Abstract
In the last two decades, our knowledge of synaptic proteomes and their relationship to normal brain function and neuropsychiatric disorders has been expanding rapidly through the use of more powerful neuroproteomic approaches. However, mass spectrometry (MS)-based neuroproteomic studies of synapses still require cell-type, spatial, and temporal proteome information. With the advancement of sample preparation and MS techniques, we have just begun to identify and understand proteomes within a given cell type, subcellular compartment, and cell-type-specific synapse. Here, we review the progress and limitations of MS-based neuroproteomics of synapses in the mammalian CNS and highlight the recent applications of these approaches in studying neuropsychiatric disorders such as major depressive disorder and substance use disorders. Combining neuroproteomic findings with other omics studies can generate an in-depth, comprehensive map of synaptic proteomes and possibly identify new therapeutic targets and biomarkers for several central nervous system disorders.
1. Introduction
The application of proteomic analyses in neuroscience has significantly increased in the past two decades [1,2]. Historically, genomic and transcriptomic analyses were extensively used to search for mutations in patients’ genomes or changes in gene expression in neuropsychiatric disorders such as autism spectrum disorder, Alzheimer’s disease, and schizophrenia [3]. However, due to the molecular complexity and heterogeneity of each of these disorders and the lack of strong coincidence between mRNA and protein expression levels, genetic and transcriptomic findings fail to fully explain the pathophysiological mechanisms of these syndromes. This discrepancy raises the need for an alternative omics approach, such as proteomics, to directly examine levels of individual proteins under these conditions.
Proteomics is the study of the proteome, the comprehensive set of proteins expressed by a genome in a cell, and neuroproteomics is the study of proteomes in the nervous system [4]. Unlike the proteomic analysis of other tissues, neuroproteomics is particularly challenging due to the need for cell-type-, region-, and temporal-specific analyses. To identify proteins in the central nervous system (CNS) and peripheral nervous system (PNS), understand their interactions, identify post-translational modifications, and discover potential biomarkers, neuroproteomic investigations require the conjunction of many biochemical techniques, including sample separation, gel electrophoresis, liquid chromatography, and mass spectrometry, and bioinformatics analyses. Several excellent reviews cover the applications and limitations of several neuroproteomic techniques [1,5,6,7,8] and synaptic neuroproteomics of PNS [9,10,11,12] and other model organisms [13,14]. This review will focus on mass spectrometry (MS)-based neuroproteomics of synapses in mammalian CNS.
Synapses interconnect approximately 86 billion neurons in a human brain, forming neural circuits [15], and mediate neuronal communication, resulting behavioral function. There are two different types of synapses, electrical and chemical, but the large majority of mammalian synapses are chemical and use neurotransmitters and neuropeptides [15]. A chemical synapse generally comprises three main constituents, a presynaptic terminal, a synaptic cleft, and a postsynaptic compartment. It contains approximately 1000–1500 distinct proteins with a turnover rate of 0.7% per hour [16].
Two decades of MS-based synaptic neuroproteomic studies have identified over a thousand synaptic proteins, tens of thousands of phosphorylation sites, and provided transient and time-resolved information on protein–protein interactions and structures. In 2019, synapse-specific gene ontology (SynGo) classification was established using published, expert-curated annotations. SynGo contains 87 synaptic locations and 179 synaptic processes and showed that genes that encode synaptic proteins are exceptionally well conserved and less tolerant to mutations than other genes [17]. In 2021, the synaptic proteome SQLite database, integrating 58 published synaptic proteomic datasets, was also established. It covered 8087 unique genes and 407,643 direct protein interactions [18]. Overall, MS-based synaptic neuroproteomic studies have significantly expanded our understanding of synapses not only in normal brain function but also in the pathophysiology of CNS disorders [19], especially based on the unbiased nature of these approaches [20,21,22,23]. However, there are several limitations to these studies.
We still lack cell-type-, subcellular compartment-, and synapse-cell-type-specific proteome information, with neuroproteomic studies of cell types, subcellular compartments, and cell-type-specific synapses now only just beginning [23,24,25,26,27]. Here, we focus on advances in MS-based neuroproteomic studies of chemical synapses. We highlight the recent application of these methods to specific cell types and subcellular compartments and cell-type-specific synapses in the context of CNS disorders. Integrating neuroproteomic approaches with other omics will improve our understanding of synapses and ultimately lead to the identification of biomarkers or new therapeutic targets.
2. Synapses
At chemical synapses, depolarizing electrical signals are rapidly converted into chemical signals by opening voltage-dependent Ca2+ channels, which allow calcium influx and change the local Ca2+ concentration critical for the release of synaptic vesicles (SVs) and large dense-core vesicles (LDCVs) [28,29,30]. The fusion of the vesicle membrane with the plasma membrane, leading to the release of the soluble contents of synaptic vesicles (SVs) and large dense-core vesicles (LDCVs), is initiated by the interaction between soluble NSF attachment protein receptor (SNARE) proteins present on the plasma membrane (t-SNARE) and vesicle (v-SNARE) [31,32,33,34,35]. This process is facilitated by the increased levels of Ca2+ in the presynaptic terminal [31,32,33,34,35]. Once released, neurotransmitters bind and activate their receptors located predominately in the postsynaptic membrane. The majority of the receptors are ligand-gated ion channels, with minor populations of G protein-coupled receptors in a small number of synapses. [29,36,37]. The intricate mechanisms underlying synaptic transmission and the release of large dense-core vesicles (LDCVs) have been comprehensively explored and elucidated in numerous outstanding reviews [29,34,35,37,38,39]. Here, we focus on the structure and isolation of synapses.
2.1. Structure of Synapses
Synapses are structurally complex despite being small. Classically, synapses were described as bipartite, containing pre- and postsynaptic compartments [15]. Now, with advancements in understanding of the bidirectional communication between neurons and astrocytes and the role of the extracellular matrix (ECM) in regulating synaptic functions, tripartite [40,41] and tetrapartite [42,43,44] synapses, in addition to bipartite synapses, have been widely studied. A tripartite synapse is one with pre- and postsynaptic neuronal compartments plus astrocytes, while a tetrapartite synapse includes the ECM as well. At these synapses, astrocytes and the ECM regulate both structural and functional aspects of synaptic plasticity. An in-depth discussion of tripartite and tetrapartite synapses is beyond the scope of this review, in which we focus mainly on bipartite synapses.
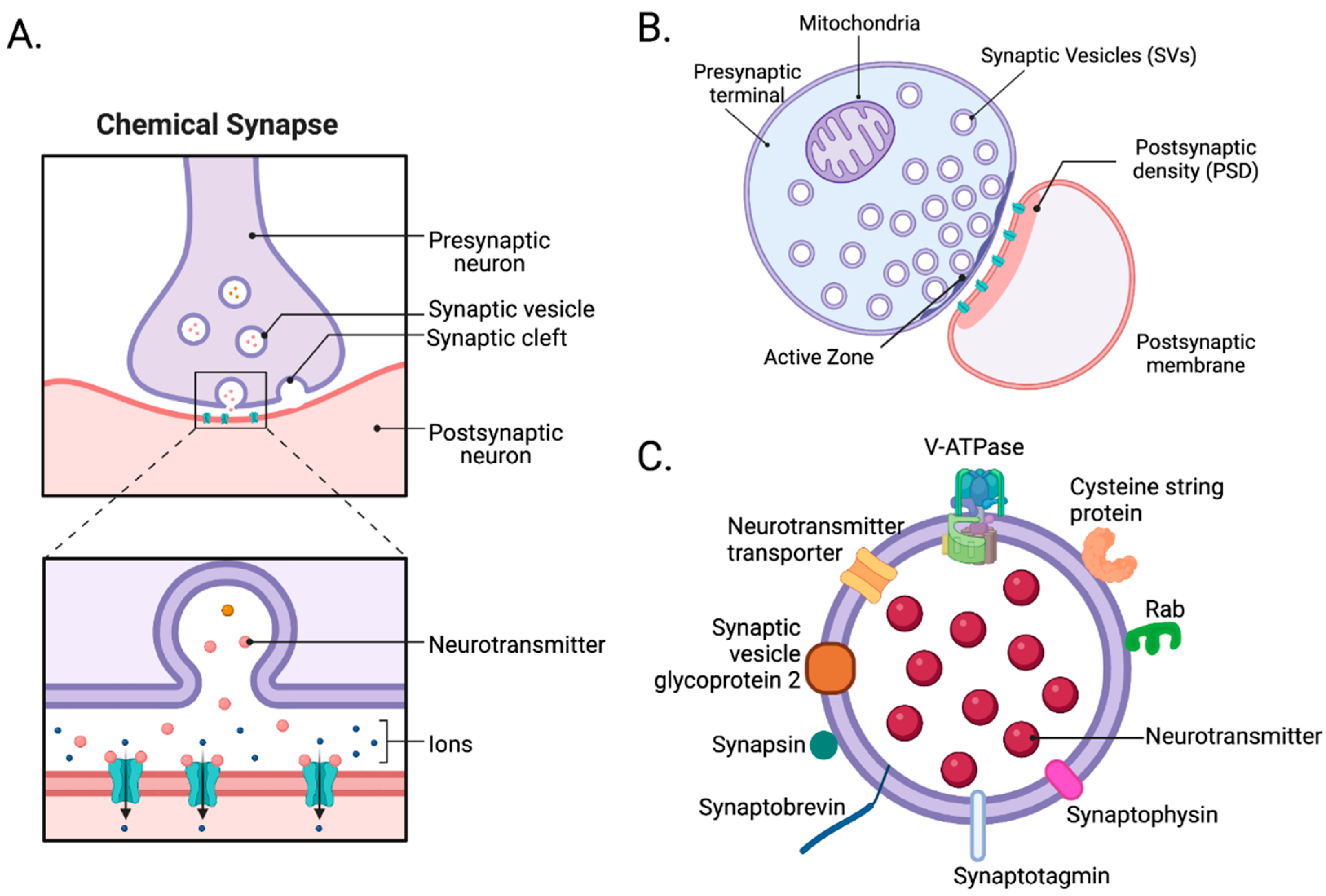
As noted, bipartite synapses contain three components, a presynaptic nerve terminal, a postsynaptic compartment, and a synaptic cleft. The presynaptic terminal includes its plasma membrane, which contains an active zone (Figure 1A) where vesicle mobilization, docking, priming, exocytosis, and endocytosis occur [45]. Both excitatory and inhibitory presynaptic terminals share similar structures. Differences primarily lie in neurotransmitter-synthesizing enzymes and transporters. Within the presynaptic terminal, numerous synaptic proteins, such as the SNARE complex and synaptotagmins, mediate the fusion of vesicles with the plasma membrane and are expressed in both excitatory and inhibitory synapses [46]. The postsynaptic compartment includes the postsynaptic plasma membrane containing the postsynaptic density (PSD). PSDs are where cell surface proteins, neurotransmitter receptors, cell-adhesion molecules, intracellular signaling molecules, and cytoskeletal filaments are densely present [47]. Unlike presynaptic terminals, postsynaptic compartments of excitatory and inhibitory synapses are more intrinsically different. In excitatory postsynaptic compartments, which typically represent the heads of dendritic spines, PSD95, SHANK, HOMER, inotropic glutamate receptors (AMPA, NMDA, and kainite-type receptors), and calcium–calmodulin-dependent protein kinase 2 (CaMK2), among many other proteins, are present [47]. By contrast, in inhibitory postsynaptic compartments, which typically occur on dendritic shafts, gephyrin, collybistin, and ionotropic GABA receptors, among many other proteins, are expressed [48]. Lastly, the synaptic cleft—the space between the pre- and postsynaptic compartments—is a protein-rich environment whose components can drive synaptogenesis and modulate synaptic maturation and transmission [39]. However, biochemical isolation of the synaptic cleft is very complicated. With advances in electron microscopy, proteomics, biotin labeling, and other biochemical approaches, researchers identified numerous proteins, including synapse-organizing adhesion proteins, such as ephrin, cadherin, and neurexins, which reside in the synaptic cleft. They also uncovered differences between excitatory glutamatergic and inhibitory GABAergic synaptic clefts [49,50]. For example, glutamatergic synaptic clefts contain NLGN1, LRRTM1, and LRRTM2 [51,52], while GABAergic synaptic clefts contain SLITRK3 and NLGN2 [53,54,55]. In glutamatergic synaptic clefts, postsynaptic NLGN1 interacts with presynaptic neurexins. However, in GABAergic synaptic clefts, postsynaptic NLGN2 interacts with presynaptic neurexins [49].
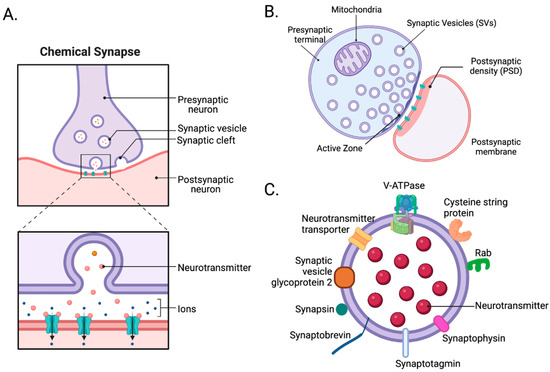
Figure 1.
The architecture of synapses. (A) At chemical synapses, depolarizing electrical signals are rapidly converted into chemical signals by neurotransmitters released through exocytosis from the presynaptic terminal. Once released, they bind and activate receptors and channels on the postsynaptic membrane to initiate excitatory or inhibitory postsynaptic currents in postsynaptic cells. (B) A synaptosome is a “pinched-off nerve ending” consisting of a presynaptic terminal with its active zone, synaptic cleft, and postsynaptic membrane with its postsynaptic density (PSD). (C) Synaptic vesicles, the carriers of neurotransmitters, contain numerous proteins, such as V-ATPase, neurotransmitter transporter, cysteine string protein (CSP), RAB, synaptophysin, synaptotagmin, and synaptobrevin [56]. Created with BioRender.com (Shiz Aoki, Toronto, ON, Canada).
2.2. Isolation of Synapses
Biochemical isolation of synapses or SVs, followed by neuroproteomic analysis, is commonly utilized to understand the architecture of synapses and the molecular mechanisms of synaptic transmission in the brain [57].
Synaptosomes are “pinched-off nerve endings” composed of several cellular fragments, including a presynaptic nerve terminal with its active zone, mitochondria, SVs, plus the associated postsynaptic membrane with its PSD (Figure 1B) [46]. To prepare synaptosomes, brain tissue is first homogenized in an isotonic sucrose solution. The homogenate is centrifuged at various speeds to remove nuclei, cytosol, and cellular debris. Then, depending on the type of experiment, crude synaptosomes are further purified using discontinuous sucrose [58,59], ficoll [60,61], or percoll [62] gradient ultracentrifugation to remove mitochondria and myelin. Additionally, crude synaptosomes can be further fractionated to obtain synaptic sub-compartments, such as presynaptic nerve terminals, postsynaptic membranes, PSDs, and synaptic cytosol. Synaptosomes are the perfect model for understanding synaptic transmission and cataloging synaptic proteins. However, the applications of synaptosomes may require careful consideration of the following limitations. The average synaptosome diameter ranges from 0.5 to 0.9 µm [57], which makes fluorescence imaging and sorting challenging. In addition, commonly used molecular techniques, such as transfections of tagged genes or RNAi knockdown, cannot be applied to synaptosomes. Instead, synaptic protein manipulation must be conducted prior to brain tissue collection. Synaptosomes also need to be depolarized by chemicals since they are not sufficiently responsive to field stimulation [57]. Despite these limitations, synaptosomes are widely used, especially in neuroproteomic studies. In Wilhelm et al., 60 proteins were localized in their respective copy numbers within a three-dimensional model of an average synapse. In total, approximately 300,000 protein molecules were found in an average synapse, including multiple copies of numerous transporters, receptors, and ion channels, along with vesicle trafficking proteins (e.g., SNAP25, VAMP2, and syntaxin1) and other presynaptic proteins critical for exocytosis (e.g., SEC1/MUNC18 [SM] proteins and MUNC13 [63]).
SVs are essential organelles in the presynaptic terminal for neurotransmission, and SV integral or membrane-associated proteins mediate the various functions. These functions include organelle transport, interactions with the nerve terminal cytoskeleton, and regulated interactions with the presynaptic plasma membrane during exo- and endocytosis, which affect synaptic function and pathophysiology (Figure 1C) [25,64]. Understanding the composition of SVs and their trafficking mechanisms is essential to understanding synaptic transmission. In neuroproteomic and other biochemical studies, three different SV isolation protocols are widely used. One involves subcellular fractionation of crude synaptosomes, and the other involves direct isolation of SVs from brain homogenates using differential and density-gradient centrifugation [65,66]. Since the 1960s, centrifugation methods used to isolate SVs have evolved to improve the yield and purity of SVs. However, today’s centrifugation methods still suffer from low final yields, low purity, and longer preparation time. Due to the small size of SVs, approximately 40–50 nm in diameter, their centrifugation purification takes ~24 h. Recently, immunoprecipitation (IP) has been more favorable in isolating SVs. Using an SV tag, such as RHO1D4, synaptotagmin1, or SV glycoprotein 2A/B/C [25,67], the IP method only takes ~2 h and is more selective than centrifugation [25]. With these advancements, several studies have successfully identified SV proteomes.
With the advent of single-cell and cell-type-specific transcriptomic techniques, neuroproteomics is now shifting toward identifying proteome changes with spatial and temporal information. While focusing on a specific brain region provides some spatial resolution, this fails to provide the cell-type- and synapse-cell-type-specific and temporal information. For this reason, we focus here on the efforts to accomplish the latter.
3. Advancements in Neuroproteomics
Most neuroproteomic studies to date have used entire brain region or large sections of the brain and, therefore, yield averaged proteome changes. Given the importance of more precise analyses for understanding a role of specific protein in the CNS, the field is pursuing numerous technical innovations, as described below.
3.1. Isolation of Cell Types, Subcellular Compartments, and Cell-Type-Specific Synapses
3.1.1. Transgenic Animals
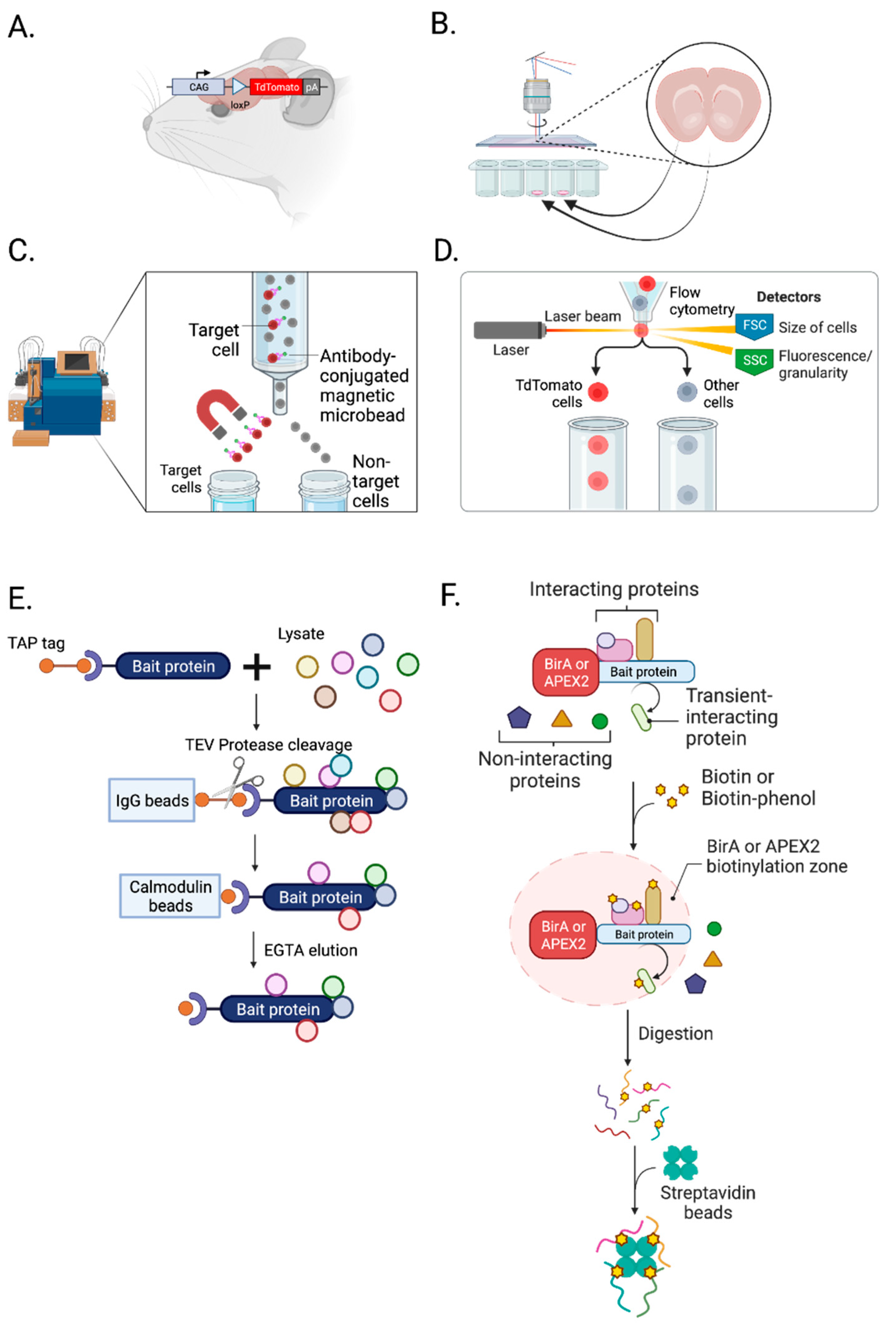
By crossbreeding fluorophore loxP “floxed” mice [68,69,70] or rats [70,71,72,73] with neuronal cell-type-specific Cre-expressing mice [74,75,76] or rats [77,78,79], we can label cell-type-specific synapses for MS-based neuroproteomic analyses and other biochemical assays (Figure 2A). In addition, Cre-dependent viruses can be applied to a specific brain region of neuron-specific Cre animals to label a subcellular compartment of synapses [67]. A list of available fluorophore-expressing loxP “floxed” and Cre recombinase animals can be found on the Jackson Laboratory website (https://www.jax.org/research-and-faculty/resources/cre-repository, (accessed on 10 May 2023)) and the Rat Resource and Research Center in the US (https://www.rrrc.us/, (accessed on 10 May 2023)).
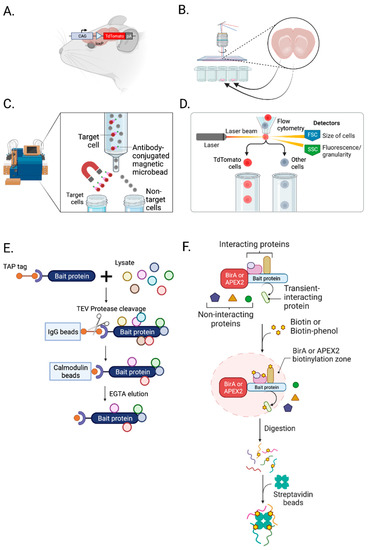
Figure 2.
Isolation and enrichment of chemical synapses. (A) Crossbreeding a loxP “floxed” rodent with Cre driver rodent labels cell-type-specific synapses with fluorophores, such as TdTomato. (B) Laser capture microdissection (LCM) can isolate a subpopulation of cells from a brain slice. (C) Magnetic-activated cell sorting (MACS) is a bulk isolation technique utilizing magnetic and cell surface markers. (D) Fluorescence-activated cell sorting (FACS) isolates cells and synaptosomes using a surface marker, size, and granularity. (E) Tandem affinity purification (TAP) uses TEV protease, and IgG and calmodulin beads to isolate not only a bait but also proteins interacting with a bait. (F) BioID and APEX2 proximity labeling techniques can label proteins in an activity-dependent manner. This labeling helps to identify neuroproteome changes and their interactions in a cell-type-, subcellular-compartment-, and activity-dependent manner. Created with BioRender.com (Shiz Aoki, Toronto, ON, Canada).
3.1.2. Laser Capture Microdissection (LCM)
LCM allows the isolation of a subpopulation of cells from tissue slices under direct microscopic visualization (Figure 2B) [80]. With 20 µm spatial resolution, it can isolate cells and specific subcellular compartments [81]. For example, LCM can dissect neuromelanin granules from substantia nigra [82] and separate neurons and amyloid plaques from Alzheimer’s disease brain tissue [83]. LCM is well validated and commonly used in transcriptomic studies but is yet to be widely used in neuroproteomic analysis, with its application limited primarily to human postmortem brain tissue [84,85,86,87,88]. Recently, LCM was applied to rat hippocampus to examine spatial proteomic changes [89]. In their study, do Canto et al. identified new signaling pathways and proteins present in specific layers and regions of the dentate gyrus. With micro-proteomics, which requires only 5000 cells [90] or nanoPOTs (see single-cell mass spectrometry), LCM has great potential for future neuroproteomic studies.
3.1.3. Magnetic-Activated Cell Sorting (MACS)
MACS, known as immunomagnetic cell separation, isolates specific cell types using tiny paramagnetic beads coupled to antibodies, enzymes, lectins, or streptavidin (Figure 2C) [91]. It is one of the most common, inexpensive, user-friendly cell separation techniques. It does not require specialized training like fluorescence-activated cell sorting (FACS) and eliminates the need for fluorophores [92]. However, it involves bulk isolation compared to FACS, which provides cell-by-cell isolation [93]. Since 1990, MACS has been widely used in neuroscience to purify several CNS cell types (neurons, astrocytes, oligodendrocytes, and microglia) from a rodent brain [26,92,94]. MACS is particularly favorable for astrocytes [95,96]. Holt et al. showed that astrocytes isolated by MACS are significantly more morphologically complex than those isolated by FACS, suggesting that MACS is gentler than FACS [92]. However, isolating neurons from an adult rodent brain using MACS results in significant contamination [92]. This major limitation of MACS has made FACS much more commonly used for neuronal neuroproteomic studies.
3.1.4. Fluorescence-Activated Cell Sorting (FACS)
FACS is a technique to isolate a homogeneous population from a heterogeneous cell population (Figure 2D). Samples are placed in a fluid stream, enter the flow cell in a cell-by-cell form through a nozzle and pass by a set of lasers, and the light scattering and fluorescence signals of each particle passing by are detected [97]. Then, individual cell types are collected into homogeneous fractions. FACS can isolate cells based on their surface marker, size, and granularity, and it allows the enrichment of even low abundant subpopulations with high purity. Nevertheless, FACS has several limitations. It can only sort suspended cells and requires several hundreds of microliters to milliliters of samples [98]. In addition, it highly depends on fluorescence signal intensity, so fluorescence compensation is necessary to sort cells accurately. Despite these limitations, FACS is commonly used in neuroproteomics and preferred over MACS for studies of neurons and synaptosomes.
Because synaptosomes are heterogenous, an additional isolation step is necessary to isolate cell-type-specific synaptosomes. However, synaptosomes are much more challenging to sort than cells: they are an order of magnitude smaller than an average cell. To successfully conduct fluorescence-activated synaptosome sorting (FASS), size standards, a non-light-scattering-dependent detection method, and longer sorting times are required [99,100]. Biesemann et al. successfully isolated glutamatergic synaptosomes using FASS and identified 163 enriched proteins in sorted glutamatergic synaptosomes [100]. Moreover, Paget-Blanc et al. successfully characterized dopaminergic (DA) synapses, with 57 proteins specifically enriched, and revealed “DA hub synapses”—those adhered to glutamatergic, GABAergic, or cholinergic synapses [23]. FASS combined with highly sensitive MS is the most widely used approach to study cell-type-specific synapses.
3.1.5. Tandem Affinity Purification
The specificity of affinity reagents, such as antibodies, peptides, and ligands, limits the isolation of synapses with affinity methods [101,102,103,104,105,106]. The recovery of the native complex is also low and potentially includes more contaminants. To overcome these weaknesses, a tandem affinity purification (TAP) tag was developed [107]. TAP is an IP-based purification technique (Figure 2E). Initially, it was made with two IgG binding domains of Straphylococus aureus protein A (ProtA), a tobacco etch virus (TEV) protease cleavage site, and calmodulin binding peptide (CBP) [107]. ProtA binds tightly to an IgG matrix, requiring TEV protease to elute material under native conditions. Then, elutants are incubated with calmodulin-coated beads in the presence of calcium, allowing the CBP of TAP to bind to the beads. After going through multiple washes, EGTA is used for elution. A protease cleavage site between two affinity tags allows rapid purification of complexes under native conditions. Despite its strength, the original TAP tag has some disadvantages. The calmodulin affinity step was inefficient since endogenous calmodulin in mammalian cells interferes with the binding of the target, causing poor protein recovery. In addition, the chelating agent used in elution can irreversibly interfere with the function of cation-dependent proteins. Lastly, the original TAP tag is relatively large, 21 kDa, and can potentially impair protein function [108]. Now, thirty alternative TAP tags with different combinations of affinity handles and lower kDa are available [108]. Both C- and N-terminus TAP tags are available to isolate the protein of interest (with its associated proteins) without impairing protein function [109]. Moreover, transgenic mice lines with TAP tags have been developed over a decade to study protein–protein interactions in disease models and signaling complexes of synapses. For example, TAP-tagged PSD95 knockin and PSD95 conditional TAP mice have been used to isolate postsynaptic compartments of synapses and perform proteomic analysis of PSD-95-associated complexes in the forebrain [109] and hippocampus and its CA3 subfield [110].
3.1.6. Protein Labeling
To overcome the limitations of MACS and FACS, numerous protein-labeling techniques have been developed to conduct subcellular compartment and cell-type-specific proteomic analysis [111]. The two most commonly used techniques are metabolic labeling and proximity labeling.
Bio-orthogonal non-canonical amino acid tagging (BONCAT) is a metabolic label that enriches cell-specific proteomes. A mutant methionyl-tRNA synthetase (MetRSL274G) labels newly translated proteins with the non-canonical amino acid [112]. This tool is very powerful in labeling newly synthesized proteins in a cell-type-specific manner when applied with Cre-loxP transgenic animals. Following copper-catalyzed azide-alkyne ligation (CLICK chemistry), labeled proteins can be isolated and analyzed by MS. Alvarez-Castelao et al. developed a protocol that labels, purifies, and identifies cell-type-specific proteomes in a Cre-recombinase-inducible mouse line expressing a mutant L274GMetRS. The authors successfully detected 2384 distinct proteins in hippocampal excitatory neurons and 1687 distinct proteins in cerebellum inhibitory neurons [113,114].
An alternative protein labeling approach is proximity labeling [115]. Genetically-encoded labeling enzymes such as BioID [116], TurboID [117], APEX2 [118], and horse radish peroxidase (HRP) [49,119,120] can be expressed and localized to a specific subcellular compartment and modify a freely diffusing biotin (Figure 2F). In situ biotinylation occurs rapidly from minutes to hours for TurboID and within seconds for APEX2 [121]. Then, with streptavidin affinity purification, labeled proteins can be isolated.
Although these methods extensively examine both cell-type- and subcellular-specific proteomes, several limitations must be addressed. Proximity labeling requires the expression of an exogenous enzyme and uses a transfection method or knockin mouse line [49]. In addition, endogenous biotinylation must be considered [122]. Currently, the application of BioID [123,124] or TurboID [125,126] to the brain of transgenic mice is very limited. To map activity-dependent changes at the proteome level, APEX2, known as fast proximity labeling, combined with Cre transgenic animals, is more favorable [121,127,128]. Despite the toxicity of biotin-phenol, H2O2, and the ex vivo application of APEX2 and HRP-mediated biotinylation, APEX2 and HRP labeling helps to identify neuroproteome changes in a cell-type-, subcellular-compartment-, and activity-dependent manner. Hobson et al. examined the DA presynaptic proteome using synaptosomes purified from the striatum of DAT-IRES-CRE mice expressing APEX2NES. From striatal synaptosomes with APEX2 expression in midbrain DA neurons, they identified 1533 proteins, including those involved in DA synthesis, release, reuptake, and degradation. Moreover, Suster et al. showed an efficient ex vivo cell surface biotinylation in the brain using Cre-dependent expression of a membrane-targeted HRP. ARMH4 was identified as a critical cell surface protein required for Purkinje cell dendrite arborization in the cerebellum [120].
3.2. Advancements in MS Approaches
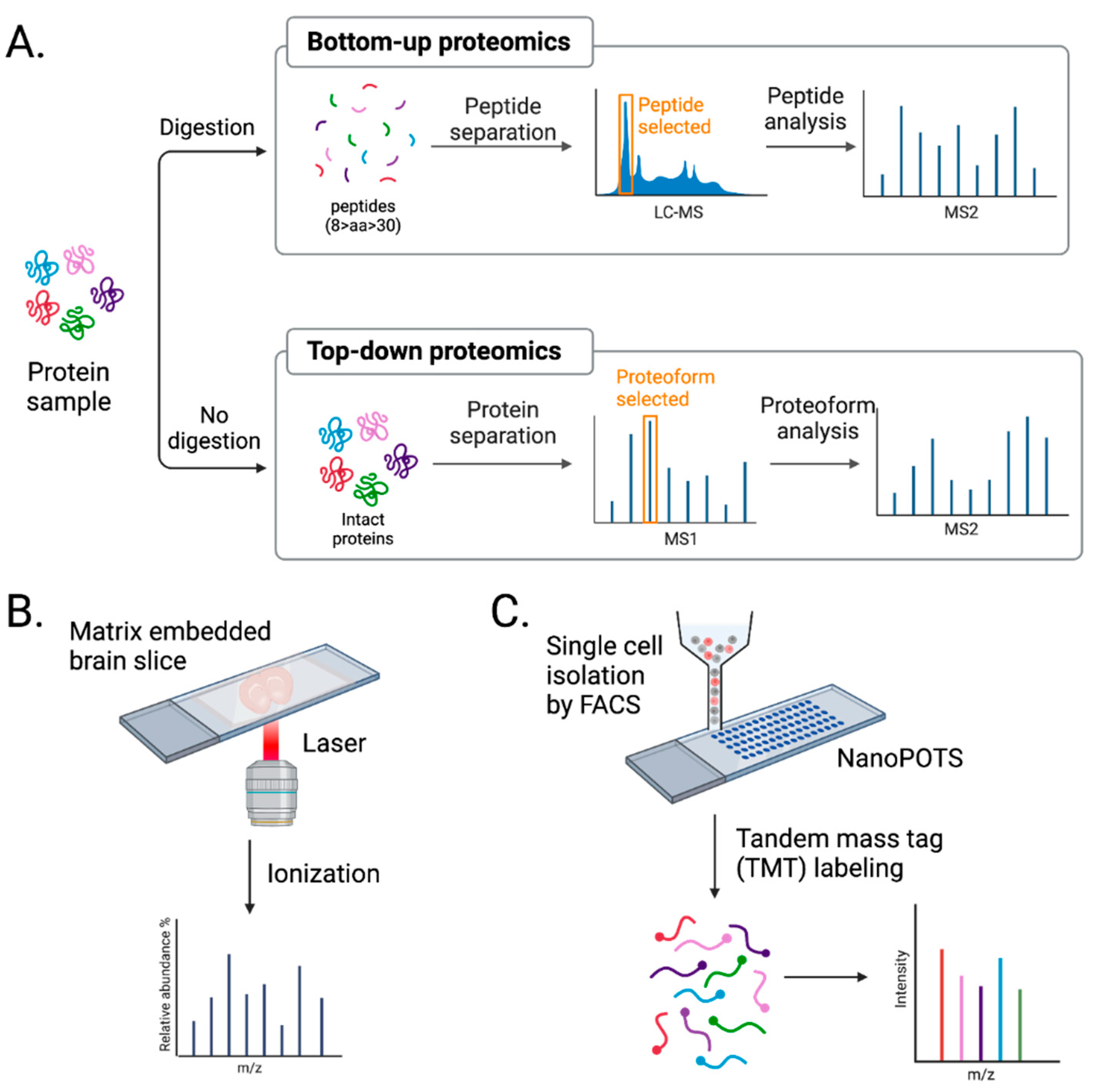
The development of MS-based neuroproteomics has enabled the characterization and quantification of brain proteomes in a high throughput manner. MS-based proteomics is divided into two approaches: bottom-up vs. top-down (Figure 3A). The main difference is the digestion step.
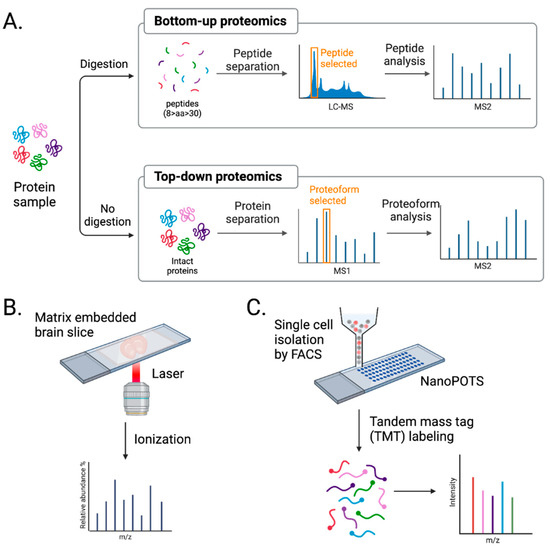
Figure 3.
Advancement in mass spectrometry techniques. (A) MS-based proteomics is largely divided into bottom-up and top-down approaches. Bottom-up proteomics analyzes peptides, while top-down proteomics analyzes intact proteins. (B) Imaging mass spectrometry (IMS) provides a spatial distribution of molecules in a tissue sample. (C) Single-cell mass spectrometry (scMS) with NanoPOTs and FACS helps to unbiasedly study proteomes in single brain cells. Created with BioRender.com (Shiz Aoki, Toronto, ON, Canada).
Bottom-up proteomics uses proteases, such as trypsin, to digest proteins into peptides, which are then analyzed by MS (Figure 3A). The mass-to-charge ratio and predicted sequences of peptides are used to search a protein database to characterize the open-reading frame these isolated peptides are from. The pros of using bottom-up proteomics are that peptides are more easily separated by reverse-phase liquid chromatography, ionize well, and fragment in a more predictable manner [129]. However, the extensive use of proteases brings caveats. Peptides identified in bottom-up proteomics are often not specific to a single protein. Data must be filtered to identify unique peptides for a given protein to accurately identify and quantify that protein. In addition, the method only covers the partial sequence of a protein. Despite these limitations, bottom-up proteomics is the most commonly used MS approach, especially in neuroproteomics [112,130].
Top-down proteomics uses intact proteins and thereby eliminates issues caused by focusing on peptides. Intact proteins are fractionated and run on high-resolution MS, where proteoform, all of the different molecular forms in which the protein product of a single gene can be found, including genetic variations, alternatively spliced RNA transcripts, and post-translational modifications [131], are selected and analyzed (Figure 3A). This approach allows for 100% sequence coverage and complete characterization of proteomes, including genetic variation, alternative splicing, and post-translational modification [132]. However, several challenges, such as protein solubility, detection of low-abundance proteins, and proteome complexity, need to be addressed, and for these reasons, it is less favorable than bottom-up proteomics. With ongoing improvements in solubilizing membrane proteins and ECM, enriching low-abundance proteins, and separating intact proteins before MS analysis [133], the application of top-down proteomics in neuroproteomics will undoubtfully increase.
Since the mid-1990s, numerous proteomic methods have been developed and widely applied in a cell-type-specific manner in neuroscience [134]. Here, we highlight the two most trending methods of MS: imaging and single-cell MS.
3.2.1. Direct In Situ Spatial Proteomics
Imaging mass spectrometry (IMS), such as matrix-assisted laser desorption/ionization IMS (MALDI-MS), provides a spatial distribution of molecules present in a sample (Figure 3B). MALDI-MS uses brain tissue embedded in a matrix, allowing the ionization of molecules in situ with a laser. Although suitable for de novo spatial proteome discovery, MALDI-MS suffers from shallow depth [2]. Proteins and peptides are challenging to ionize in this manner. Therefore, IMS is more applicable to studies of metabolites and lipids.
Imaging mass cytometry (IMC) with IMS is another approach that can be used to study cell types, subcellular compartments, and cell-type-specific synapses [2]. IMC uses antibodies coupled to heavy metal species to label proteins, allowing the simultaneous imaging of up to 40 different proteins, with labeled proteins identified by IMS [135,136]. In Van Deusen et al., protein-based cell atlases of the developing mouse telencephalon, diencephalon, mesencephalon, and rhombencephalon were mapped using this approach. They quantified 85 molecularly distinct cell populations, including neurons and myelin [136].
3.2.2. Single-Cell Mass Spectrometry
Single-cell transcriptomics has transformed our understanding of the brain. However, mRNA and protein expression are often inconsistent [26,137,138,139]. This gap led to the development of single-cell mass spectrometry (scMS) [140]. Comparing the results of single-cell transcriptomics with single-cell proteomics can yield new insights into the mechanisms of circuit formation and function. With the advancement in liquid chromatography (LC), tandem mass spectrometry (MS/MS), and sample preparation, scMS has just begun to be applied in neuroproteomics.
Current MS-based proteomic approaches require samples containing a minimum of thousands of cells to provide in-depth profiling [90]. Because proteomics does not allow for amplification steps like PCR-based transcriptomics, many cells are required for proteomic analysis, which is the biggest hurdle of scMS. To overcome this, nanoPOTS, a nanodroplet process in one pot for trace samples, was developed [81]. nanoPOTS consists of two glass pieces, a slide and a spacer, which are micropatterned with hydrophilic nanowells surrounded by a hydrophobic surface. Nanowells serve as microreactors for cells or other protein samples. They can undergo chemical treatments to extract, reduce, alkylate, and digest in volumes as small as 200 nl while avoiding sample loss due to surface exposure. Using LCM and nanoPOTS, the quantitative profiling of >3000 proteins was achieved from 10 HeLa cells [81]. In addition, nearly 1000 proteins were detected from a 100 µm diameter section of a 12 µm thick slice of rat cerebral cortex [81].
scMS with multiplexed isobaric tandem mass tags (TMTs), including single-cell proteomics by mass spectrometry (ScoPE-MS) [141] and improved boosting to amplify the signal with isobaric labeling (iBASIL) strategy [142], are, so far, the most successful approaches used for single-cell, cell-type-specific proteomic analysis (Figure 3C). These methods enhance protein detection and minimize sample surface losses of labeled samples by using a pool of cells or standard peptides from proteins of interest, known as “carrier” or “boosting.” Both carrier and single cells are labeled with TMTs, adding the same total mass to the peptides but having a different isotope composition. This results in one isobaric mass signal on MS1 spectra, but once the peptide precursors are fragmented, the difference is found in the low m/z region in the MS2 or MS3 spectrum [143]. Then, the ratio of those reported ions from single cells quantifies the previously labeled sample. These methods allow the more accurate quantification of detected proteins compared to label-free proteomic analysis. However, quantifying proteins using these tags requires optimizing a carrier signal. Larger carrier proteomes may promote losses in quantifying low-abundance peptides. Furthermore, co-eluting and co-fragmented peptide signals may interfere with quantifying a peptide of interest. So far, these scMS efforts with TMTs have not been readily applied to study neural cells. Combining these with other cell-type-specific isolation techniques, such as FACS, will help characterize single brain cells [143]. Unbiased classification of neuron types by large-scale scMS and combining results with other available omics should improve quantification of brain proteomes.
4. Application of Neuroproteomic Analysis to Neuropsychiatric Disorders
Disordered functioning of synapses is known to contribute to a wide range of neuropsychiatric disorders [112]. Thus, an in-depth understanding of the molecular and functional organization of synapses and synaptic dysfunction in these neuropsychiatric disorders is essential.
Many high-throughput genomic and transcriptomic studies of such disorders have examined mutations in patient genomes or changes in their transcriptomes, yielding numerous key discoveries [144]. However, these approaches fail to offer a complete picture of disease states because they cannot detect the abundance of proteins and examine the protein networks. To fill this gap, neuroproteomics is increasingly used to discover biomarkers and explore underlying pathological mechanisms. Indeed, synaptic proteomic changes have been identified for several psychiatric disorders [145,146,147]. Here, we highlight studies across several disorders that use proteomic analysis of synapses to further refine the mechanisms of various disease states and identify new targets for possible treatments.
4.1. Autism Spectrum Disorder
Neurodevelopmental disorders are multifaceted conditions characterized by impairments in cognition, communication, behavior, and/or motor skills resulting from abnormal brain development [148]. Autism spectrum disorders (ASDs) are among the most well-studied neurodevelopmental disorders in neuroproteomics. ASDs are highly heritable, heterogeneous disorders characterized by impairments in social communication and sensory perception, often accompanied by repetitive behaviors [149]. Due to the varied genetic underpinnings of ASDs, the contribution of identified de novo mutations and rare or common variants found in ASDs is not always clear. Genetic variations associated with ASDs are highly enriched in genes encoding synaptic proteins, such as group 1 mGLURs, NMDARs, and SHANK, to name a few [149]. To further understand the signaling network of ASDs, various neuroproteomic approaches have been utilized [145,150,151,152,153,154,155,156].
SHANK3 is a large scaffold protein that organizes the PSD of glutamatergic synapses [155]. Mutation of SHANK3 is hypothesized to perturb synaptic transmission in neural circuits throughout the brain and cause diverse neuropsychiatric phenotypes. With improvements in biochemical subcellular fractionation of synapses, the effect of SHANK3 mutations was examined in striatal and hippocampal PSDs in SHANK3 mutant mice [145]. Reim et al. identified changes in 55 and 61 proteins, out of ~2500, in striatal PSDs and hippocampal PSDs, respectively, from SHANK3 mutants [145]. The findings of this study mirrored results from previous work using two different ASD mouse models, Pten mutant [157] and Fmr1 knockout [150] mice. Together, the work highlights the value of unbiased and comprehensive screening of subcellular synapse anatomy in ASD-associated brain regions to understand the molecular consequences of the corresponding mutation and the big picture of ASD pathology [145]. Recently, using BioID and MS-based neuroproteomic approaches, protein–protein interaction (PPI) networks for 41 ASD risk genes were identified. The PPI network revealed the convergent pathways of ASDs as well as other pathways that are affected in only a subset of ASDs [156].
4.2. Alzheimer’s Disease
Alzheimer’s disease (AD), the most common form of progressive, age-related dementia, is a neurodegenerative disorder involving the gradual loss of synapses and the accumulation of amyloid β (Aβ) oligomers [158,159] and tau-containing neurofibrillary tangles [160,161]. Aβ and tau are also present in normal, healthy individuals, but under certain circumstances, which yet remain to be learned, Aβ and tau aggregate and start AD progression [162]. In AD patients, soluble Aβ and tau oligomers cause synaptic and cognitive dysfunctions by enhancing long-term depression (LTD) and inhibiting long-term potentiation (LTP), accelerating neuronal cell death [160,163,164,165,166]. Despite the well-known genetic underpinnings and molecular hallmarks of AD, treatment options for AD remain limited. Recent neuroproteomic analysis of AD synapses suggests new potential therapeutic targets.
Neuroproteomic analysis of synaptosomes from the human AD postmortem hippocampus and inferior temporal gyrus was first reported in 2013 [167]. Chang et al. identified expression changes in several synaptic proteins, such as synaptotagmin 1 and V-ATPase, located at the SV membrane. Kadoyama et al. later detected V-ATPase components in the hippocampus of bicuculline-treated Apposk-Tg mice, a transgenic mouse model of AD. Moreover, the synaptic vulnerability caused by the genetic factor of sporadic AD, apolipoprotein E 4 alleles (APOE4), was identified using neuroproteomic analysis of superior temporal gyrus (BA41/42) and primary visual cortex (BA17) from human APOE4+ brain tissue. In total, 5678 expressed proteins, including 1532 differentially expressed proteins important for synaptic and mitochondrial function, neuroimmune interactions, and intracellular signaling, were detected [168]. Cafeliello et al. is another exemplary study utilizing synaptosomes to identify local translation in TgCRND8 mice—another mouse model of AD. Using radioisotope labeling and BONCAT, this study showed that amyloid precursor protein (APP), which yields Aβ, is synaptically synthesized in the cerebral cortex and cerebellum of TgCRND8 mice. Overall, neuroproteomic analysis in AD studies has shed new light on AD pathophysiology and has suggested bicuculline, a GABAA receptor blocker, as a potential treatment to improve cognition [169].
4.3. Schizophrenia
Schizophrenia (SCZ) is a heterogeneous psychotic disorder characterized by delusions, hallucinations, disorganized speech or behavior, and impaired cognitive ability [170]. The pathophysiology of SCZ is complex, and many factors are yet to be discovered [171]. SCZ involves numerous genetic loci and is highly pleiotropic [172]. Reduction in synaptic densities and abnormalities in neurotransmission are reported as pathophysiological signatures of SCZ [173,174,175,176,177]. Neuroproteomic analysis of the human SCZ postmortem brain revealed PSD proteins, such as SHANK3, MAPK3, and SNYPO, differentially expressed in SCZ [178]. Moreover, PPI and pathway analyses of proteomic experiments using primary hippocampal neurons treated with shRNA of SCZ risk genes, such as TBR1, TCF4, and TOP3B, suggested that syntaxin-mediated neurotransmitter release in SCZ may be affected owing to subtle dysregulation via an indirect upstream gene regulatory mechanism rather than dysregulation of the involved proteins, such as TCF4, TBR1, and TOP3B, per se [177]. These findings complement genomic analysis of schizophrenia risk genes that encode PSD proteins [154,179] and, most importantly, highlight the need for neuroproteomic studies to identify the network of protein changes.
4.4. Major Depressive Disorder
Major depressive disorder (MDD) is one of the most common mental disorders worldwide. In 2020, about 8.4% of all U.S. adults experienced at least one major depressive episode, and the lifetime prevalence is 17% [180]. It is also a multifactorial disorder. Studies suggest that MDD is caused by a combination of genetic predisposition (~35%) and environmental factors [181]. Our understanding of MDD‘s pathophysiology remains incomplete. Since up to 50% of MDD patients are not fully treated with available therapies, there is a tremendous unmet need for new therapeutics.
Proteome changes in MDD have been extensively studied. Postmortem anterior cingulate cortex [182], frontal cortex [183], and dorsolateral prefrontal cortex (DLPFC) [184] from MDD patients were analyzed with proteomic approaches. These studies have highlighted differentially expressed proteins involved in energy metabolisms, such as carbonic anhydrase, aldolase C, histidine triad nucleotide-binding proteins, and several subunits of oxidative phosphorylation complexes, in MDD. In addition, lower levels of adenosine triphosphatase (ATPase) were observed in MDD [184]. A phosphoproteomic study of DLPFC in MDD brains revealed differential phosphorylation levels of numerous synaptic proteins, including SNARE complex, SNAP25, and synapsin 1 [185]. Neuroproteomic analyses of cerebrospinal fluid [186] and plasma [187] from MDD patients have identified potential biomarkers. The discovery of biomarkers that can identify subtypes of MDD patients would be a major advance in the field.
4.5. Substance Use Disorders
The persistence of addiction is thought to be mediated by drug-induced changes in the physiology of reward-processing brain regions. Dysregulated signaling within brain reward regions, such as the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), and basolateral amygdala (BLA), appears to play an especially critical role in promoting drug-seeking and relapse [27,188,189,190,191]. Determining these changes will reveal more effective targets for treating drug addiction and relapse. However, our understanding of the molecular mechanisms underlying these adaptations and alterations of signaling remains incomplete.
A broad-scale investigation of molecular alterations in brain reward regions through proteomics will help capture the biological basis of addiction-related behaviors. Recent neuroproteomics work has demonstrated that addiction-related behaviors emerge from converging subtle molecular changes. Bosch et al. showed 84 differentially regulated protein changes, including proteins with known roles in SVs and cytoskeleton in dorsal striatum synaptosomes of methamphetamine self-administering rats [192]. Furthermore, utilizing labeling techniques such as TMT and fluorophore, Lull et al. compared the PFC proteome in cocaine self-administering rats. They identified 20 significant changes, such as heat shock protein 73 and SNAP25 [193]. Recently, Puig et al. identified changes in 56 and 161 proteins from synaptosomes of postmortem NAc and DLPFC, respectively, in opioid use disorder patients. In NAc synaptosomes, proteins involved in inflammatory, mitochondria, and metabolic signaling pathways were identified. In contrast, proteins involved in inflammatory signaling, serotonergic, DA, cholinergic, and oxytocin neurotransmission were identified in DLPFC synaptosomes [194]. In both brain regions, proteins involved in GABAergic and glutamatergic synaptic functions, as well as circadian rhythms, were demonstrated, suggesting molecular disruption of circadian regulation of synaptic signaling in the human brain as a critical factor in opioid addiction [194]. Although these neuroproteomic studies have successfully identified critical intracellular signaling [195] and circuit-level networks [196] in synapses associated with drug-seeking, we still need to gain an understanding of neuronal cell-type-specific synaptic changes.
5. Limitations and Future Perspectives
With improvements in sample preparation and MS, MS-based proteomics has become an even more powerful tool in recent years. Throughout this review, we have highlighted the advantages and limitations of techniques used in MS neuroproteomics studies of synapses. In this section, we will highlight several limitations which must be considered, especially when examining the proteomic landscape of synapses in a cell-type-specific manner.
Unlike genomic technologies, which can capture the vast majority of all expressed RNAs, our ability to detect proteins remains limited. Out of perhaps tens of thousands of distinct types of proteins expressed in a given tissue, the best proteomic method can detect several thousand, with low-abundance proteins especially difficult to detect. This lack of sensitivity with proteomics is due to the inability to amplify signals as routinely performed for RNAs. Moreover, because only a partial protein sequence is used in most proteomic studies, proteins with low abundance, alternative splicing, alternative translation initiation sites, and point mutations are much more challenging to detect. In addition, a relatively large quantity of samples is needed in neuroproteomic studies. This is the biggest hurdle for neuroproteomics in investigating cell-type-specific synapses within a specific brain region. The top-down MS approach, in which intact proteins instead of peptides are analyzed, helps to overcome some of these issues regarding the partial sequence coverage. However, in top-down MS, it is difficult to accurately determine the monoisotopic mass and identify proteins larger than 50 kDa [112]. Further advancement in the sensitivity and resolution of MS technology [140,197,198,199], including the recent development of ultra-high sensitive true single-cell-derived proteomics (T-SCP) [200], along with associated enrichment and purification techniques, may close the gap between proteomics and other omics analyses.
Conducting cell-type-specific neuroproteomics is essential to advance the field. For example, in the NAc, two populations of medium spiny neurons (MSNs) generally exert opposite effects on behavior. D1-MSNs promote positive reinforcement and increase the formation of cocaine reward–context associations, whereas D2-MSNs appear to produce aversion and decrease cocaine reward [201,202]. Likewise, acute cocaine administration enhances D1-MSNs and suppresses D2-MSNs activity [203]. These cell types work in a subregion-dependent, complex, interweaving manner to drive drug-seeking and relapse behavior in the NAc [204,205,206]. To further understand substance-induced synaptic proteome changes in the brain’s reward circuitry, it is necessary to examine cocaine-induced D1- and D2-MSNs-specific synaptic proteome changes in the NAc. Such proteomic adaptations will likely drive reciprocal interactions between drug-induced transcriptional responses and synaptic dysfunction, perpetuating the “addiction cycle.” Delineating these complex reciprocal interactions will reveal more effective targets for treating drug addiction and relapse. Such advances will require technological improvements since current methods would require isolation of D1- or D2-MSNs from transgenic mice, where D1- or D2-MSNs are labeled with a fluorophore, for deep neuroproteomic analysis. Our lab has generated D1- or D2-MSNs labeled transgenic animals and isolated not only bipartite synapses of D1- or D2-MSNs but also tripartite synapses using FASS. By completing this study, we aim to demonstrate cell-type-specific synaptic changes in both bipartite and tripartite synapses.
With advances in imaging and genetic labeling methods, the spatiotemporal organization of synaptic proteins can now be visualized by identifying synaptic proteins at single-synapse resolution across mouse brain regions [207]. Proteomic characterization of PSD95-positive synapses have been conducted for 20 different human brain regions [208] and for mouse brain from postnatal day 1 to 18 months [209]. These studies help to understand the diversity of synapses as brain regions become dissimilar and shed light on how the protein constituents and architecture of synapses change through development. These studies also connect transcriptomic and neuroproteomic analyses to the structural synaptic development and plasticity of synapses within the subcellular compartment.
By integrating transcriptomics, translatomics, neuroproteomics, and super-resolution structural imaging, we are now at the next step of investigating the mechanistic links between behavioral changes, psychological function, and synaptic pathology associated with specific gene mutations in a particular brain region, with cell-type specificity and temporal information. Deep learning methods offer exciting promise for linking multi-omics and spatial data across cell types and structural organization [210]. An in-depth, comprehensive understanding of synaptic proteomes, especially in a cell-type-specific manner, along with the links between mRNA and protein, local regulation of protein synthesis, and changes in subsynaptic molecular organization, will expand the potential therapeutic targets for synapse-linked diseases. This understanding will not only correct abnormal neurotransmitter-mediated signaling but also change the translational perspectives of synaptic proteins.
Author Contributions
Y.Y.Y. wrote the article; E.J.N. and Y.Y.Y. reviewed and edited the article before submission. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support from the NIH (NIDA R01DA007359; P01DA047233; and P50DA018343).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All figures were created with BioRender.com. Some figures are adapted from BioRender templates, including electrical synapses vs. chemical synapses, magnetic sorting of leukocytes, FACS sorting principle, and types of proteomic workflows. The authors thank Rita Futamura and Corrine Azizian for checking the references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Y.; Song, X.; Wang, D.; Wang, Y.; Li, P.; Li, J. Proteomic insights into synaptic signaling in the brain: The past, present and future. Mol. Brain 2021, 14, 37. [Google Scholar] [CrossRef]
- Marcassa, G.; Dascenco, D.; de Wit, J. Proteomics-based synapse characterization: From proteins to circuits. Curr. Opin. Neurobiol. 2023, 79, 102690. [Google Scholar] [CrossRef]
- Lake, J.; Storm, C.S.; Makarious, M.B.; Bandres-Ciga, S. Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead. Cells 2021, 10, 1030. [Google Scholar] [CrossRef]
- Husain, I.; Ahmad, W.; Ali, A.; Anwar, L.; Nuruddin, S.M.; Ashraf, K.; Kamal, M.A. Functional Neuroproteomics: An Imperative Approach for Unravelling Protein Implicated Complexities of Brain. CNS Neurol. Disord. Drug. Targets 2021, 20, 613–624. [Google Scholar] [CrossRef]
- Alzate, O. Neuroproteomics. In Neuroproteomics; Alzate, O., Ed.; Frontiers in Neuroscience: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bai, F.; Witzmann, F.A. Synaptosome proteomics. Subcell. Biochem. 2007, 43, 77–98. [Google Scholar] [CrossRef]
- Bayes, A.; Grant, S.G. Neuroproteomics: Understanding the molecular organization and complexity of the brain. Nat. Rev. Neurosci. 2009, 10, 635–646. [Google Scholar] [CrossRef]
- Murtaza, N.; Uy, J.; Singh, K.K. Emerging proteomic approaches to identify the underlying pathophysiology of neurodevelopmental and neurodegenerative disorders. Mol. Autism. 2020, 11, 27. [Google Scholar] [CrossRef]
- Patzig, J.; Jahn, O.; Tenzer, S.; Wichert, S.P.; de Monasterio-Schrader, P.; Rosfa, S.; Kuharev, J.; Yan, K.; Bormuth, I.; Bremer, J.; et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci. 2011, 31, 16369–16386. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, K.; Nishimune, H. Similarity and Diversity of Presynaptic Molecules at Neuromuscular Junctions and Central Synapses. Biomolecules 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Straka, T.; Schroder, C.; Roos, A.; Kollipara, L.; Sickmann, A.; Williams, M.P.I.; Hafner, M.; Khan, M.M.; Rudolf, R. Regulatory Function of Sympathetic Innervation on the Endo/Lysosomal Trafficking of Acetylcholine Receptor. Front. Physiol. 2021, 12, 626707. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Harrison, C.; Eaton, S.L.; Llavero Hurtado, M.; Graham, L.C.; Alkhammash, L.; Oladiran, O.A.; Gale, A.; Lamont, D.J.; Simpson, H.; et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017, 21, 2348–2356. [Google Scholar] [CrossRef]
- Traeger, L.L.; Sabat, G.; Barrett-Wilt, G.A.; Wells, G.B.; Sussman, M.R. A tail of two voltages: Proteomic comparison of the three electric organs of the electric eel. Sci. Adv. 2017, 3, e1700523. [Google Scholar] [CrossRef]
- Forne, I.; Abian, J.; Cerda, J. Fish proteome analysis: Model organisms and non-sequenced species. Proteomics 2010, 10, 858–872. [Google Scholar] [CrossRef]
- Caire, M.J.; Reddy, V.; Varacallo, M. Physiology, Synapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Landgraf, P.; Antileo, E.R.; Schuman, E.M.; Dieterich, D.C. BONCAT: Metabolic labeling, click chemistry, and affinity purification of newly synthesized proteomes. Methods Mol. Biol. 2015, 1266, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234.e214. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, O.; McLean, C.; Croning, M.D.R.; Heil, K.F.; Wysocka, E.; He, X.; Sterratt, D.; Grant, S.G.N.; Simpson, T.I.; Armstrong, J.D. A unified resource and configurable model of the synapse proteome and its role in disease. Sci. Rep. 2021, 11, 9967. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, C.; Altelaar, M. Neuroproteomics of the Synapse: Subcellular Quantification of Protein Networks and Signaling Dynamics. Mol. Cell Proteom. 2021, 20, 100087. [Google Scholar] [CrossRef]
- Natividad, L.A.; Buczynski, M.W.; McClatchy, D.B.; Yates, J.R., 3rd. From Synapse to Function: A Perspective on the Role of Neuroproteomics in Elucidating Mechanisms of Drug Addiction. Proteomes 2018, 6, 50. [Google Scholar] [CrossRef]
- Martins-de-Souza, D. Proteomics, metabolomics, and protein interactomics in the characterization of the molecular features of major depressive disorder. Dialogues Clin. Neurosci. 2014, 16, 63–73. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Devi, L.A. Neuroproteomics of the synapse and drug addiction. J. Pharmacol. Exp. Ther. 2006, 318, 461–468. [Google Scholar] [CrossRef]
- Paget-Blanc, V.; Pfeffer, M.E.; Pronot, M.; Lapios, P.; Angelo, M.F.; Walle, R.; Cordelieres, F.P.; Levet, F.; Claverol, S.; Lacomme, S.; et al. A synaptomic analysis reveals dopamine hub synapses in the mouse striatum. Nat. Commun. 2022, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, D.; Kater, M.S.J.; Sakers, K.; Nygaard, K.R.; Liu, Y.; Koester, S.K.; Fass, S.B.; Lake, A.M.; Khazanchi, R.; Khankan, R.R.; et al. Activity-dependent translation dynamically alters the proteome of the perisynaptic astrocyte process. Cell Rep. 2022, 41, 111474. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, M.M.; Mishra, S.; Zhang, Z.; Wu, L.; McKetney, J.M.; Vestling, M.M.; Coon, J.J.; Chapman, E.R. Rapid and Gentle Immunopurification of Brain Synaptic Vesicles. J. Neurosci. 2022, 42, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Schmitt, S.; Bergner, C.G.; Tyanova, S.; Kannaiyan, N.; Manrique-Hoyos, N.; Kongi, K.; Cantuti, L.; Hanisch, U.K.; Philips, M.A.; et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 2015, 18, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Li, H.; Siemsen, B.M.; Healey, K.L.; Tran, P.K.; Woronoff, N.; Boger, H.A.; Kalivas, P.W.; Reissner, K.J. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol. Psychiatry 2016, 80, 207–215. [Google Scholar] [CrossRef]
- Schoch, S.; Gundelfinger, E.D. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006, 326, 379–391. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef]
- Zhai, R.G.; Bellen, H.J. Hauling t-SNAREs on the microtubule highway. Nat. Cell Biol. 2004, 6, 918–919. [Google Scholar] [CrossRef]
- Pang, Z.P.; Sudhof, T.C. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 2010, 22, 496–505. [Google Scholar] [CrossRef]
- Kreutzberger, A.J.B.; Kiessling, V.; Stroupe, C.; Liang, B.; Preobraschenski, J.; Ganzella, M.; Kreutzberger, M.A.B.; Nakamoto, R.; Jahn, R.; Castle, J.D.; et al. In vitro fusion of single synaptic and dense core vesicles reproduces key physiological properties. Nat. Commun. 2019, 10, 3904. [Google Scholar] [CrossRef]
- Birinci, Y.; Preobraschenski, J.; Ganzella, M.; Jahn, R.; Park, Y. Isolation of large dense-core vesicles from bovine adrenal medulla for functional studies. Sci. Rep. 2020, 10, 7540. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C.; Malenka, R.C. Understanding synapses: Past, present, and future. Neuron 2008, 60, 469–476. [Google Scholar] [CrossRef]
- Park, Y.; Kim, K.T. Short-term plasticity of small synaptic vesicle (SSV) and large dense-core vesicle (LDCV) exocytosis. Cell Signal. 2009, 21, 1465–1470. [Google Scholar] [CrossRef]
- Dresbach, T.; Qualmann, B.; Kessels, M.M.; Garner, C.C.; Gundelfinger, E.D. The presynaptic cytomatrix of brain synapses. Cell Mol. Life Sci. 2001, 58, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.; Nuwer, J.L.; Jacob, T.C. The Yin and Yang of GABAergic and Glutamatergic Synaptic Plasticity: Opposites in Balance by Crosstalking Mechanisms. Front. Synaptic Neurosci. 2022, 14, 911020. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Chelini, G.; Pantazopoulos, H.; Durning, P.; Berretta, S. The tetrapartite synapse: A key concept in the pathophysiology of schizophrenia. Eur. Psychiatry 2018, 50, 60–69. [Google Scholar] [CrossRef]
- Kruyer, A.; Chioma, V.C.; Kalivas, P.W. The Opioid-Addicted Tetrapartite Synapse. Biol. Psychiatry 2020, 87, 34–43. [Google Scholar] [CrossRef]
- Chaves Filho, A.J.M.; Mottin, M.; Los, D.B.; Andrade, C.H.; Macedo, D.S. The tetrapartite synapse in neuropsychiatric disorders: Matrix metalloproteinases (MMPs) as promising targets for treatment and rational drug design. Biochimie 2022, 201, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.N.; De Camilli, P. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 2003, 26, 701–728. [Google Scholar] [CrossRef] [PubMed]
- Yim, Y.Y.; Zurawski, Z.; Hamm, H. GPCR regulation of secretion. Pharmacol. Ther. 2018, 192, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Lepeta, K.; Lourenco, M.V.; Schweitzer, B.C.; Martino Adami, P.V.; Banerjee, P.; Catuara-Solarz, S.; de La Fuente Revenga, M.; Guillem, A.M.; Haidar, M.; Ijomone, O.M.; et al. Synaptopathies: Synaptic dysfunction in neurological disorders-A review from students to students. J. Neurochem. 2016, 138, 785–805. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, E. The postsynaptic organization of synapses. Cold Spring Harb. Perspect. Biol. 2011, 3, a005678. [Google Scholar] [CrossRef]
- Loh, K.H.; Stawski, P.S.; Draycott, A.S.; Udeshi, N.D.; Lehrman, E.K.; Wilton, D.K.; Svinkina, T.; Deerinck, T.J.; Ellisman, M.H.; Stevens, B.; et al. Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts. Cell 2016, 166, 1295–1307.e1221. [Google Scholar] [CrossRef]
- Biederer, T.; Kaeser, P.S.; Blanpied, T.A. Transcellular Nanoalignment of Synaptic Function. Neuron 2017, 96, 680–696. [Google Scholar] [CrossRef]
- Song, J.Y.; Ichtchenko, K.; Sudhof, T.C.; Brose, N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA 1999, 96, 1100–1105. [Google Scholar] [CrossRef]
- Linhoff, M.W.; Lauren, J.; Cassidy, R.M.; Dobie, F.A.; Takahashi, H.; Nygaard, H.B.; Airaksinen, M.S.; Strittmatter, S.M.; Craig, A.M. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron 2009, 61, 734–749. [Google Scholar] [CrossRef]
- Chih, B.; Gollan, L.; Scheiffele, P. Alternative Splicing Controls Selective Trans-Synaptic Interactions of the Neuroligin-Neurexin Complex. Neuron 2006, 51, 171–178. [Google Scholar] [CrossRef]
- Takahashi, H.; Katayama, K.-I.; Sohya, K.; Miyamoto, H.; Prasad, T.; Matsumoto, Y.; Ota, M.; Yasuda, H.; Tsumoto, T.; Aruga, J.; et al. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 2012, 15, 389–398. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Jamain, S.; Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004, 83, 449–456. [Google Scholar] [CrossRef]
- Witzmann, F.A.; Arnold, R.J.; Bai, F.; Hrncirova, P.; Kimpel, M.W.; Mechref, Y.S.; McBride, W.J.; Novotny, M.V.; Pedrick, N.M.; Ringham, H.N.; et al. A proteomic survey of rat cerebral cortical synaptosomes. Proteomics 2005, 5, 2177–2201. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Robinson, P.J. Synaptosome Preparations: Which Procedure Should I Use?. In Synaptosomes; Neuromethods, Murphy, K., Eds.; Humana Press: New York, NY, USA, 2018; Volume 141, pp. 27–53. [Google Scholar] [CrossRef]
- Gray, E.G.; Whittaker, V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962, 96, 79–88. [Google Scholar]
- Dodd, P.R.; Hardy, J.A.; Oakley, A.E.; Edwardson, J.A.; Perry, E.K.; Delaunoy, J.P. A rapid method for preparing synaptosomes: Comparison, with alternative procedures. Brain Res. 1981, 226, 107–118. [Google Scholar] [CrossRef]
- Cotman, C.W.; Matthews, D.A. Synaptic plasma membranes from rat brain synaptosomes: Isolation and partial characterization. Biochim. Biophys. Acta 1971, 249, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.F.; Clark, J.B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem. J. 1978, 176, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Krohnert, K.; Schafer, C.; Rammner, B.; Koo, S.J.; Classen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Burre, J.; Volknandt, W. The synaptic vesicle proteome. J. Neurochem. 2007, 101, 1448–1462. [Google Scholar] [CrossRef]
- Ahmed, S.; Holt, M.; Riedel, D.; Jahn, R. Small-scale isolation of synaptic vesicles from mammalian brain. Nat. Protoc. 2013, 8, 998–1009. [Google Scholar] [CrossRef]
- Hell, J.W.; Maycox, P.R.; Stadler, H.; Jahn, R. Uptake of GABA by rat brain synaptic vesicles isolated by a new procedure. EMBO J. 1988, 7, 3023–3029. [Google Scholar] [CrossRef]
- Chantranupong, L.; Saulnier, J.L.; Wang, W.; Jones, D.R.; Pacold, M.E.; Sabatini, B.L. Rapid purification and metabolomic profiling of synaptic vesicles from mammalian brain. Elife 2020, 9, e59699. [Google Scholar] [CrossRef]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- De Gasperi, R.; Rocher, A.B.; Sosa, M.A.G.; Wearne, S.L.; Perez, G.M.; Friedrich, V.L., Jr.; Hof, P.R.; Elder, G.A. The IRG mouse: A two-color fluorescent reporter for assessing Cre-mediated recombination and imaging complex cellular relationships in situ. Genesis 2008, 46, 308–317. [Google Scholar] [CrossRef]
- Igarashi, H.; Koizumi, K.; Kaneko, R.; Ikeda, K.; Egawa, R.; Yanagawa, Y.; Muramatsu, S.-i.; Onimaru, H.; Ishizuka, T.; Yawo, H. A Novel Reporter Rat Strain That Conditionally Expresses the Bright Red Fluorescent Protein tdTomato. PLoS ONE 2016, 11, e0155687. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, L.; Pan, S.; Gao, S.; Chen, W.; Zhang, X.; Dong, W.; Li, J.; Zhou, R.; Huang, L.; et al. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre–loxP-mediated lineage tracing. FEBS J. 2017, 284, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Bryda, E.C.; Men, H.; Davis, D.J.; Bock, A.S.; Shaw, M.L.; Chesney, K.L.; Hankins, M.A. A novel conditional ZsGreen-expressing transgenic reporter rat strain for validating Cre recombinase expression. Sci. Rep. 2019, 9, 13330. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Endo, H.; Ajiki, T.; Hakamata, Y.; Okada, T.; Murakami, T.; Kobayashi, E. Establishment of Cre/LoxP recombination system in transgenic rats. Biochem. Biophys. Res. Commun. 2004, 319, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Im, S.-K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Hirokawa, K.E.; Sorensen, S.A.; Gu, H.; Mills, M.; Ng, L.L.; Bohn, P.; Mortrud, M.; Ouellette, B.; Kidney, J.; et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front. Neural Circuits 2014, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Shcholok, T.; Eftekharpour, E. Cre-recombinase systems for induction of neuron-specific knockout models: A guide for biomedical researchers. Neural Regen. Res. 2023, 18, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, Q.; Chen-Tsai, R.Y. Establishment of a Cre-rat resource for creating conditional and physiological relevant models of human diseases. Transgenic Res. 2021, 30, 91–104. [Google Scholar] [CrossRef]
- Witten, I.B.; Steinberg, E.E.; Lee, S.Y.; Davidson, T.J.; Zalocusky, K.A.; Brodsky, M.; Yizhar, O.; Cho, S.L.; Gong, S.; Ramakrishnan, C.; et al. Recombinase-Driver Rat Lines: Tools, Techniques, and Optogenetic Application to Dopamine-Mediated Reinforcement. Neuron 2011, 72, 721–733. [Google Scholar] [CrossRef]
- Liu, Z.; Brown, A.; Fisher, D.; Wu, Y.; Warren, J.; Cui, X. Tissue Specific Expression of Cre in Rat Tyrosine Hydroxylase and Dopamine Active Transporter-Positive Neurons. PLoS ONE 2016, 11, e0149379. [Google Scholar] [CrossRef]
- Espina, V.; Wulfkuhle, J.D.; Calvert, V.S.; VanMeter, A.; Zhou, W.; Coukos, G.; Geho, D.H.; Petricoin, E.F.; Liotta, L.A. Laser-capture microdissection. Nat. Protoc. 2006, 1, 586–603. [Google Scholar] [CrossRef]
- Zhu, Y.; Piehowski, P.D.; Zhao, R.; Chen, J.; Shen, Y.; Moore, R.J.; Shukla, A.K.; Petyuk, V.A.; Campbell-Thompson, M.; Mathews, C.E.; et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat. Commun. 2018, 9, 882. [Google Scholar] [CrossRef]
- Plum, S.; Steinbach, S.; Attems, J.; Keers, S.; Riederer, P.; Gerlach, M.; May, C.; Marcus, K. Proteomic characterization of neuromelanin granules isolated from human substantia nigra by laser-microdissection. Sci. Rep. 2016, 6, 37139. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. The use of localized proteomics to identify the drivers of Alzheimer’s disease pathogenesis. Neural Regen. Res. 2017, 12, 912–913. [Google Scholar] [CrossRef]
- Nijholt, D.A.T.; Stingl, C.; Luider, T.M. Laser Capture Microdissection of Fluorescently Labeled Amyloid Plaques from Alzheimer’s Disease Brain Tissue for Mass Spectrometric Analysis. In Clinical Proteomics: Methods and Protocols; Vlahou, A., Makridakis, M., Eds.; Springer: New York, NY, USA, 2015; pp. 165–173. [Google Scholar]
- Garcia-Berrocoso, T.; Llombart, V.; Colas-Campas, L.; Hainard, A.; Licker, V.; Penalba, A.; Ramiro, L.; Simats, A.; Bustamante, A.; Martinez-Saez, E.; et al. Single Cell Immuno-Laser Microdissection Coupled to Label-Free Proteomics to Reveal the Proteotypes of Human Brain Cells After Ischemia. Mol. Cell Proteom. 2018, 17, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Bogdanovic, N.; Nakagawa, H.; Volkmann, I.; Aoki, M.; Winblad, B.; Sakai, J.; Tjernberg, L.O. Analysis of microdissected neurons by 18O mass spectrometry reveals altered protein expression in Alzheimer’s disease. J. Cell. Mol. Med. 2012, 16, 1686–1700. [Google Scholar] [CrossRef]
- MacDonald, M.L.; Favo, D.; Garver, M.; Sun, Z.; Arion, D.; Ding, Y.; Yates, N.; Sweet, R.A.; Lewis, D.A. Laser capture microdissection–targeted mass spectrometry: A method for multiplexed protein quantification within individual layers of the cerebral cortex. Neuropsychopharmacology 2019, 44, 743–748. [Google Scholar] [CrossRef]
- Griesser, E.; Wyatt, H.; Ten Have, S.; Stierstorfer, B.; Lenter, M.; Lamond, A.I. Quantitative Profiling of the Human Substantia Nigra Proteome from Laser-capture Microdissected FFPE Tissue*. Mol. Cell. Proteom. 2020, 19, 839–851. [Google Scholar] [CrossRef] [PubMed]
- do Canto, A.M.; Vieira, A.S.; Matos, H.B.A.; Carvalho, B.S.; Henning, B.; Norwood, B.A.; Bauer, S.; Rosenow, F.; Gilioli, R.; Cendes, F.; et al. Laser microdissection-based microproteomics of the hippocampus of a rat epilepsy model reveals regional differences in protein abundances. Sci. Rep. 2020, 10, 4412. [Google Scholar] [CrossRef] [PubMed]
- Bensaddek, D.; Narayan, V.; Nicolas, A.; Brenes Murillo, A.; Gartner, A.; Kenyon, C.J.; Lamond, A.I. Micro-proteomics with iterative data analysis: Proteome analysis in C. elegans at the single worm level. Proteomics 2016, 16, 381–392. [Google Scholar] [CrossRef]
- Kaur, R.P.; Ludhiadch, A.; Munshi, A. Chapter 9-Single-Cell Genomics: Technology and Applications. In Single-Cell Omics; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 179–197. [Google Scholar]
- Holt, L.M.; Olsen, M.L. Novel Applications of Magnetic Cell Sorting to Analyze Cell-Type Specific Gene and Protein Expression in the Central Nervous System. PLoS ONE 2016, 11, e0150290. [Google Scholar] [CrossRef]
- Rayaprolu, S.; Gao, T.; Xiao, H.; Ramesha, S.; Weinstock, L.D.; Shah, J.; Duong, D.M.; Dammer, E.B.; Webster, J.A., Jr.; Lah, J.J.; et al. Flow-cytometric microglial sorting coupled with quantitative proteomics identifies moesin as a highly-abundant microglial protein with relevance to Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 28. [Google Scholar] [CrossRef]
- Jungblut, M.; Tiveron, M.C.; Barral, S.; Abrahamsen, B.; Knöbel, S.; Pennartz, S.; Schmitz, J.; Perraut, M.; Pfrieger, F.W.; Stoffel, W.; et al. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia 2012, 60, 894–907. [Google Scholar] [CrossRef]
- Stokum, J.A.; Shim, B.; Huang, W.; Kane, M.; Smith, J.A.; Gerzanich, V.; Simard, J.M. A large portion of the astrocyte proteome is dedicated to perivascular endfeet, including critical components of the electron transport chain. J. Cereb. Blood Flow. Metab. 2021, 41, 2546–2560. [Google Scholar] [CrossRef]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Gao, T.; Xiao, H.; Betarbet, R.; Duong, D.M.; Webster, J.A.; Hales, C.M.; Lah, J.J.; et al. Quantitative proteomics of acutely-isolated mouse microglia identifies novel immune Alzheimer’s disease-related proteins. Mol. Neurodegener. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Maes, E.; Cools, N.; Willems, H.; Baggerman, G. FACS-Based Proteomics Enables Profiling of Proteins in Rare Cell Populations. Int. J. Mol. Sci. 2020, 21, 6557. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for Single-Cell Isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef] [PubMed]
- Postupna, N.O.; Latimer, C.S.; Keene, C.D.; Montine, K.S.; Montine, T.J.; Darvas, M. Flow cytometric evaluation of crude synaptosome preparation as a way to study synaptic alteration in neurodegenerative diseases. Neuromethods 2018, 141, 297–310. [Google Scholar] [CrossRef]
- Biesemann, C.; Gronborg, M.; Luquet, E.; Wichert, S.P.; Bernard, V.; Bungers, S.R.; Cooper, B.; Varoqueaux, F.; Li, L.; Byrne, J.A.; et al. Proteomic screening of glutamatergic mouse brain synaptosomes isolated by fluorescence activated sorting. EMBO J. 2014, 33, 157–170. [Google Scholar] [CrossRef]
- Husi, H.; Ward, M.A.; Choudhary, J.S.; Blackstock, W.P.; Grant, S.G.N. Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nat. Neurosci. 2000, 3, 661–669. [Google Scholar] [CrossRef]
- Dosemeci, A.; Makusky, A.J.; Jankowska-Stephens, E.; Yang, X.; Slotta, D.J.; Markey, S.P. Composition of the synaptic PSD-95 complex. Mol. Cell Proteom. 2007, 6, 1749–1760. [Google Scholar] [CrossRef]
- Klemmer, P.; Smit, A.B.; Li, K.W. Proteomics analysis of immuno-precipitated synaptic protein complexes. J. Proteom. 2009, 72, 82–90. [Google Scholar] [CrossRef]
- Paulo, J.A.; Brucker, W.J.; Hawrot, E. Proteomic Analysis of an α7 Nicotinic Acetylcholine Receptor Interactome. J. Proteome Res. 2009, 8, 1849–1858. [Google Scholar] [CrossRef]
- Farr, C.D.; Gafken, P.R.; Norbeck, A.D.; Doneanu, C.E.; Stapels, M.D.; Barofsky, D.F.; Minami, M.; Saugstad, J.A. Proteomic analysis of native metabotropic glutamate receptor 5 protein complexes reveals novel molecular constituents. J. Neurochem. 2004, 91, 438–450. [Google Scholar] [CrossRef]
- Collins, M.O.; Husi, H.; Yu, L.; Brandon, J.M.; Anderson, C.N.G.; Blackstock, W.P.; Choudhary, J.S.; Grant, S.G.N. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 2006, 97, 16–23. [Google Scholar] [CrossRef]
- Rigaut, G.; Shevchenko, A.; Rutz, B.; Wilm, M.; Mann, M.; Séraphin, B. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 1999, 17, 1030–1032. [Google Scholar] [CrossRef]
- Li, Y. The tandem affinity purification technology: An overview. Biotechnol. Lett. 2011, 33, 1487–1499. [Google Scholar] [CrossRef]
- Fernandez, E.; Collins, M.O.; Uren, R.T.; Kopanitsa, M.V.; Komiyama, N.H.; Croning, M.D.; Zografos, L.; Armstrong, J.D.; Choudhary, J.S.; Grant, S.G. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol. Syst. Biol. 2009, 5, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Collins, M.O.; Harmse, J.; Choudhary, J.S.; Grant, S.G.N.; Komiyama, N.H. Cell-type-specific visualisation and biochemical isolation of endogenous synaptic proteins in mice. Eur. J. Neurosci. 2020, 51, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.E.; Glenn, W.S.; Hamblin, G.D.; Tirrell, D.A. Cell-selective proteomics for biological discovery. Curr. Opin. Chem. Biol. 2017, 36, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, E.; Li, Y.; Roche, K.W. Advances in Proteomics Allow Insights Into Neuronal Proteomes. Front. Mol. Neurosci. 2021, 14, 647451. [Google Scholar] [CrossRef]
- Alvarez-Castelao, B.; Schanzenbacher, C.T.; Hanus, C.; Glock, C.; Tom Dieck, S.; Dorrbaum, A.R.; Bartnik, I.; Nassim-Assir, B.; Ciirdaeva, E.; Mueller, A.; et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat. Biotechnol. 2017, 35, 1196–1201. [Google Scholar] [CrossRef]
- Alvarez-Castelao, B.; Schanzenbacher, C.T.; Langer, J.D.; Schuman, E.M. Cell-type-specific metabolic labeling, detection and identification of nascent proteomes in vivo. Nat. Protoc. 2019, 14, 556–575. [Google Scholar] [CrossRef]
- Mathew, B.; Bathla, S.; Williams, K.R.; Nairn, A.C. Deciphering Spatial Protein-Protein Interactions in Brain Using Proximity Labeling. Mol. Cell Proteom. 2022, 21, 100422. [Google Scholar] [CrossRef]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 2016, 27, 1188–1196. [Google Scholar] [CrossRef]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 2015, 12, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Cijsouw, T.; Ramsey, A.M.; Lam, T.T.; Carbone, B.E.; Blanpied, T.A.; Biederer, T. Mapping the Proteome of the Synaptic Cleft through Proximity Labeling Reveals New Cleft Proteins. Proteomes 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.A.; Li, J.; Chon, U.; Sinantha-Hu, M.C.; Luginbuhl, D.J.; Udeshi, N.D.; Carey, D.K.; Takeo, Y.H.; Xie, Q.; Xu, C.; et al. In situ cell-type-specific cell-surface proteomic profiling in mice. Neuron 2022, 110, 3882–3896.e3889. [Google Scholar] [CrossRef]
- Dumrongprechachan, V.; Salisbury, R.B.; Soto, G.; Kumar, M.; MacDonald, M.L.; Kozorovitskiy, Y. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat. Commun. 2021, 12, 4855. [Google Scholar] [CrossRef]
- Brewer, K.D.; Shi, S.M.; Wyss-Coray, T. Unraveling protein dynamics to understand the brain-the next molecular frontier. Mol. Neurodegener. 2022, 17, 45. [Google Scholar] [CrossRef]
- Uezu, A.; Kanak, D.J.; Bradshaw, T.W.A.; Soderblom, E.J.; Catavero, C.M.; Burette, A.C.; Weinberg, R.J.; Soderling, S.H. Identification of an elaborate complex mediating postsynaptic inhibition. Science 2016, 353, 1123–1129. [Google Scholar] [CrossRef]
- Spence, E.F.; Dube, S.; Uezu, A.; Locke, M.; Soderblom, E.J.; Soderling, S.H. In vivo proximity proteomics of nascent synapses reveals a novel regulator of cytoskeleton-mediated synaptic maturation. Nat. Commun. 2019, 10, 386. [Google Scholar] [CrossRef]
- Rayaprolu, S.; Bitarafan, S.; Santiago, J.V.; Betarbet, R.; Sunna, S.; Cheng, L.; Xiao, H.; Nelson, R.S.; Kumar, P.; Bagchi, P.; et al. Cell type-specific biotin labeling in vivo resolves regional neuronal and astrocyte proteomic differences in mouse brain. Nat. Commun. 2022, 13, 2927. [Google Scholar] [CrossRef]
- Takano, T.; Wallace, J.T.; Baldwin, K.T.; Purkey, A.M.; Uezu, A.; Courtland, J.L.; Soderblom, E.J.; Shimogori, T.; Maness, P.F.; Eroglu, C.; et al. Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 2020, 588, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hobson, B.D.; Choi, S.J.; Mosharov, E.V.; Soni, R.K.; Sulzer, D.; Sims, P.A. Subcellular proteomics of dopamine neurons in the mouse brain. Elife 2022, 11, e70921. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.T.; Kim, J.; Doan, T.T.; Lee, M.-W.; Lee, M. APEX Proximity Labeling as a Versatile Tool for Biological Research. Biochemistry 2020, 59, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R., 3rd. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Smith, L.M.; Kelleher, N.L.; Linial, M.; Goodlett, D.; Langridge-Smith, P.; Ah Goo, Y.; Safford, G.; Bonilla*, L.; Kruppa, G.; Zubarev, R.; et al. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef]
- Catherman, A.D.; Skinner, O.S.; Kelleher, N.L. Top Down proteomics: Facts and perspectives. Biochem. Biophys. Res. Commun. 2014, 445, 683–693. [Google Scholar] [CrossRef]
- Melby, J.A.; Roberts, D.S.; Larson, E.J.; Brown, K.A.; Bayne, E.F.; Jin, S.; Ge, Y. Novel Strategies to Address the Challenges in Top-Down Proteomics. J. Am. Soc. Mass. Spectrom. 2021, 32, 1278–1294. [Google Scholar] [CrossRef]
- Wilson, R.S.; Nairn, A.C. Cell-Type-Specific Proteomics: A Neuroscience Perspective. Proteomes 2018, 6, 51. [Google Scholar] [CrossRef]
- Giesen, C.; Wang, H.A.O.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schüffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef]
- Amy, L.V.D.; Sarah, M.G.; Corey, M.W.; Austin, B.K.; Kristen, I.F.; Irene, C.; Christopher, D.D.; Eli, R.Z. A developmental atlas of the mouse brain by single-cell mass cytometry. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. BioSyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef]
- Mansuri, M.S.; Williams, K.; Nairn, A.C. Uncovering biology by single-cell proteomics. Commun. Biol. 2023, 6, 381. [Google Scholar] [CrossRef]
- Budnik, B.; Levy, E.; Harmange, G.; Slavov, N. SCoPE-MS: Mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018, 19, 161. [Google Scholar] [CrossRef]
- Tsai, C.F.; Zhao, R.; Williams, S.M.; Moore, R.J.; Schultz, K.; Chrisler, W.B.; Pasa-Tolic, L.; Rodland, K.D.; Smith, R.D.; Shi, T.; et al. An Improved Boosting to Amplify Signal with Isobaric Labeling (iBASIL) Strategy for Precise Quantitative Single-cell Proteomics. Mol. Cell Proteom. 2020, 19, 828–838. [Google Scholar] [CrossRef]
- Goto-Silva, L.; Junqueira, M. Single-cell proteomics: A treasure trove in neurobiology. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140658. [Google Scholar] [CrossRef]
- Han, G.; Sun, J.; Wang, J.; Bai, Z.; Song, F.; Lei, H. Genomics in neurological disorders. Genom. Proteom. Bioinform. 2014, 12, 156–163. [Google Scholar] [CrossRef]
- Reim, D.; Distler, U.; Halbedl, S.; Verpelli, C.; Sala, C.; Bockmann, J.; Tenzer, S.; Boeckers, T.M.; Schmeisser, M.J. Proteomic Analysis of Post-synaptic Density Fractions from Shank3 Mutant Mice Reveals Brain Region Specific Changes Relevant to Autism Spectrum Disorder. Front. Mol. Neurosci. 2017, 10, 26. [Google Scholar] [CrossRef]
- Al Shweiki, M.R.; Oeckl, P.; Steinacker, P.; Barschke, P.; Dorner-Ciossek, C.; Hengerer, B.; Schonfeldt-Lecuona, C.; Otto, M. Proteomic analysis reveals a biosignature of decreased synaptic protein in cerebrospinal fluid of major depressive disorder. Transl. Psychiatry 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.; Beasley, C.L.; Dicker, P.; Fagan, A.; English, J.; Pariante, C.M.; Wait, R.; Dunn, M.J.; Cotter, D.R. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol. Psychiatry 2008, 13, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Mullin, A.P.; Gokhale, A.; Moreno-De-Luca, A.; Sanyal, S.; Waddington, J.L.; Faundez, V. Neurodevelopmental disorders: Mechanisms and boundary definitions from genomes, interactomes and proteomes. Transl. Psychiatry 2013, 3, e329. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Tang, B.; Wang, T.; Wan, H.; Han, L.; Qin, X.; Zhang, Y.; Wang, J.; Yu, C.; Berton, F.; Francesconi, W.; et al. Fmr1 deficiency promotes age-dependent alterations in the cortical synaptic proteome. Proc. Natl. Acad. Sci. USA 2015, 112, E4697–E4706. [Google Scholar] [CrossRef]
- Yi, J.J.; Paranjape, S.R.; Walker, M.P.; Choudhury, R.; Wolter, J.M.; Fragola, G.; Emanuele, M.J.; Major, M.B.; Zylka, M.J. The autism-linked UBE3A T485A mutant E3 ubiquitin ligase activates the Wnt/beta-catenin pathway by inhibiting the proteasome. J. Biol. Chem. 2017, 292, 12503–12515. [Google Scholar] [CrossRef]
- Matic, K.; Eninger, T.; Bardoni, B.; Davidovic, L.; Macek, B. Quantitative phosphoproteomics of murine Fmr1-KO cell lines provides new insights into FMRP-dependent signal transduction mechanisms. J. Proteome Res. 2014, 13, 4388–4397. [Google Scholar] [CrossRef]
- Collins, M.O.; Yu, L.; Coba, M.P.; Husi, H.; Campuzano, I.; Blackstock, W.P.; Choudhary, J.S.; Grant, S.G. Proteomic analysis of in vivo phosphorylated synaptic proteins. J. Biol. Chem. 2005, 280, 5972–5982. [Google Scholar] [CrossRef]
- Li, J.; Wilkinson, B.; Clementel, V.A.; Hou, J.; O’Dell, T.J.; Coba, M.P. Long-term potentiation modulates synaptic phosphorylation networks and reshapes the structure of the postsynaptic interactome. Sci. Signal. 2016, 9, rs8. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatry 2020, 25, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, N.; Cheng, A.A.; Brown, C.O.; Meka, D.P.; Hong, S.; Uy, J.A.; El-Hajjar, J.; Pipko, N.; Unda, B.K.; Schwanke, B.; et al. Neuron-specific protein network mapping of autism risk genes identifies shared biological mechanisms and disease-relevant pathologies. Cell Rep. 2022, 41, 111678. [Google Scholar] [CrossRef] [PubMed]
- Tilot, A.K.; Bebek, G.; Niazi, F.; Altemus, J.B.; Romigh, T.; Frazier, T.W.; Eng, C. Neural transcriptome of constitutional Pten dysfunction in mice and its relevance to human idiopathic autism spectrum disorder. Mol. Psychiatry 2016, 21, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s beta-amyloid precursor protein. J. Neurochem. 2012, 120 (Suppl. 1), 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, K.; Lee, Y.C.; Kim, S.; Won, H.H.; Yu, T.Y.; Lee, E.M.; Kang, J.M.; Lewis, M.; Kim, D.K.; et al. Associations between vascular risk factors and subsequent Alzheimer’s disease in older adults. Alzheimers Res. Ther. 2020, 12, 117. [Google Scholar] [CrossRef]
- Virgilio, E.; De Marchi, F.; Contaldi, E.; Dianzani, U.; Cantello, R.; Mazzini, L.; Comi, C. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. Biomedicines 2022, 10, 760. [Google Scholar] [CrossRef]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Dejanovic, B.; Huntley, M.A.; De Maziere, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e1327. [Google Scholar] [CrossRef]
- Robbins, M.; Clayton, E.; Kaminski Schierle, G.S. Synaptic tau: A pathological or physiological phenomenon? Acta Neuropathol. Commun. 2021, 9, 149. [Google Scholar] [CrossRef]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef]
- Chang, R.Y.; Nouwens, A.S.; Dodd, P.R.; Etheridge, N. The synaptic proteome in Alzheimer’s disease. Alzheimers Dement. 2013, 9, 499–511. [Google Scholar] [CrossRef]
- Hesse, R.; Hurtado, M.L.; Jackson, R.J.; Eaton, S.L.; Herrmann, A.G.; Colom-Cadena, M.; Tzioras, M.; King, D.; Rose, J.; Tulloch, J.; et al. Comparative profiling of the synaptic proteome from Alzheimer’s disease patients with focus on the APOE genotype. Acta Neuropathol. Commun. 2019, 7, 214. [Google Scholar] [CrossRef]
- Kadoyama, K.; Matsuura, K.; Takano, M.; Otani, M.; Tomiyama, T.; Mori, H.; Matsuyama, S. Proteomic analysis involved with synaptic plasticity improvement by GABA(A) receptor blockade in hippocampus of a mouse model of Alzheimer’s disease. Neurosci. Res. 2021, 165, 61–68. [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Luvsannyam, E.; Jain, M.S.; Pormento, M.K.L.; Siddiqui, H.; Balagtas, A.R.A.; Emuze, B.O.; Poprawski, T. Neurobiology of Schizophrenia: A Comprehensive Review. Cureus 2022, 14, e23959. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68, image 45. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, R.; Weinberger, D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef]
- Rosato, M.; Stringer, S.; Gebuis, T.; Paliukhovich, I.; Li, K.W.; Posthuma, D.; Sullivan, P.F.; Smit, A.B.; van Kesteren, R.E. Combined cellomics and proteomics analysis reveals shared neuronal morphology and molecular pathway phenotypes for multiple schizophrenia risk genes. Mol. Psychiatry 2021, 26, 784–799. [Google Scholar] [CrossRef]
- Focking, M.; Lopez, L.M.; English, J.A.; Dicker, P.; Wolff, A.; Brindley, E.; Wynne, K.; Cagney, G.; Cotter, D.R. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol. Psychiatry 2015, 20, 424–432. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Yang, H.; Howrigan, D.P.; Wilkinson, B.; Souaiaia, T.; Evgrafov, O.V.; Genovese, G.; Clementel, V.A.; Tudor, J.C.; et al. Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat. Neurosci. 2017, 20, 1150–1161. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; HHS Publication No. PEP21-07-01-003, NSDUH Series H-56; Center for Behavioral Health Statistics and Quality; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021. Available online: https://www.samhsa.gov/data/ (accessed on 12 June 2023).
- Martins-de-Souza, D. Comprehending depression through proteomics. Int. J. Neuropsychopharmacol. 2012, 15, 1373–1374. [Google Scholar] [CrossRef][Green Version]
- Beasley, C.L.; Pennington, K.; Behan, A.; Wait, R.; Dunn, M.J.; Cotter, D. Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: Evidence for disease-associated changes. Proteomics 2006, 6, 3414–3425. [Google Scholar] [CrossRef]
- Johnston-Wilson, N.L.; Sims, C.D.; Hofmann, J.P.; Anderson, L.; Shore, A.D.; Torrey, E.F.; Yolken, R.H. Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol. Psychiatry 2000, 5, 142–149. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Guest, P.C.; Harris, L.W.; Vanattou-Saifoudine, N.; Webster, M.J.; Rahmoune, H.; Bahn, S. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl. Psychiatry 2012, 2, e87. [Google Scholar] [CrossRef] [PubMed]
- Martins-de-Souza, D.; Guest, P.C.; Vanattou-Saifoudine, N.; Rahmoune, H.; Bahn, S. Phosphoproteomic differences in major depressive disorder postmortem brains indicate effects on synaptic function. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ditzen, C.; Tang, N.; Jastorff, A.M.; Teplytska, L.; Yassouridis, A.; Maccarrone, G.; Uhr, M.; Bronisch, T.; Miller, C.A.; Holsboer, F.; et al. Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology 2012, 37, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Zhang, R.F.; Luo, D.; Zhou, Y.; Wang, Y.; Fang, L.; Li, W.J.; Mu, J.; Zhang, L.; Zhang, Y.; et al. Comparative proteomic analysis of plasma from major depressive patients: Identification of proteins associated with lipid metabolism and immunoregulation. Int. J. Neuropsychopharmacol. 2012, 15, 1413–1425. [Google Scholar] [CrossRef]
- See, R.E. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 2002, 71, 517–529. [Google Scholar] [CrossRef]
- Everitt, B.J.; Wolf, M.E. Psychomotor stimulant addiction: A neural systems perspective. J. Neurosci. 2002, 22, 3312–3320. [Google Scholar] [CrossRef]
- Jasinska, A.J.; Chen, B.T.; Bonci, A.; Stein, E.A. Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: Implications for drug addiction therapies. Addict. Biol. 2015, 20, 215–226. [Google Scholar] [CrossRef]
- Van den Oever, M.C.; Spijker, S.; Smit, A.B.; De Vries, T.J. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci. Biobehav. Rev. 2010, 35, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.J.; Peng, L.; Kivell, B.M. Proteomics Analysis of Dorsal Striatum Reveals Changes in Synaptosomal Proteins following Methamphetamine Self-Administration in Rats. PLoS ONE 2015, 10, e0139829. [Google Scholar] [CrossRef]
- Lull, M.E.; Erwin, M.S.; Morgan, D.; Roberts, D.C.; Vrana, K.E.; Freeman, W.M. Persistent proteomic alterations in the medial prefrontal cortex with abstinence from cocaine self-administration. Proteom. Clin. Appl. 2009, 3, 462–472. [Google Scholar] [CrossRef]
- Puig, S.; Xue, X.; Salisbury, R.; Shelton, M.A.; Kim, S.M.; Hildebrand, M.A.; Glausier, J.R.; Freyberg, Z.; Tseng, G.C.; Yocum, A.K.; et al. Uncovering circadian rhythm disruptions of synaptic proteome signaling in prefrontal cortex and nucleus accumbens associated with opioid use disorder. bioRxiv 2023. [Google Scholar] [CrossRef]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Hendrickson, C.L. High-resolution mass spectrometers. Annu. Rev. Anal. Chem. 2008, 1, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chu, S.; Tan, S.; Yin, X.; Jiang, Y.; Dai, X.; Gong, X.; Fang, X.; Tian, D. Towards Higher Sensitivity of Mass Spectrometry: A Perspective From the Mass Analyzers. Front. Chem. 2021, 9, 813359. [Google Scholar] [CrossRef] [PubMed]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass. Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.D.; Thielert, M.; Vasilopoulou, C.; Ammar, C.; Coscia, F.; Mund, A.; Hoerning, O.B.; Bache, N.; Apalategui, A.; Lubeck, M.; et al. Ultra-high sensitivity mass spectrometry quantifies single-cell proteome changes upon perturbation. Mol. Syst. Biol. 2022, 18, e10798. [Google Scholar] [CrossRef]
- Lobo, M.K.; Covington, H.E., 3rd; Chaudhury, D.; Friedman, A.K.; Sun, H.; Damez-Werno, D.; Dietz, D.M.; Zaman, S.; Koo, J.W.; Kennedy, P.J.; et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010, 330, 385–390. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Tye, L.D.; Kreitzer, A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 2012, 15, 816–818. [Google Scholar] [CrossRef]
- Calipari, E.S.; Bagot, R.C.; Purushothaman, I.; Davidson, T.J.; Yorgason, J.T.; Pena, C.J.; Walker, D.M.; Pirpinias, S.T.; Guise, K.G.; Ramakrishnan, C.; et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. USA 2016, 113, 2726–2731. [Google Scholar] [CrossRef]
- Lutz, P.E.; Kieffer, B.L. The multiple facets of opioid receptor function: Implications for addiction. Curr. Opin. Neurobiol. 2013, 23, 473–479. [Google Scholar] [CrossRef]
- Turner, B.D.; Kashima, D.T.; Manz, K.M.; Grueter, C.A.; Grueter, B.A. Synaptic Plasticity in the Nucleus Accumbens: Lessons Learned from Experience. ACS Chem. Neurosci. 2018, 9, 2114–2126. [Google Scholar] [CrossRef]
- Chartoff, E.H.; Connery, H.S. It’s MORe exciting than mu: Crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front. Pharmacol. 2014, 5, 116. [Google Scholar] [CrossRef]
- Zhu, F.; Cizeron, M.; Qiu, Z.; Benavides-Piccione, R.; Kopanitsa, M.V.; Skene, N.G.; Koniaris, B.; DeFelipe, J.; Fransen, E.; Komiyama, N.H.; et al. Architecture of the Mouse Brain Synaptome. Neuron 2018, 99, 781–799.e710. [Google Scholar] [CrossRef] [PubMed]
- Curran, O.E.; Qiu, Z.; Smith, C.; Grant, S.G.N. A single-synapse resolution survey of PSD95-positive synapses in twenty human brain regions. Eur. J. Neurosci. 2021, 54, 6864–6881. [Google Scholar] [CrossRef] [PubMed]
- Cizeron, M.; Qiu, Z.; Koniaris, B.; Gokhale, R.; Komiyama, N.H.; Fransen, E.; Grant, S.G.N. A brainwide atlas of synapses across the mouse life span. Science 2020, 369, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Minehart, J.A.; Speer, C.M. A Picture Worth a Thousand Molecules-Integrative Technologies for Mapping Subcellular Molecular Organization and Plasticity in Developing Circuits. Front. Synaptic Neurosci. 2020, 12, 615059. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).