enhance the bioactivity of the composite hydrogel, BGM is commonly employed as a bioactive agent, owing to its unique biological properties, particularly its capacity to stimulate osteogenesis and angiogenesis [
41]. In this study, a multi-component hydrogel was successfully synthesized by combining inorganic bioactive particles (BGM) and a polymer matrix (GelMA and SA), which was then processed into GelMA/SA/BGM composite hydrogel scaffolds by 3D printing technology. The physicochemical characteristics and in vitro osteogenic ability of the scaffolds were evaluated. Subsequently, the GelMA/SA/BGM composite hydrogel was explored as a bio-ink for the fabrication of cell and growth factor (BMP2 and PDGF)-laden scaffolds using 3D bioprinting for the regeneration of periodontal tissue.
The regular arrangement of pore structure with similar pore size was observed in GelMA/SA and GelMA/SA/BGM 3D-printed scaffolds. Elemental analysis revealed the presence of Ca, Si and P in the GelMA/SA/BGM scaffold due to the addition of BGM particles, while the GelMA/SA scaffold also contained Ca, potentially resulting from the cross-linking of CaCl
2 with SA. Both GelMA/SA and GelMA/SA/BGM scaffolds exhibited high water content, facilitating nutrient and metabolite flow and transportation, and creating a favorable microenvironment for tissue regeneration and repair. The GelMA/SA/BGM scaffold exhibited a slightly lower water content compared to the GelMA/SA scaffold. This can be attributed to the potential interaction between the Ca ions dissolved from BGM and the carboxyl groups of GelMA, leading to a decrease in the binding capacity of the GelMA and water molecules [
28]. Additionally, the uniform dispersion of BGM within the scaffolds resulted in a decreased binding rate of water molecules to GelMA and SA, ultimately contributing to the reduction in water content. The degradation rate of the two scaffolds in the presence of type II collagenase exhibited an initial rapid decline followed by a gradual decrease, with the scaffold containing BGM demonstrating a slower degradation rate. This phenomenon may be attributed to the superior water absorption capacity of the GelMA/SA scaffold compared to the GelMA/SA/BGM scaffold, which facilitated rapid adsorption of the type II collagenase solution and subsequent degradation. Consequently, the incorporation of BGM into the GelMA/SA scaffold resulted in a marked improvement in the stability of the hydrogel system. The mechanical testing results indicated a reduction in compression modulus upon the inclusion of BGM, attributable to the presence of BGM impeding the interaction of GelMA molecules activated by PI-2959. The ability to induce apatite formation in vitro may indirectly reflect the in vivo osseointegration ability of biomaterials [
42]. The biomineralization assay conducted in vitro demonstrated the formation of a layer of apatite on the GelMA/SA/BGM scaffold surface, signifying the favorable bioactivity of this composite material system, suitable for the fabrication of bone tissue engineering scaffolds. The GelMA/SA-based scaffolds exhibited good biocompatibility and cell viability. The incorporation of BGM into the scaffold resulted in enhanced cell adhesion, proliferation, ALP activity, calcium nodule formation and the promotion of osteogenesis-related gene expression. The improvement of the biological performance of the BGM-loaded scaffold can be attributed to the bioactive ions (Si, Ca and P) released from BGM, which have been shown to promote the osteogenesis and angiogenesis of mesenchymal stem cells in many studies [
43,
44].
Based on the above experimental results, the composite hydrogel composed of GelMA, SA and BGM presented great potential as a bio-ink for 3D bioprinting of cell-laden scaffolds. Both BMP2 and PDGF are growth factors that are associated with regenerative repair of maxillofacial tissues, and they are capable of recruiting mesenchymal stem cells (MSCs). BMP2 primarily induces bone regeneration by promoting the differentiation of MSCs into osteoblasts [
45,
46]. On the other hand, PDGF is a potent chemotactic agent that can attract MSCs, fibroblasts, repair proteins, and other factors, thereby promoting soft tissue regeneration [
47]. Consequently, we developed a scaffold laden with mBMSCs, BMP2, and PDGF via 3D bioprinting to address the repair of the defects resulting from periodontal disease. The utilization of PDGF-B facilitates the restoration of gingival tissue, whereas BMP2 expedites the generation of fresh osseous tissue. Our investigation involved in vitro and in vivo experiments to evaluate the scaffold’s efficacy for bone and soft tissue repair, with potential applications for personalized tissue defect therapy.
Natural polymer hydrogels, such as GelMA and SA, exhibit favorable biocompatibility and low cytotoxicity, rendering them suitable carriers for delivering diverse biomolecules, including living cells [
48]. Bioactive glass is classified as a type of bioactive material that possesses favorable biocompatibility and osteoinductivity [
41]. Therefore, the 3D bioprinted GelMA/SA/BGM scaffolds with cells and growth factors exhibited favorable cell viability and proliferation activity. The ALP activity of the scaffold was enhanced after the addition of BMP2. OCN, OPN and Runx2 are markers for different stages of bone matrix mineralization and osteogenic differentiation. FN, as one of the components of the extracellular matrix, promotes fibroblast migration [
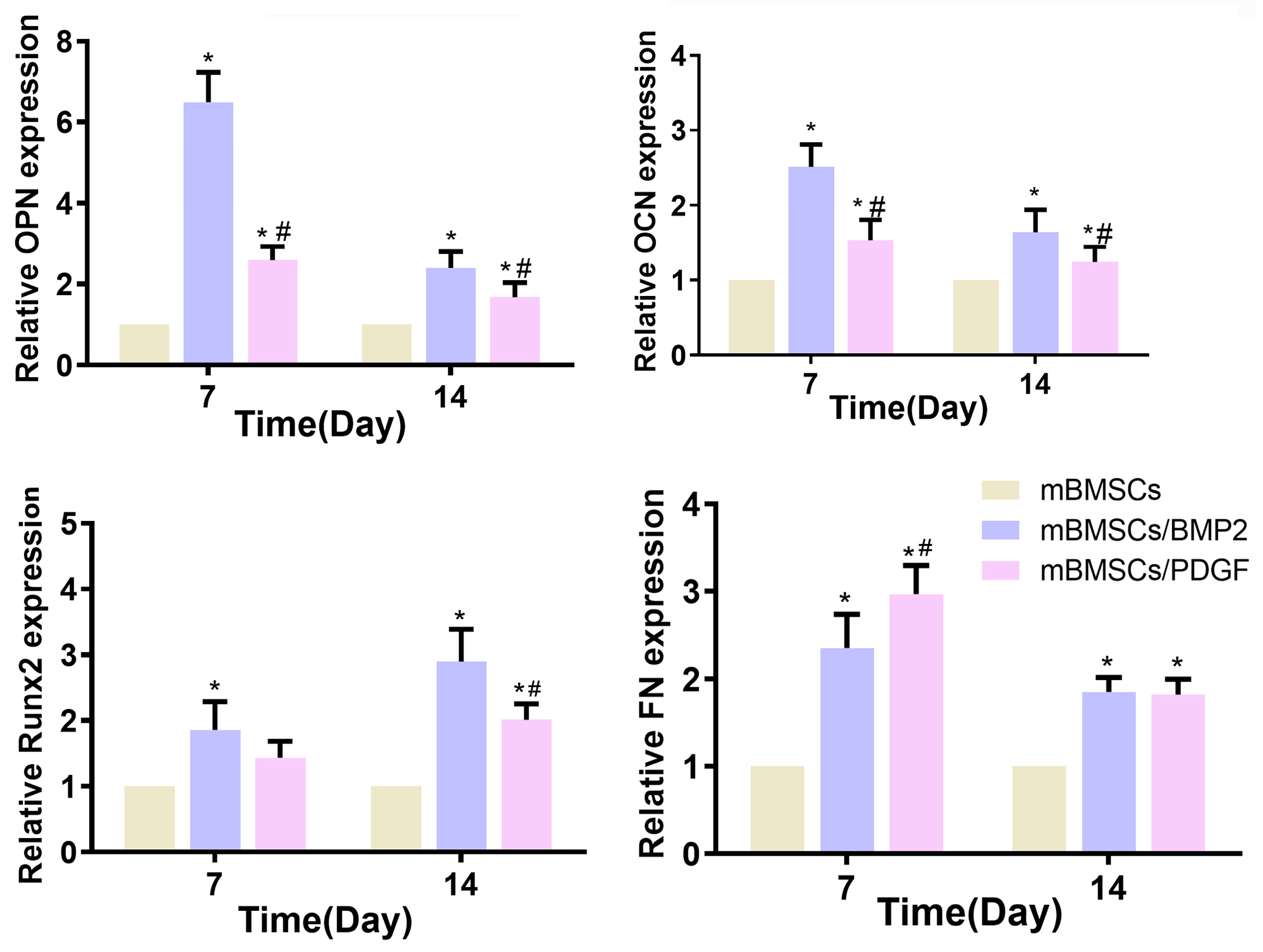
49] and is thus considered a marker for soft tissue repair. RT-qPCR results showed that the mBMSCs/BMP2 scaffold exhibited a greater capacity to enhance the expression of OCN, OPN and Runx2 in mBMSCs, while the mBMSCs/PDGF scaffold demonstrated a superior ability to promote the expression of FN in mBMSCs. Additionally, the expression of OCN, OPN and FN was observed to be more pronounced during the initial stage, while Runx2 exhibited greater activity during later stages.
In vivo, we constructed the mBMSCs/BMP2/PDGF scaffold by combining the mBMSCs/PDGF scaffold, which promotes soft tissue repair, and the mBMSCs/BMP2 scaffold, which promotes bone regeneration. The results indicated that postoperative wound healing was satisfactory, with improvements in ΔPD and ΔSI. The mBMSCs/BMP2/PDGF scaffold exhibited the most significant improvement, providing evidence that the mBMSCs/BMP2/PDGF scaffold is an effective means of promoting gingival repair. Additionally, new bone tissue was observed in all groups, with the mMSCs/BMP2/PDGF group exhibiting the most significant improvement in buccal alveolar bone height, bone tissue volume, and bone trabecular number, confirming that adding BMP2 in cell-laden scaffolds can effectively promote bone formation. Thus, it can be concluded that cell-laden scaffolds in combination with BMP2 and PDGF can effectively repair periodontal soft and hard tissue defects when compared with the scaffold only containing BMP2 and PDGF.
Although we successfully established a bio-ink that can be used for 3D bioprinting and achieved the desired bone and soft tissue regeneration in an in vivo periodontal defect experiment, the research still has some shortcomings. The low mechanical strength of the scaffold should be further improved to be more suitable for the repair of periodontal bone tissue defects. Additionally, an acute trauma model of periodontal tissue was used in this study, and the repair of periodontal injury caused by periodontal inflammation and the regulation effect of hydrogel on inflammation should be carried out in future studies.