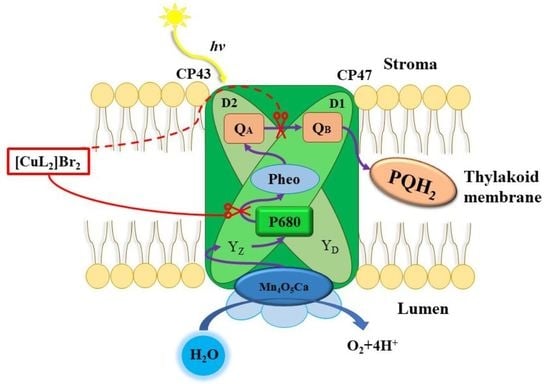
Probing the Influence of Novel Organometallic Copper(II) Complexes on Spinach PSII Photochemistry Using OJIP Fluorescence Transient Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of PSII-Containing Membranes
2.2. Fast Induction Kinetics of Chlorophyll Fluorescence
2.3. Spectrophotometric Measurements
2.4. Solutions of Inhibitory Agents
3. Results
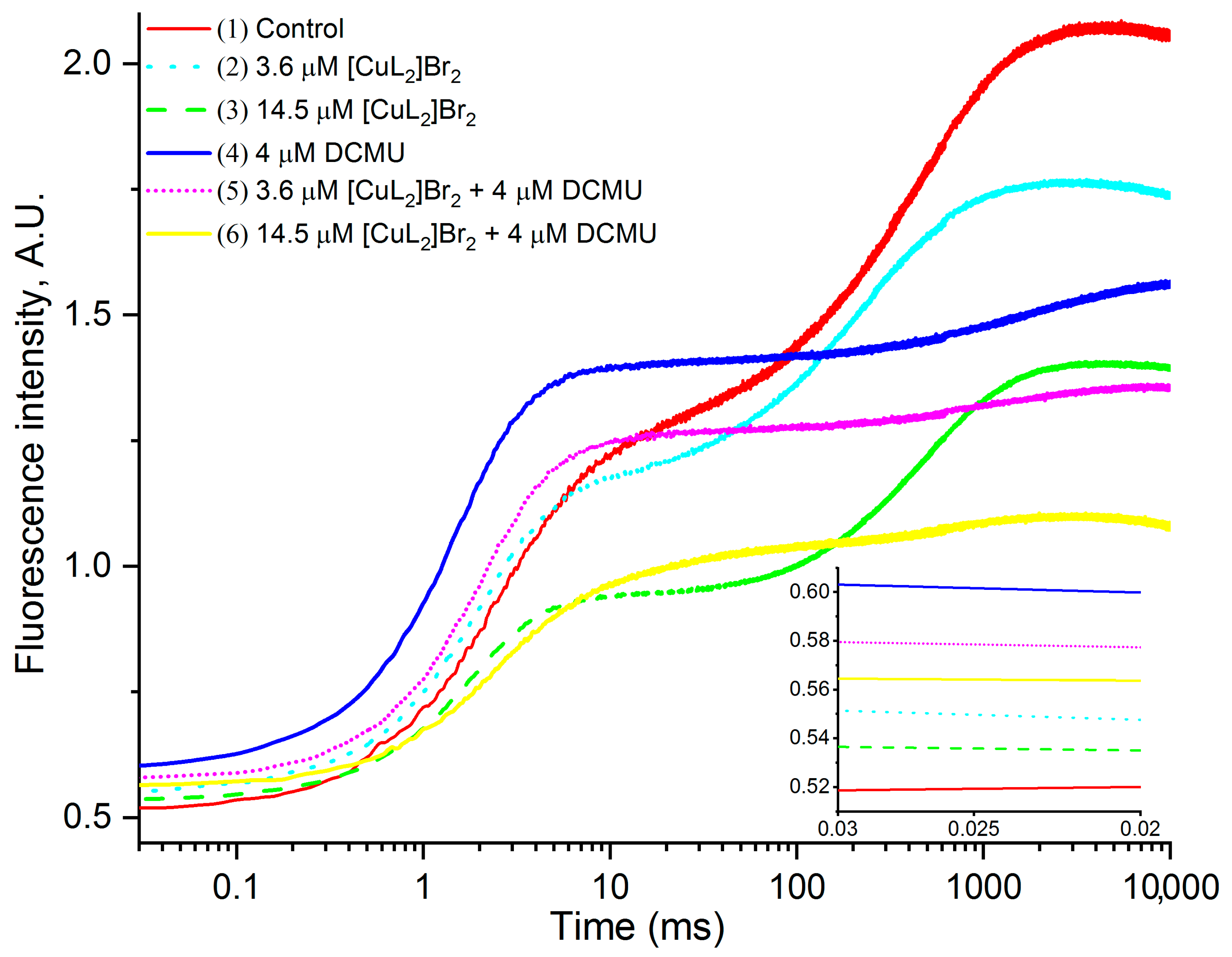
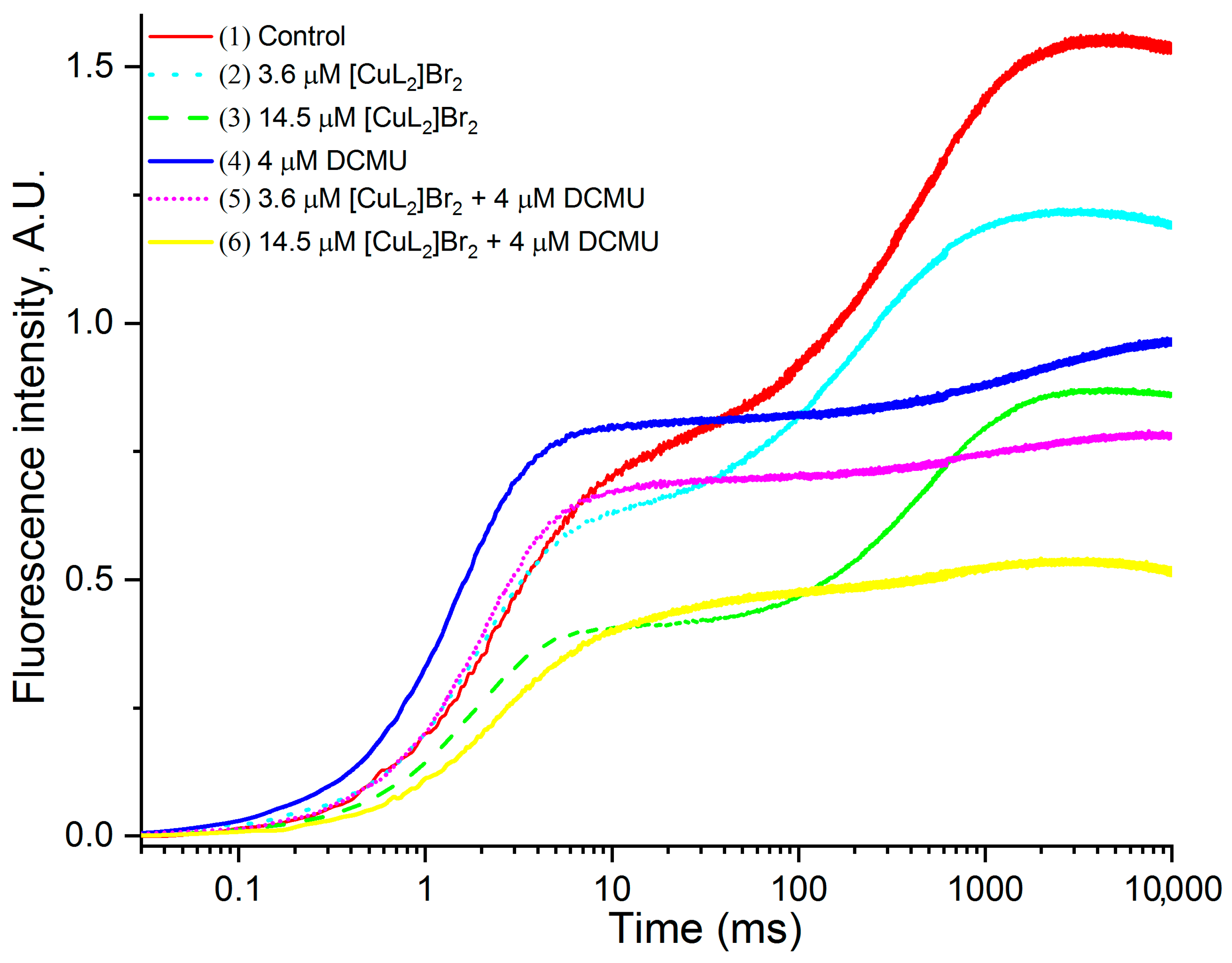
3.1. Original OJIP Kinetics
3.2. Original OJIP Kinetics Normalized Relative to F0 (F0.02ms)
3.3. OJIP Kinetics Normalized Relative to F20µs and FM
3.4. Comparison [CuL2]Br2 and DCMU Effects
3.4.1. Effects of [CuL2]Br2 in the Absence of DCMU
3.4.2. Effects of [CuL2]Br2 in the Presence of DCMU
3.5. Estimation of the Rate of Photoinduced Reduction of QA
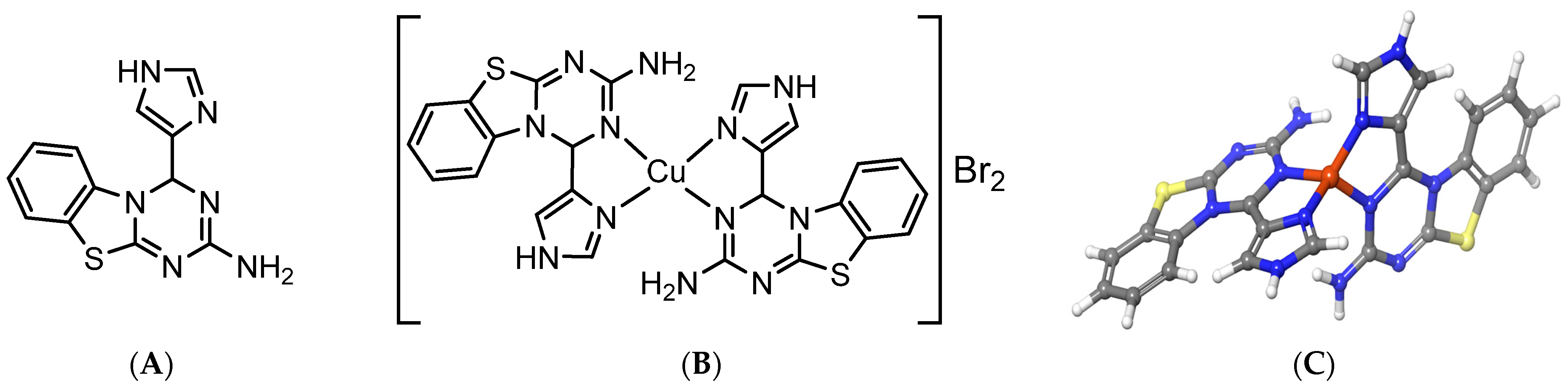
3.6. Absorption Spectrum of [CuL2]Br2
4. Discussion
4.1. Main Inhibitory Impact of [CuL2]Br2 on OJP Transients
4.2. Impact of [CuL2]Br2 on J and 0 Peaks
4.2.1. Impact of [CuL2]Br2 on J and 0 Peaks in the Absence DCMU
4.2.2. Impact of [CuL2]Br2 on J and 0 Peaks in the Presence DCMU
4.3. The Rate of Photoinduced Reduction of QA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, N.; Mukhtar, Z. Genetic Manipulations in Crops: Challenges and Opportunities. Genomics 2017, 109, 494–505. [Google Scholar] [CrossRef]
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, A.G.W.; Mertens, M. Herbicide Resistance and Biodiversity: Agronomic and Environmental Aspects of Genetically Modified Herbicide-Resistant Plants. Environ. Sci. Eur. 2017, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Karki, L.; Lakshmi, K.V.; Szalai, V.A.; Brudvig, G.W. Low-Temperature Turnover Control of Photosystem II Using Novel Metal- Containing Redox-Active Herbicides. J. Am. Chem. Soc. 2000, 122, 5180–5188. [Google Scholar] [CrossRef]
- Trebst, A. Inhibitors in the Functional Dissection of the Photosynthetic Electron Transport System. Photosynth. Res. 2007, 92, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zharmukhamedov, S.K.; Allakhverdiev, S.I. Chemical Inhibitors of Photosystem II. Russ. J. Plant Physiol. 2021, 68, 212–227. [Google Scholar] [CrossRef]
- Ran, C.; Yu, X.; Jin, M.; Zhang, W. Role of Carbonyl Cyanide M-Chlorophenylhydrazone in Enhancing Photobiological Hydrogen Production by Marine Green Alga Platymonas Subcordiformis. Biotechnol. Prog. 2006, 22, 438–443. [Google Scholar] [CrossRef]
- Nelson, N.; Junge, W. Structure and Energy Transfer in Photosystems of Oxygenic Photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef]
- Vass, I. Molecular Mechanisms of Photodamage in the Photosystem II Complex. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Semelkova, L.; Konecna, K.; Paterova, P.; Kubicek, V.; Kunes, J.; Novakova, L.; Marek, J.; Naesens, L.; Pesko, M.; Kralova, K.; et al. Synthesis and Biological Evaluation of N-Alkyl-3-(Alkylamino)-Pyrazine-2-Carboxamides. Molecules 2015, 20, 8687–8711. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K.; Pesko, M.; Kos, J. Ring-Substituted 8-Hydroxyquinoline-2-Carboxanilides as Photosystem II Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. [Google Scholar] [CrossRef]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-Alkoxyphenylhydroxynaphthalenecarboxamides Affecting Photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-Hydroxynaphthalene-2-Carboxanilides Affecting Photosynthetic Electron Transport in Photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef] [PubMed]
- Karacan, M.S.; Rodionova, M.V.; Tunç, T.; Venedik, K.B.; Mamaş, S.; Shitov, A.V.; Zharmukhamedov, S.K.; Klimov, V.V.; Karacan, N.; Allakhverdiev, S.I. Characterization of Nineteen Antimony(III) Complexes as Potent Inhibitors of Photosystem II, Carbonic Anhydrase, and Glutathione Reductase. Photosynth. Res. 2016, 130, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Sersen, F.; Kralova, K.P.M.C.M. Electrophysiological Variability in the SH-SY5Y Cellular Line. Gen. Physiol. Biophys. 2014, 31, 375–382. [Google Scholar] [CrossRef]
- Karacan, M.S.; Zharmukhamedov, S.K.; Mamaş, S.; Kupriyanova, E.V.; Shitov, A.V.; Klimov, V.V.; Özbek, N.; Özmen, Ü.; Gündüzalp, A.; Schmitt, F.J.; et al. Screening of Novel Chemical Compounds as Possible Inhibitors of Carbonic Anhydrase and Photosynthetic Activity of Photosystem II. J. Photochem. Photobiol. B Biol. 2014, 137, 156–167. [Google Scholar] [CrossRef]
- Sersen, F.; Kral’Ova, K.; Bumbalova, A.; Svajlenova, O. The Effect of Cu(II) Ions Bound with Tridentate Schiff Base Ligands upon the Photosynthetic Apparatus. J. Plant Physiol. 1997, 151, 299–305. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Zharmukhamedov, S.K.; Karacan, M.S.; Venedik, K.B.; Shitov, A.V.; Tunç, T.; Mamaş, S.; Kreslavski, V.D.; Karacan, N.; Klimov, V.V.; et al. Evaluation of New Cu(II) Complexes as a Novel Class of Inhibitors against Plant Carbonic Anhydrase, Glutathione Reductase, and Photosynthetic Activity in Photosystem II. Photosynth. Res. 2017, 133, 139–153. [Google Scholar] [CrossRef]
- Good, N.E.; Winget, G.D.; Winter, W.; Conolly, N.T.; Izawa, S.; Singh, R.M.M. Hydrogen Ion Buffers for Biological Research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef]
- Balt, S.; Van Dalen, E. The Reactions of Diphenylcarbazide and Diphenylcarbazone with Cations. Anal. Chim. Acta 1963, 29, 466–471. [Google Scholar] [CrossRef]
- Crespo, G.A.; Andrade, F.J.; Iñón, F.A.; Tudino, M.B. Kinetic Method for the Determination of Trace Amounts of Copper(II) in Water Matrices by Its Catalytic Effect on the Oxidation of 1,5-Diphenylcarbazide. Anal. Chim. Acta 2005, 539, 317–325. [Google Scholar] [CrossRef]
- Weissberger, A.; Lu Valle, J.E. Oxidation Processes. XVII. The Autoxidation of Ascorbic Acid in the Presence of Copper. J. Am. Chem. Soc. 1944, 66, 700–705. [Google Scholar] [CrossRef]
- Anderson, J.H. The Copper-Catalysed Oxidation of Hydroxylamine. Analyst 1964, 89, 357–362. [Google Scholar] [CrossRef]
- Keller, R.N.; Wrcoff, H.D. Copper(I) Chloride. In Inorganic Syntheses; Fernelius, W.C., Ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1946; pp. 1–4. ISBN 9780470131619. [Google Scholar]
- Yuan, X.; Pham, A.N.; Miller, C.J.; Waite, T.D. Copper-Catalyzed Hydroquinone Oxidation and Associated Redox Cycling of Copper under Conditions Typical of Natural Saline Waters. Environ. Sci. Technol. 2013, 47, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- López-Cueto, L.; Casado-Riobó, J.A. Catalytic Effect of Copper on the Hexacyanoferrate(III)-Cyanide Redox Reaction-I. The Uncatalysed and Catalysed Reactions. Talanta 1979, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zharmukhamedov, S.K.; Shabanova, M.S.; Rodionova, M.V.; Huseynova, I.M.; Karacan, M.S.; Karacan, N.; Aşık, K.B.; Kreslavski, V.D.; Alwasel, S.; Allakhverdiev, S.I. Effects of Novel Photosynthetic Inhibitor [CuL2]Br2 Complex on Photosystem II Activity in Spinach. Cells 2022, 11, 2680. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J.; et al. The Use of Chlorophyll Fluorescence Kinetics Analysis to Study the Performance of Photosynthetic Machinery in Plants. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 347–384. ISBN 978-0-12-800875-1. [Google Scholar]
- Kalaji, M.H.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence, Understanding Crop Performance; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-6449-0. [Google Scholar]
- Velthuys, B.R. Electron-Dependent Competition between Plastoquinone and Inhibitors for Binding to Photosystem II. FEBS Lett. 1981, 126, 277–281. [Google Scholar] [CrossRef]
- Wraight, C.A. Oxidation-Reduction Physical Chemistry of the Acceptor Quinone Complex in Bacterial Photosynthetic Reaction Centers: Evidence for a New Model of Herbicide Activity. Isr. J. Chem. 1981, 21, 348–354. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal Structure of Oxygen-Evolving Photosystem II at a Resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native Structure of Photosystem II at 1.95 Å Resolution Viewed by Femtosecond X-Ray Pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef]
- Zharmukhamedov, S.K.; Klimov, V.V.; Allakhverdiev, S.I. The Absence of Competition for the Binding Site between Diuron and Novel Electron Transfer Inhibitors in Photosystem 2—Derivatives of Perfluoroisopropyldinitrobenzene. Biochem. Mosc. 1995, 60, 723–728. [Google Scholar]
- Rehman, A.U.; Kodru, S.; Vass, I. Chloramphenicol Mediates Superoxide Production in Photosystem II and Enhances Its Photodamage in Isolated Membrane Particles. Front. Plant Sci. 2016, 7, 479. [Google Scholar] [CrossRef]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A Highly Resolved, Oxygen-Evolving Photosystem II Preparation from Spinach Thylakoid Membranes. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Ford, R.C.; Evans, M.C.W. Isolation of a Photosystem 2 Preparation from Higher Plants with Highly Enriched Oxygen Evolution Activity. FEBS Lett. 1983, 160, 159–164. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Shuvalov, V.A.; Krasnovsky, A.A. Effect of Extraction and Re-Addition of Manganese on Light Reactions of Photosystem-II Preparations. FEBS Lett. 1982, 148, 307–312. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Yanykin, D.; Sundyreva, M.; Khorobrykh, A.; Semenova, G.; Savchenko, T. Functional Characterization of the Corticular Photosynthetic Apparatus in Grapevine. Biochim. Biophys. acta. Bioenerg. 2020, 1861, 148260. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. In Intact Leaves, the Maximum Fluorescence Level (FM) Is Independent of the Redox State of the Plastoquinone Pool: A DCMU-Inhibition Study. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Van Rensen, J.J.S.; Vredenberg, W.J.; Wakabayashi, K. Characterization of the Alterations of the Chlorophyll a Fluorescence Induction Curve after Addition of Photosystem II Inhibiting Herbicides. Photosynth. Res. 2003, 78, 35–46. [Google Scholar] [CrossRef]
- Pospíšil, P.; Dau, H. Chlorophyll Fluorescence Transients of Photosystem II Membrane Particles as a Tool for Studying Photosynthetic Oxygen Evolution. Photosynth. Res. 2000, 65, 41–52. [Google Scholar] [CrossRef]
- Heredia, P.; Rivas, J.D. Las Fluorescence Induction of Photosystem II Membranes Shows the Steps till Reduction and Protonation of the Quinone Pool. J. Plant Physiol. 2003, 160, 1499–1506. [Google Scholar] [CrossRef]
- Lovyagina, E.R.; Semin, B.K. Mechanism of Inhibition and Decoupling of Oxygen Evolution from Electron Transfer in Photosystem II by Fluoride, Ammonia and Acetate. J. Photochem. Photobiol. B Biol. 2016, 158, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Padhi, B.; Chauhan, G.; Kandoi, D.; Stirbet, A.; Tripathy, B.C.; Govindjee, G. A Comparison of Chlorophyll Fluorescence Transient Measurements, Using Handy Pea and Fluorpen Fluorometers. Photosynthetica 2021, 59, 399–408. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.F.; Hussain, S.; Mbarki, S.; et al. JIP-Test as a Tool to Identify Salinity Tolerance in Sweet Sorghum Genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef]
- Viljevac Vuletić, M.; Mihaljević, I.; Tomaš, V.; Horvat, D.; Zdunić, Z.; Vuković, D. Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus Domestica L.) Cultivars. Horticulturae 2022, 8, 72. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, C.; Wang, G.; Li, Q.; Shao, A. Exogenously Spermidine Alleviates Damage from Drought Stress in the Photosystem II of Tall Fescue. Plant, Soil Environ. 2021, 67, 558–566. [Google Scholar] [CrossRef]
- Pospíšil, P.; Dau, H. Valinomycin Sensitivity Proves That Light-Induced Thylakoid Voltages Result in Millisecond Phase of Chlorophyll Fluorescence Transients. Biochim. Biophys. Acta Bioenerg. 2002, 1554, 94–100. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Egorova, E.A.; Govindachary, S.; Carpentier, R. Changes in Polyphasic Chlorophyll a Fluorescence Induction Curve upon Inhibition of Donor or Acceptor Side of Photosystem II in Isolated Thylakoids. Biochim. Biophys. Acta Bioenerg. 2004, 1657, 121–130. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Perboni, A.T.; Bacarin, M.A. The Effect of Inhibitors on Photosynthetic Electron Transport Chain in Canola Leaf Discs. Acta Sci. Biol. Sci. 2015, 37, 159–167. [Google Scholar] [CrossRef]
- De Carvalho, A.C.; Salvador, J.P.; Pereira, T.d.M.; Ferreira, P.H.A.; Lira, J.C.S.; Veiga, T.A.M. Fluorescence of Chlorophyll for Discovering Inhibitors of Photosynthesis in Plant Extracts. Am. J. Plant Sci. 2016, 7, 1545–1554. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative Effect of Tenuazonic Acid, Diuron, Bentazone, Dibromothymoquinone and Methyl Viologen on the Kinetics of Chl a Fluorescence Rise OJIP and the MR820 Signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Klimov, V.V.; Shuvalov, V.A.; Heber, U. Photoreduction of Pheophytin as a Result of Electron Donation from the Water-Splitting System to Photosystem-II Reaction Centers. Biochim. Biophys. Acta Bioenerg. 1985, 809, 345–350. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Ladygin, V.G. Photoreduction of Pheophytin in Photosystem II of the Whole Cells of Green Algae and Cyanobacteria. Photosynth. Res. 1986, 10, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.D.; Lee, J.Y. Toxic Effects of Copper on Photosystem II of Spinach Chloroplasts. Plant Physiol. 1988, 87, 116–119. [Google Scholar] [CrossRef]
- Kamachi, H.; Tamura, N.; Inoué, H. Putative Second Binding Site of DCMU on the Oxidizing Side of Photosystem II in Photosystem II Membranes Depleted of Functional Mn. Plant Cell Physiol. 1992, 33, 437–443. [Google Scholar] [CrossRef]
- Srivastava, A.; Strasser, R.J. Govindjee Polyphasic Rise of Chlorophyll a Fluorescence in Herbicide-Resistant D1 Mutants of Chlamydomonas Reinardtii. Photosynth. Res. 1995, 43, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A. Govindjee Polyphasic Chlorophyll a Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Haldimann, P.; Strasser, R.J. Effects of Anaerobiosis as Probed by the Polyphasic Chlorophyll a Fluorescence Rise Kinetic in Pea (Pisum Sativum L.). Photosynth. Res. 1999, 62, 67–83. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll A Fluorescence: A Signature of Photosynthesis; Springer: New York, NY, USA, 2004; pp. 321–362. [Google Scholar]
- Tsimilli-Michael, M. Special Issue in Honour of Prof. Reto J. Strasser-Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Srivastava, A.; Strasser, R.J. Govindjee Differential Effects of Dimethylbenzoquinone and Dichlorobenzoquinone on Chlorophyll Fluorescence Transient in Spinach Thylakoids. J. Photochem. Photobiol. B Biol. 1995, 31, 163–169. [Google Scholar] [CrossRef]
- Petrova, N.; Paunov, M.; Petrov, P.; Velikova, V.; Goltsev, V.; Krumova, S. Polymer-Modified Single-Walled Carbon Nanotubes Affect Photosystem II Photochemistry, Intersystem Electron Transport Carriers and Photosystem I End Acceptors in Pea Plants. Molecules 2021, 26, 5958. [Google Scholar] [CrossRef]
- Yruela, I.; Pueyo, J.J.; Alonso, P.J.; Picorel, R. Photoinhibition of Photosystem II from Higher Plants: Effect of Copper Inhibition. J. Biol. Chem. 1996, 271, 27408–27415. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Guissé, B.; Greppin, H.; Strasser, R.J. Regulation of Antenna Structure and Electron Transport in Photosystem II of Pisum Sativum under Elevated Temperature Probed by the Fast Polyphasic Chlorophyll a Fluorescence Transient: OKJIP. Biochim. Biophys. Acta Bioenerg. 1997, 1320, 95–106. [Google Scholar] [CrossRef]
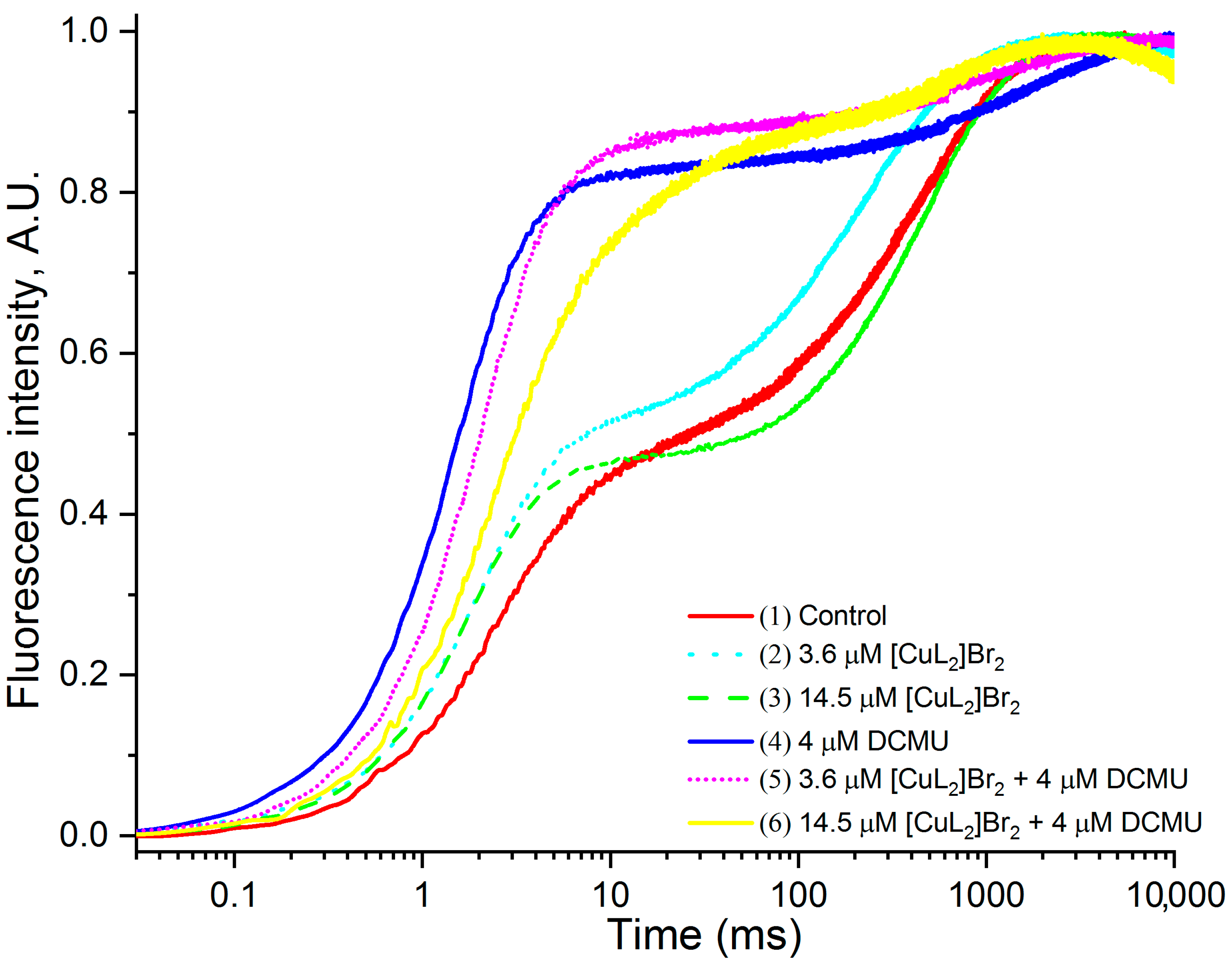
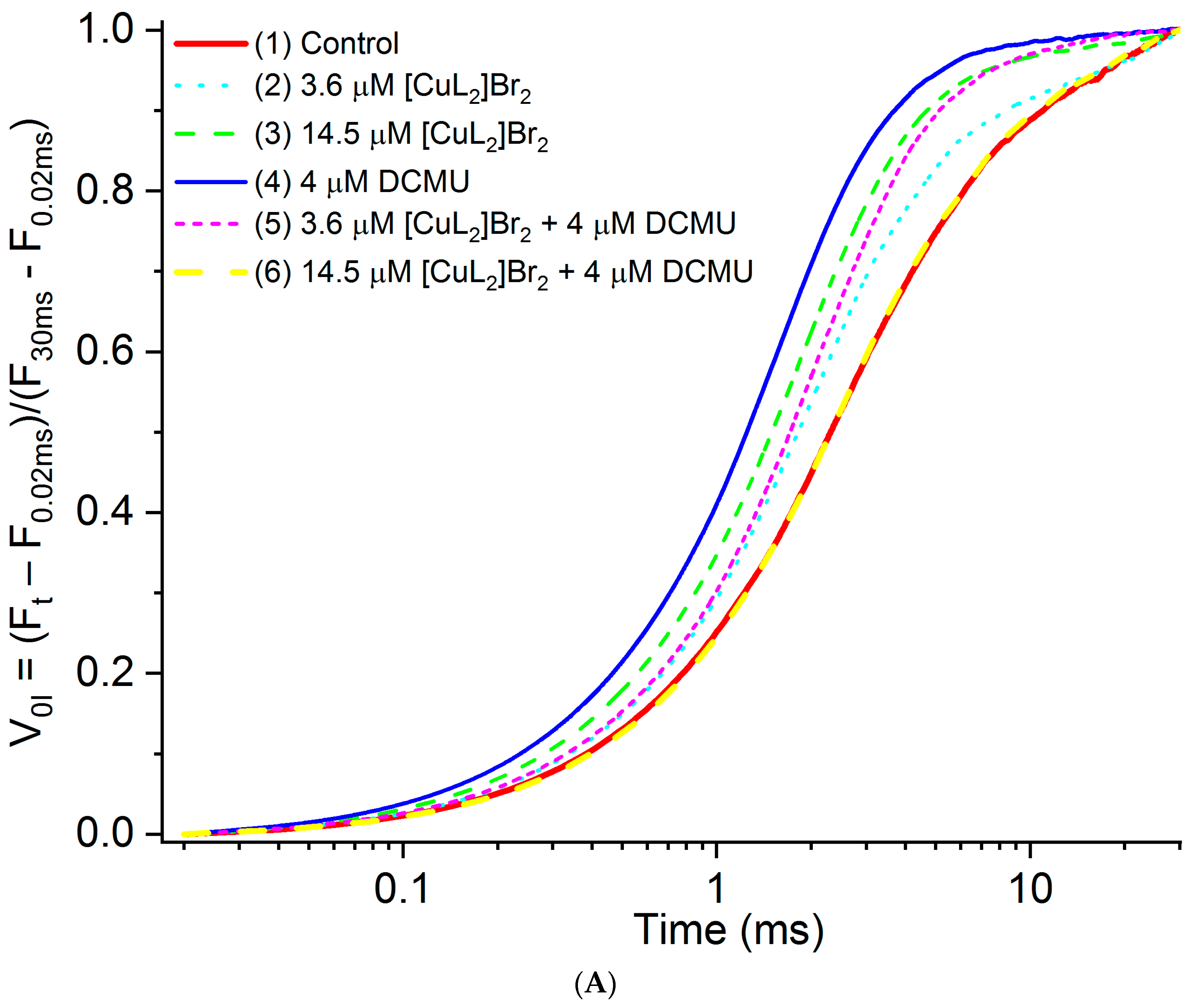
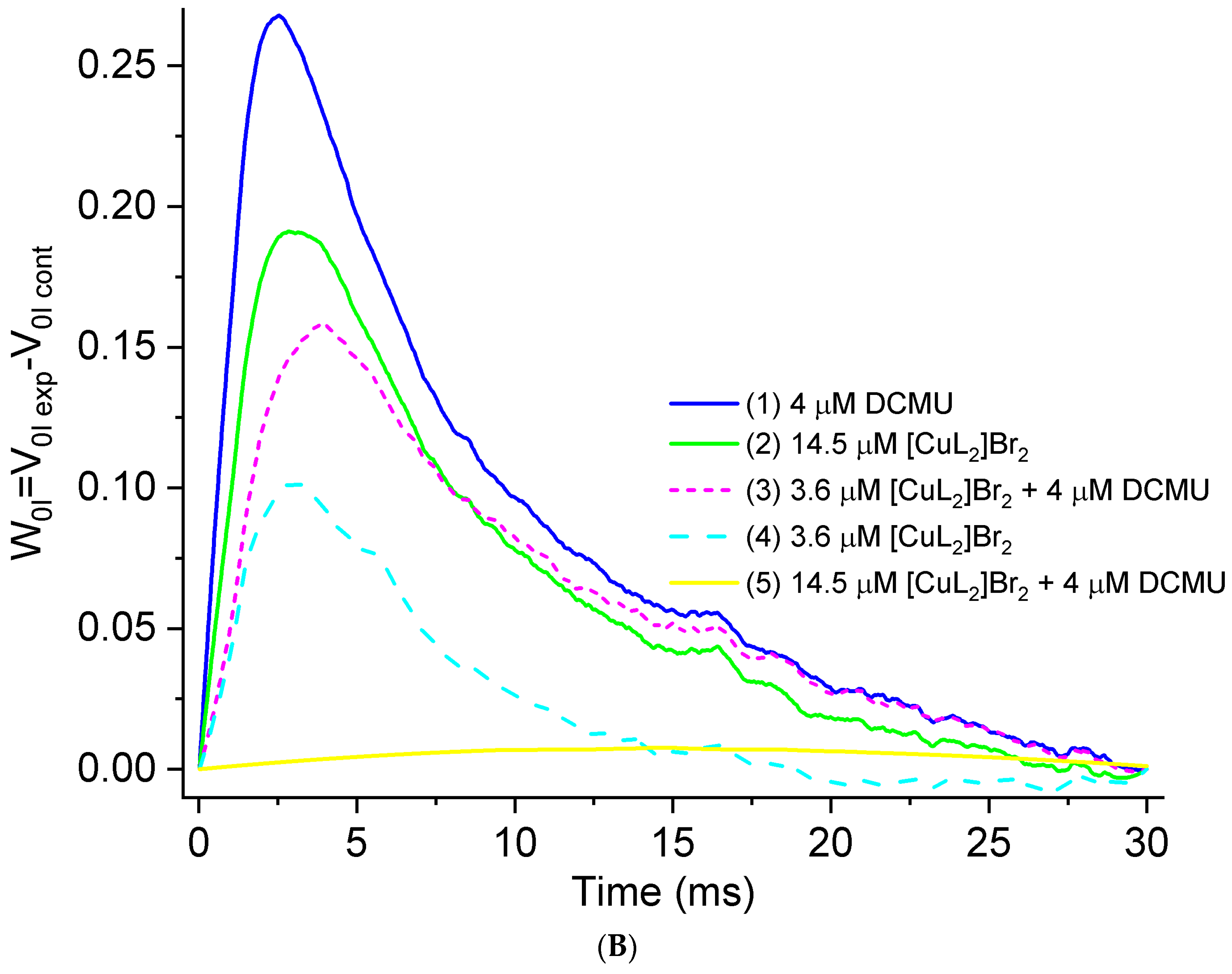
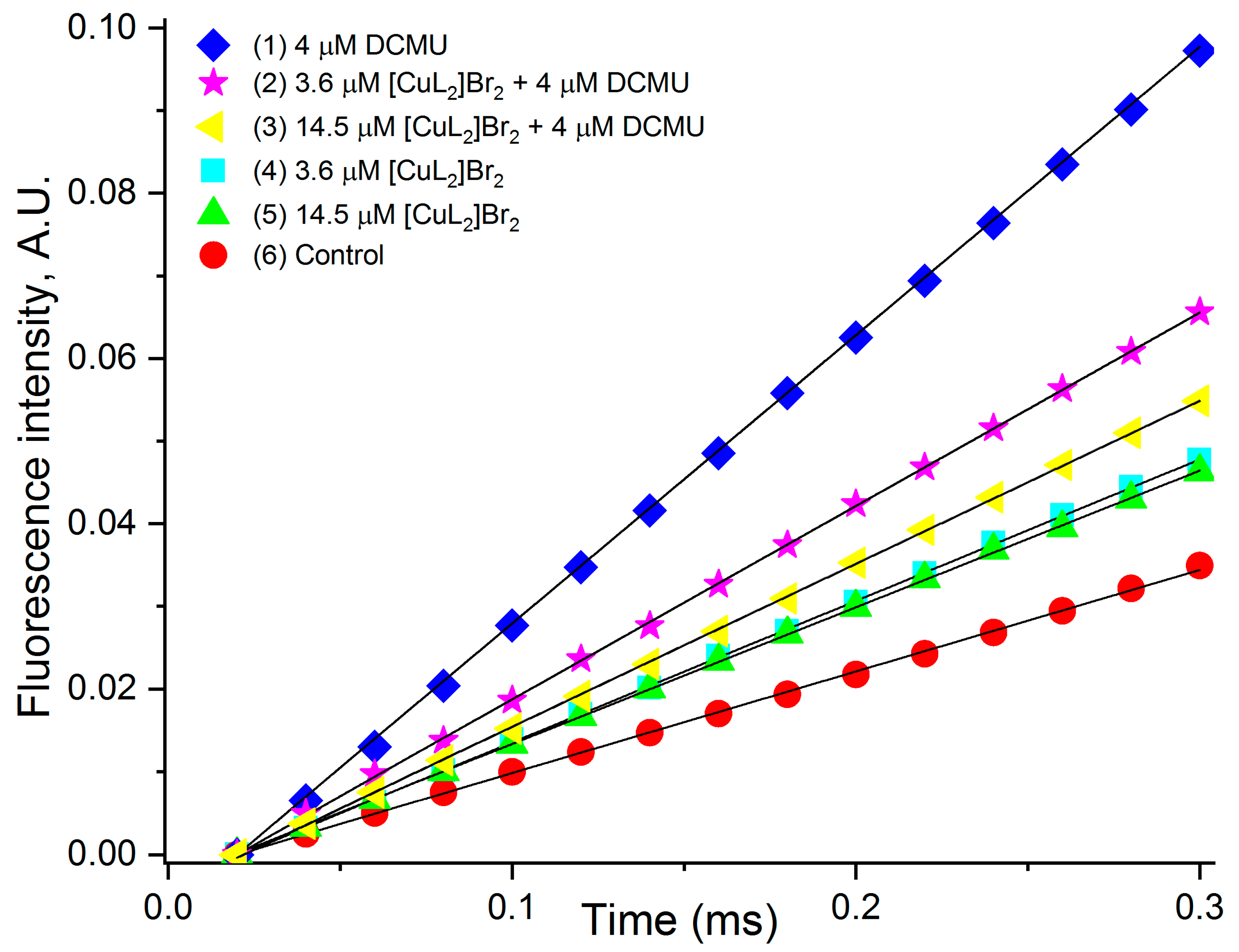

| Variants | FM, % of Control | FM Decreases, % of Control |
|---|---|---|
| Control | 100 | 0 |
| 3.6 µM [CuL2]Br2 | 78 | 22 |
| 14.5 µM [CuL2]Br2 | 55 | 45 |
| 4 µM DCMU | 62 (100) | 38 (0) |
| 3.6 µM [CuL2]Br2 + 4 µM DCMU | 50 (81) | 50 (19) |
| 14.5 µM [CuL2]Br2 + 4 µM DCMU | 34 (56) | 66 (44) |
| Variants | FJ, % of FJ with DCMU |
|---|---|
| 4 µM DCMU | 100 |
| 3.6 µM M [CuL2]Br2 | 37.9 ± 0.3 |
| 14.5 µM M [CuL2]Br2 | 71.3 ± 0.3 |
| 3.6 µM [CuL2]Br2 + 4 µM DCMU | 59.0 ± 0.3 |
| 14.5 µM [CuL2]Br2 +4 µM DCMU | 2.9 ± 0.3 |
| Variants | M0, % of Control | M0, % of DCMU |
|---|---|---|
| 4 µM DCMU | 290 | 100 |
| 3.6 µM [CuL2]Br2 + 4 µM DCMU | 199 | 69 |
| 14.5 µM [CuL2]Br2 + 4 µM DCMU | 161 | 56 |
| 3.6 µM [CuL2]Br2 | 146 | 50 |
| 14.5 µM [CuL2]Br2 | 132 | 46 |
| Control | 100 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharmukhamedov, S.K.; Shabanova, M.S.; Huseynova, I.M.; Karacan, M.S.; Karacan, N.; Akar, H.; Kreslavski, V.D.; Alharby, H.F.; Bruce, B.D.; Allakhverdiev, S.I. Probing the Influence of Novel Organometallic Copper(II) Complexes on Spinach PSII Photochemistry Using OJIP Fluorescence Transient Measurements. Biomolecules 2023, 13, 1058. https://doi.org/10.3390/biom13071058
Zharmukhamedov SK, Shabanova MS, Huseynova IM, Karacan MS, Karacan N, Akar H, Kreslavski VD, Alharby HF, Bruce BD, Allakhverdiev SI. Probing the Influence of Novel Organometallic Copper(II) Complexes on Spinach PSII Photochemistry Using OJIP Fluorescence Transient Measurements. Biomolecules. 2023; 13(7):1058. https://doi.org/10.3390/biom13071058
Chicago/Turabian StyleZharmukhamedov, Sergei K., Mehriban S. Shabanova, Irada M. Huseynova, Mehmet Sayım Karacan, Nurcan Karacan, Hande Akar, Vladimir D. Kreslavski, Hesham F. Alharby, Barry D. Bruce, and Suleyman I. Allakhverdiev. 2023. "Probing the Influence of Novel Organometallic Copper(II) Complexes on Spinach PSII Photochemistry Using OJIP Fluorescence Transient Measurements" Biomolecules 13, no. 7: 1058. https://doi.org/10.3390/biom13071058
APA StyleZharmukhamedov, S. K., Shabanova, M. S., Huseynova, I. M., Karacan, M. S., Karacan, N., Akar, H., Kreslavski, V. D., Alharby, H. F., Bruce, B. D., & Allakhverdiev, S. I. (2023). Probing the Influence of Novel Organometallic Copper(II) Complexes on Spinach PSII Photochemistry Using OJIP Fluorescence Transient Measurements. Biomolecules, 13(7), 1058. https://doi.org/10.3390/biom13071058