Intravitreal Neuroglobin Mitigates Primate Experimental Glaucomatous Structural Damage in Association with Reduced Optic Nerve Microglial and Complement 3-Astrocyte Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use and Ethical Approval
2.2. Animal Anaesthesia
2.3. EG Induction and IOP Measurements
2.4. Optical Coherence Tomography Imaging
2.5. Anterior Segment Imaging and Fundus Examination
2.6. Intravitreal Injections
2.7. Histology and Immunohistochemistry
2.8. Statistical Analyses
3. Results
3.1. Baseline Parameters before EG Induction Are Similar
3.2. IOP Profiles from Six Primates Show Similar IOP Elevations
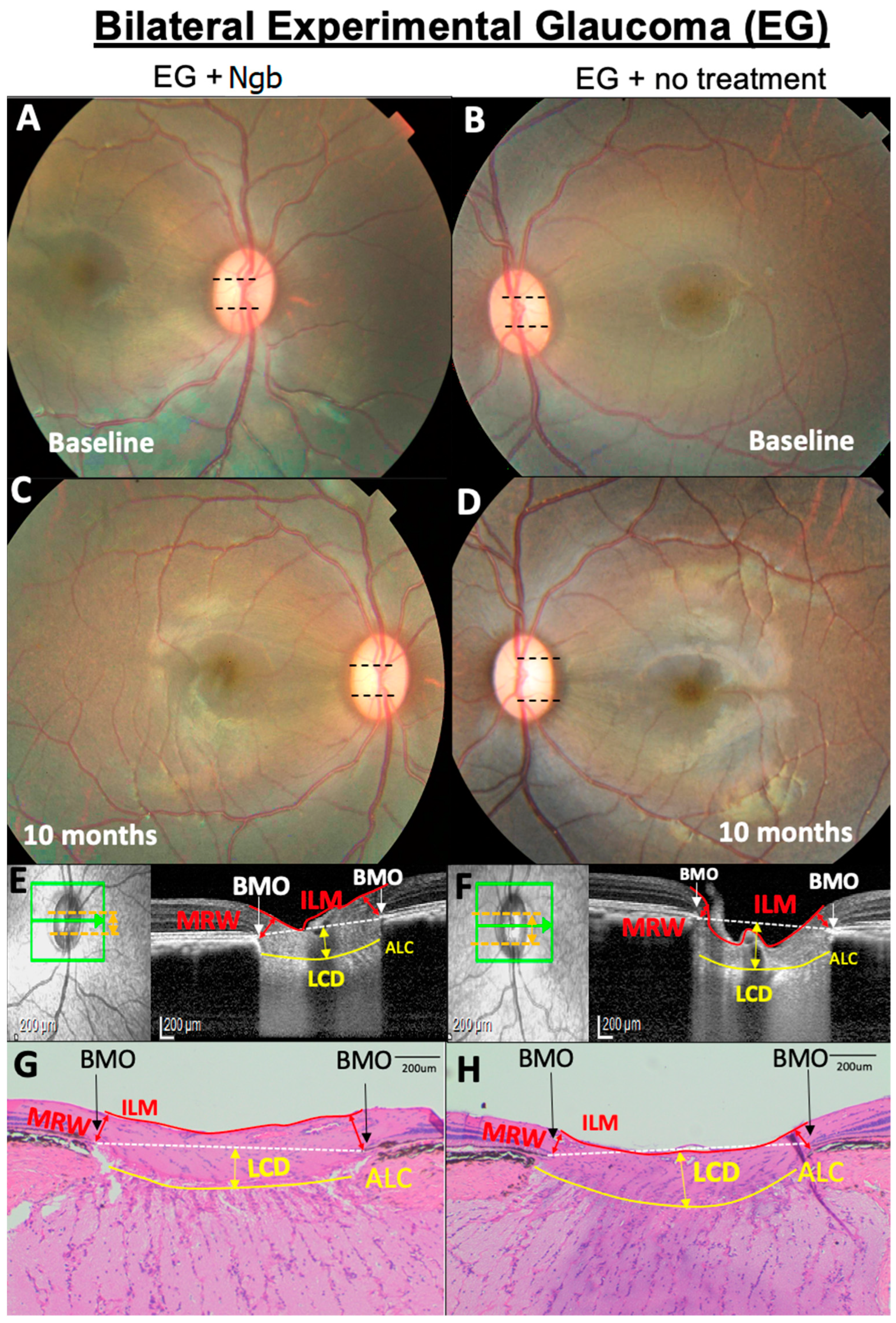
3.3. OCT Structural Changes with EG: Before and after Intervention
3.4. IVT-Ngb Increased the Cup–Disc Ratio after EG
3.5. Histological Changes in the ONH Corroborate OCT Structural Changes
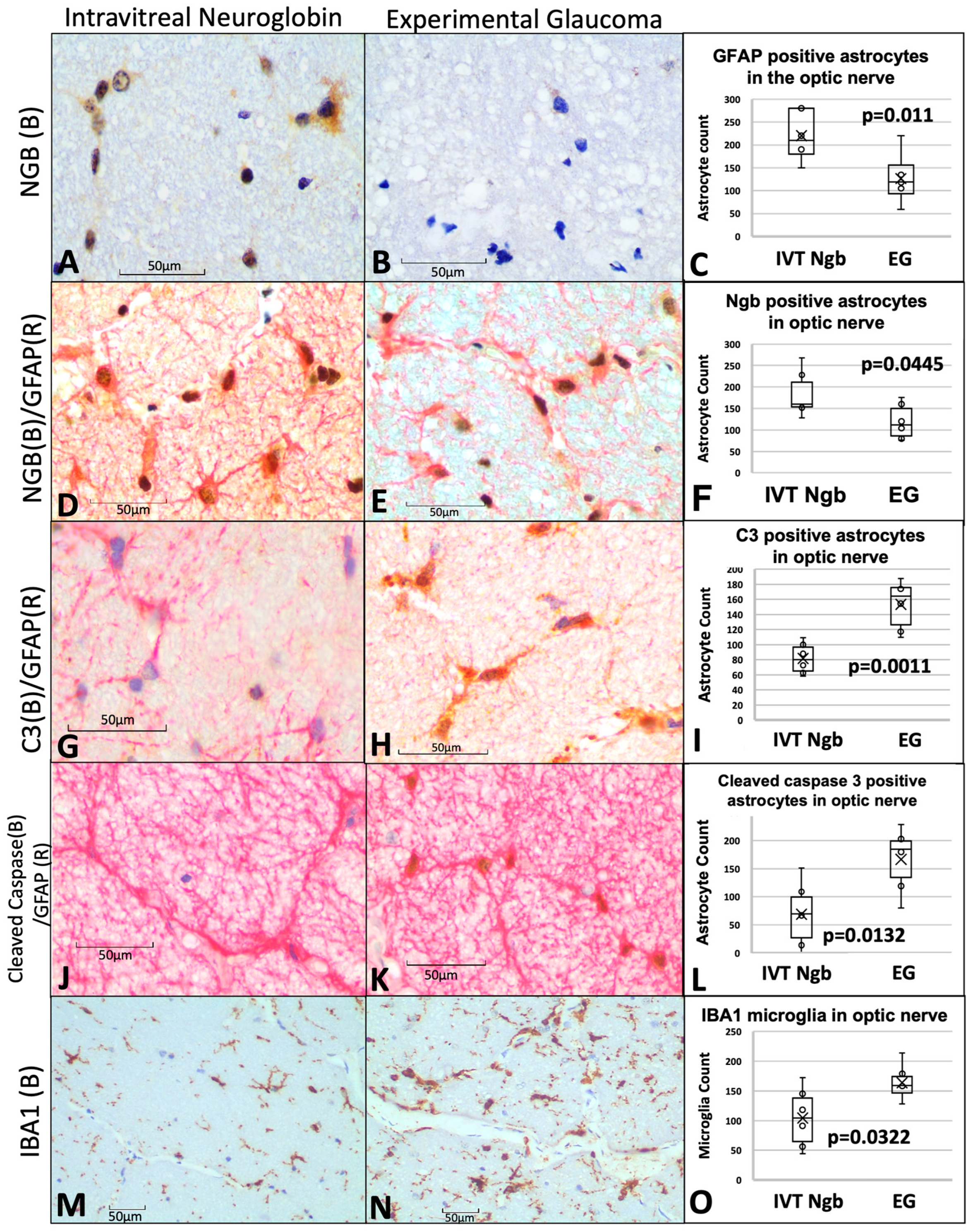
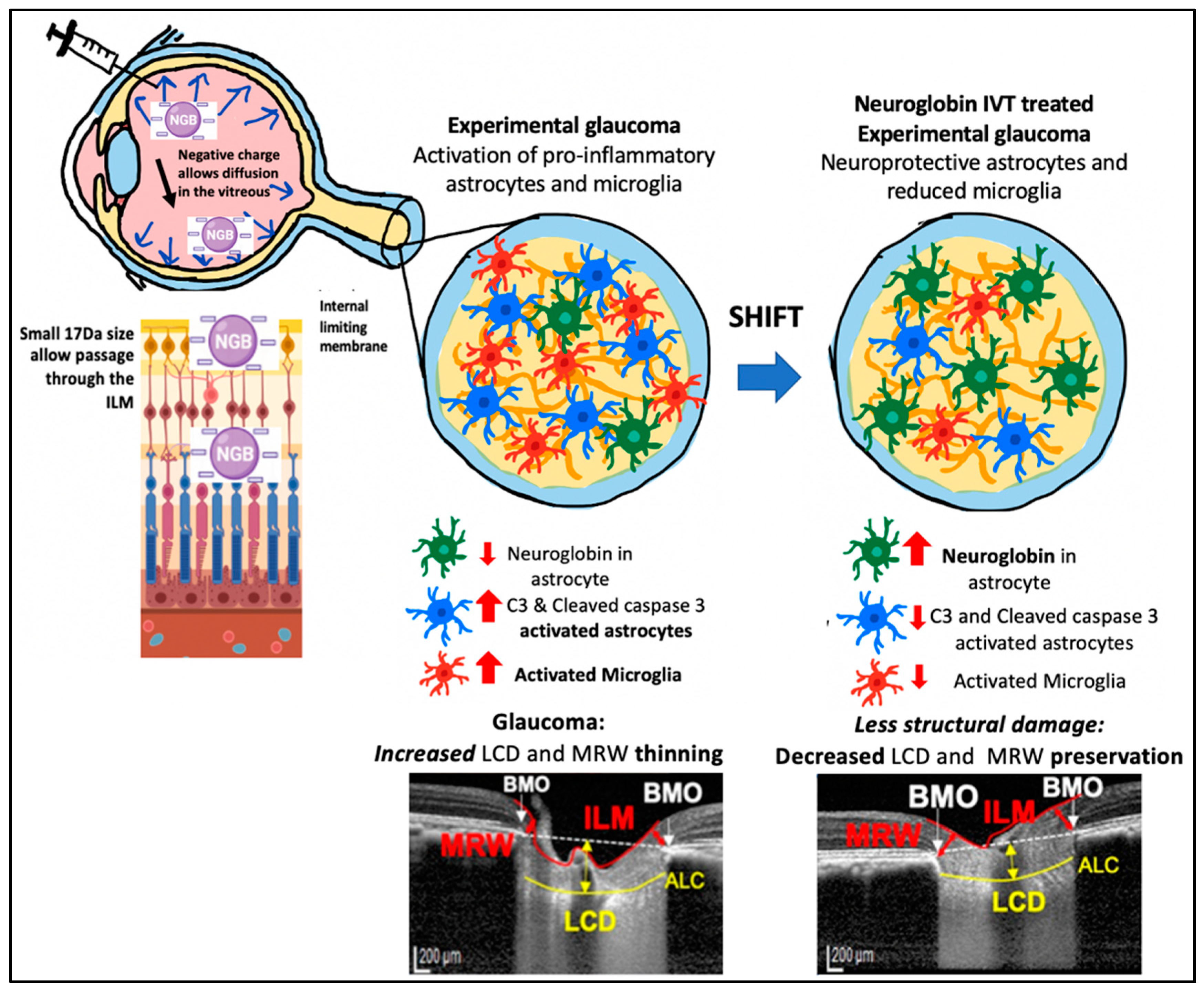
3.6. IVT-Ngb Increases Ngb Expression in the Optic Nerve and Reduces Infiltration by Activated Astrocytes and Microglial Cells
3.7. Complications Post-IVT Injection included a Bilateral Transient Uveitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOP | Intraocular pressure |
| Ngb | Neuroglobin |
| ONH | Optic nerve head |
| OCT | Optical coherence tomography |
| LCD | Lamina cribrosa depth |
| MRW | Minimum rim width |
| RNFL | Retinal nerve fibre layer |
| EG | Experimental glaucoma |
| IVT | Intravitreal treatment |
| IVT-Ngb | Intravitreal treatment with neuroglobin |
| RGC | Retinal ganglion cell |
| IL | Interleukin |
| C3 | Complement 3 |
| AAALAC | American Association for Accreditation of Laboratory Animal Care |
| ARVO | Association for Research in Vision and Ophthalmology |
| IACUC | Institutional Animal Care and Use Committee |
| GA | General anaesthesia |
| OHT | Ocular hypertension |
| SD | Spectral domain |
| EDI | Enhanced depth imaging |
| LC | Lamina cribrosa |
| BMO | Bruch’s membrane opening |
| ILM | Internal limiting membrane |
| CDR | Cup–disc ratio |
| HE | Haematoxylin and eosin |
| IHC | Immunohistochemistry |
| GFAP | Glial fibrillary acid phosphatase |
| IBA1 | Ionised calcium-binding adapter molecule 1 |
| GFAP+ | GFAP-positive |
| Ngb+ | Ngb-positive |
| C3+ | Complement 3-positive |
| M | Month |
| A | Reactive astrocytes |
References
- Artero-Castro, A.; Rodriguez-Jimenez, F.J.; Jendelova, P.; VanderWall, K.B.; Meyer, J.S.; Erceg, S. Glaucoma as a Neurodegenerative Disease Caused by Intrinsic Vulnerability Factors. Prog. Neurobiol. 2020, 193, 101817. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Levin, L.A. Glaucoma as a neurodegenerative disease. J. Neuroophthalmol. 2015, 35 (Suppl. S1), S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Yücel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Guymer, C.; Wood, J.P.; Chidlow, G.; Casson, R.J. Neuroprotection in glaucoma: Recent advances and clinical translation. Clin. Exp. Ophthalmol. 2019, 47, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R. Neuroprotection: Is it already applicable to glaucoma therapy? Curr. Opin. Ophthalmol. 2000, 11, 78–84. [Google Scholar] [CrossRef]
- Burmester, T.; Hankeln, T. Neuroglobin: A respiratory protein of the nervous system. News Physiol. Sci. 2004, 19, 110–113. [Google Scholar] [CrossRef]
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From structure to function in health and disease. Mol. Aspects Med. 2016, 52, 1–48. [Google Scholar] [CrossRef]
- Greenberg, D.A.; Jin, K.; Khan, A.A. Neuroglobin: An endogenous neuroprotectant. Curr. Opin. Pharmacol. 2008, 8, 20–24. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Peel, A.; Mao, X.O.; Xie, L.; Greenberg, D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 3497–3500. [Google Scholar] [CrossRef]
- Baez, E.; Echeverria, V.; Cabezas, R.; Ávila-Rodriguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Protection by Neuroglobin Expression in Brain Pathologies. Front. Neurol. 2016, 7, 146. [Google Scholar] [CrossRef]
- Jin, K.; Mao, Y.; Mao, X.; Xie, L.; Greenberg, D.A. Neuroglobin expression in ischemic stroke. Stroke 2010, 41, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Raida, Z.; Hundahl, C.A.; Kelsen, J.; Nyengaard, J.R.; Hay-Schmidt, A. Reduced infarct size in neuroglobin-null mice after experimental stroke in vivo. Exp. Transl. Stroke Med. 2012, 4, 15. [Google Scholar] [CrossRef]
- Raida, Z.; Hundahl, C.A.; Nyengaard, J.R.; Hay-Schmidt, A. Neuroglobin over expressing mice: Expression pattern and effect on brain ischemic infarct size. PLoS ONE 2013, 8, e76565. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, N.; Liu, J.; Yang, K.; Wang, X. Neuroglobin, a novel target for endogenous neuroprotection against stroke and neurodegenerative disorders. Int. J. Mol. Sci. 2012, 13, 6995–7014. [Google Scholar] [CrossRef]
- Wen, H.; Liu, L.; Zhan, L.; Liang, D.; Li, L.; Liu, D.; Sun, W.; Xu, E. Neuroglobin mediates neuroprotection of hypoxic postconditioning against transient global cerebral ischemia in rats through preserving the activity of Na+/K+ ATPases. Cell Death Dis. 2018, 9, 635. [Google Scholar] [CrossRef]
- Cwerman-Thibault, H.; Lechauve, C.; Augustin, S.; Roussel, D.; Reboussin, E.; Mohammad, A.; Degardin-Chicaud, J.; Simonutti, M.; Liang, H.; Brignole-Baudouin, F.; et al. Neuroglobin Can Prevent or Reverse Glaucomatous Progression in DBA/2J Mice. Mol. Ther. Methods Clin. Dev. 2017, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, Z.; Cho, K.S.; Chen, H.; Malik, M.T.; Chen, X.; Lo, E.H.; Wang, X.; Chen, D.F. Neuroglobin is an endogenous neuroprotectant for retinal ganglion cells against glaucomatous damage. Am. J. Pathol. 2011, 179, 2788–2797. [Google Scholar] [CrossRef]
- Chan, A.S.; Saraswathy, S.; Rehak, M.; Ueki, M.; Rao, N.A. Neuroglobin protection in retinal ischemia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 704–711. [Google Scholar] [CrossRef]
- Cwerman-Thibault, H.; Lechauve, C.; Malko-Baverel, V.; Augustin, S.; Le Guilloux, G.; Reboussin, É.; Degardin-Chicaud, J.; Simonutti, M.; Debeir, T.; Corral-Debrinski, M. Neuroglobin effectively halts vision loss in Harlequin mice at an advanced stage of optic nerve degeneration. Neurobiol. Dis. 2021, 159, 105483. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Augustin, S.; Roussel, D.; Sahel, J.A.; Corral-Debrinski, M. Neuroglobin involvement in visual pathways through the optic nerve. Biochim. Biophys. Acta 2013, 1834, 1772–1778. [Google Scholar] [CrossRef]
- Tun, S.B.B.; Barathi, V.A.; Luu, C.D.; Lynn, M.N.; Chan, A.S.Y. Effects of Exogenous Neuroglobin (Ngb) on retinal inflammatory chemokines and microglia in a rat model of transient hypoxia. Sci. Rep. 2019, 9, 18799. [Google Scholar] [CrossRef]
- Sugitani, K.; Koriyama, Y.; Sera, M.; Arai, K.; Ogai, K.; Wakasugi, K. A novel function of neuroglobin for neuroregeneration in mice after optic nerve injury. Biochem. Biophys. Res. Commun. 2017, 493, 1254–1259. [Google Scholar] [CrossRef]
- Lechauve, C.; Augustin, S.; Cwerman-Thibault, H.; Reboussin, E.; Roussel, D.; Lai-Kuen, R.; Saubamea, B.; Sahel, J.A.; Debeir, T.; Corral-Debrinski, M. Neuroglobin gene therapy prevents optic atrophy and preserves durably visual function in Harlequin mice. Mol. Ther. 2014, 22, 1096–1109. [Google Scholar] [CrossRef]
- Cai, B.; Lin, Y.; Xue, X.H.; Fang, L.; Wang, N.; Wu, Z.Y. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp. Neurol. 2011, 227, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.M.; Risner, M.L.; Cooper, M.L.; Wareham, L.K.; Calkins, D.J. Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration. Cells 2021, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Münch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Smith, M.D.; Jin, J.; Garton, T.; Taylor, M.; Chao, A.; Meyers, K.; Kornberg, M.D.; Zack, D.J.; Ohayon, J.; et al. Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation. Acta Neuropathol. 2021, 142, 899–915. [Google Scholar] [CrossRef]
- Chan, A.S.Y.; Tun, T.A.; Allen, J.C.; Lynn, M.N.; Tun, S.B.B.; Barathi, V.A.; Girard, M.J.A.; Aung, T.; Aihara, M. Longitudinal assessment of optic nerve head changes using optical coherence tomography in a primate microbead model of ocular hypertension. Sci. Rep. 2020, 10, 14709. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F. The non-human primate experimental glaucoma model. Exp. Eye Res. 2015, 141, 57–73. [Google Scholar] [CrossRef]
- Rasmussen, C.A.; Kaufman, P.L. Primate glaucoma models. J. Glaucoma 2005, 14, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Fortune, B.; Hardin, C.; Reynaud, J.; Cull, G.; Yang, H.; Wang, L.; Burgoyne, C.F. Comparing Optic Nerve Head Rim Width, Rim Area, and Peripapillary Retinal Nerve Fiber Layer Thickness to Axon Count in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT404–OCT412. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.; Gardiner, S.K.; Williams, G.; Hardin, C.; Strouthidis, N.G.; Fortune, B.; Burgoyne, C.F. Longitudinal detection of optic nerve head changes by spectral domain optical coherence tomography in early experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 574–586. [Google Scholar] [CrossRef]
- Pardon, L.P.; Harwerth, R.S.; Patel, N.B. Neuroretinal rim response to transient changes in intraocular pressure in healthy non-human primate eyes. Exp. Eye Res. 2020, 193, 107978. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Lynn, M.N.; Tun, S.B.B.; Barathi, V.A.; Aung, T. Bilateral intraocular pressure (IOP) changes in a non human primate (NHP) microbead model of chronic IOP elevation: Can both eyes achieve similar elevations for therapeutics evalution. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2001. [Google Scholar]
- Lambert, W.S.; Carlson, B.J.; Ghose, P.; Vest, V.D.; Yao, V.; Calkins, D.J. Towards A Microbead Occlusion Model of Glaucoma for a Non-Human Primate. Sci. Rep. 2019, 9, 11572. [Google Scholar] [CrossRef]
- Weber, A.J.; Zelenak, D. Experimental glaucoma in the primate induced by latex microspheres. J. Neurosci. Methods 2001, 111, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tun, T.A.; Sun, C.H.; Baskaran, M.; Girard, M.J.; de Leon, J.M.; Cheng, C.Y.; Htoon, H.M.; Wong, T.Y.; Aung, T.; Strouthidis, N.G. Determinants of optical coherence tomography-derived minimum neuroretinal rim width in a normal Chinese population. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3337–3344. [Google Scholar] [CrossRef]
- Tun, T.A.; Thakku, S.G.; Png, O.; Baskaran, M.; Htoon, H.M.; Sharma, S.; Nongpiur, M.E.; Cheng, C.Y.; Aung, T.; Strouthidis, N.G.; et al. Shape Changes of the Anterior Lamina Cribrosa in Normal, Ocular Hypertensive, and Glaucomatous Eyes Following Acute Intraocular Pressure Elevation. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4869–4877. [Google Scholar] [CrossRef]
- Hou, H.; Moghimi, S.; Zangwill, L.M.; Shoji, T.; Ghahari, E.; Manalastas, P.I.C.; Penteado, R.C.; Weinreb, R.N. Inter-eye Asymmetry of Optical Coherence Tomography Angiography Vessel Density in Bilateral Glaucoma, Glaucoma Suspect, and Healthy Eyes. Am. J. Ophthalmol. 2018, 190, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Structural basis of glaucoma: The fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 2012, 60, 13–28. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Koizumi, S. Potential roles of astrocytes and Muller cells in the pathogenesis of glaucoma. J. Pharmacol. Sci. 2021, 145, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, R.; Luo, X.; Wang, F.; Sun, X. The Interaction Between Microglia and Macroglia in Glaucoma. Front. Neurosci. 2021, 15, 610788. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fan, Z.; Wang, S.; Ma, L.; Wang, J.; Yu, D.; Zhang, Z.; Wu, L.; Peng, Z.; Liu, W.; et al. Astrocytic A1/A2 paradigm participates in glycogen mobilization mediated neuroprotection on reperfusion injury after ischemic stroke. J. Neuroinflamm. 2021, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Acarin, L.; Villapol, S.; Faiz, M.; Rohn, T.T.; Castellano, B.; González, B. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia 2007, 55, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.C.; Riegelsberger, U.M.; Michalk, S.; Härtig, W.; Kranz, A.; Boltze, J. Cleaved caspase-3 expression after experimental stroke exhibits different phenotypes and is predominantly non-apoptotic. Brain Res. 2011, 1381, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Anderson, S.R.; Breen, K.T.; Romero, C.O.; Steele, M.R.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Tomlinson, S.; Vetter, M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018, 26, 2379–2396. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Amri, F.; Ghouili, I.; Amri, M.; Carrier, A.; Masmoudi-Kouki, O. Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J. Neurochem. 2017, 140, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Qin, L.Y.; Zhang, C.G.; Yang, L.T.; Gao, Z.; Liu, S.; Lau, L.T.; Fung, Y.W.; Greenberg, D.A.; Yu, A.C. Presence of neuroglobin in cultured astrocytes. Glia 2005, 50, 182–186. [Google Scholar] [CrossRef]
- Huber, H.F.; Jenkins, S.L.; Li, C.; Nathanielsz, P.W. Strength of nonhuman primate studies of developmental programming: Review of sample sizes, challenges, and steps for future work. J. Dev. Orig. Health Dis. 2020, 11, 297–306. [Google Scholar] [CrossRef]
- DellaValle, B.; Hempel, C.; Kurtzhals, J.A.; Penkowa, M. In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia 2010, 58, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J. Negative surface charges in neuroglobin modulate the interaction with cytochrome c. Biochem Biophys Res. Commun 2020, 523, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Sebag, J.; Cunningham, E.T., Jr. II. E. Vitreoretinal Interface and Inner Limiting Membrane. In Vitreous: In Health and Disease; Sebag, J., Ed.; Springer: New York, NY, USA, 2014; pp. 165–191. [Google Scholar]
- Morgan, J.E. Optic nerve head structure in glaucoma: Astrocytes as mediators of axonal damage. Eye 2000, 14 Pt 3B, 437–444. [Google Scholar] [CrossRef] [PubMed]


| Baseline for IVT-Ngb (Right) Eyes | Baseline for EG (Left) Eyes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | IOP, mmHg | CDR | LCD, µm | MRW, µm | RNFL, µm | IOP, mmHg | CDR | LCD, µm | MRW, µm | RNFL, µm |
| 1 | 13.0 ± 1.2 | 0.1 | 200.7 ± 29.3 | 292.6 ± 43.6 | 108.9 ± 42.7 | 15.0 ± 1.7 | 0.1 | 192.1 ± 21.3 | 264.3 ± 52.9 | 108.9 ± 42.7 |
| 2 | 16.0 ± 1.7 | 0.2 | 213.7 ± 33.5 | 293.4 ± 57.3 | 118.6 ± 43.6 | 15.0 ± 2.3 | 0.2 | 223.6 ± 31.2 | 291.0 ± 53.1 | 118.6 ± 43.6 |
| 3 | 14.0 ± 1.9 | 0.3 | 236.4 ± 6.7 | 304.7 ± 57.4 | 119.7 ± 42.9 | 12.0 ± 1.7 | 0.3 | 221.2 ± 14.1 | 289.0 ± 55.3 | 119.7 ± 42.9 |
| 4 | 15.0 ± 0.5 | 0.2 | 221.4 ± 21.4 | 277.0 ± 47.6 | 110.0 ± 36.2 | 14.0 ± 0.6 | 0.2 | 225.4 ± 21.2 | 277.5 ± 52.4 | 110. ± 36.2 |
| 5 | 16.0 ± 2.9 | 0.3 | 193.0 ± 33.6 | 341.1 ± 66.6 | 122.6 ± 44.4 | 17.0 ± 2.1 | 0.2 | 178.1 ± 32.1 | 360.7 ± 62.3 | 122.6 ± 44.4 |
| 6 | 12.0 ± 2.3 | 0.2 | 197.7 ± 9.2 | 366.2 ± 46.8 | 115.9 ± 44.7 | 16.0 ± 1.0 | 0.2 | 217.4 ± 4.7 | 372.7 ± 46.4 | 115.9 ± 44.7 |
| Mean | 14.3 ± 1.6 | 0.2 ± 0.1 | 210.5 ± 16.5 | 312.7 ± 34.2 | 115.9 ± 5.5 | 14.8 ± 1.7 | 0.2 ± 0.1 | 209.6 ± 19.7 | 309.2 ± 45.7 | 113.2 ± 4.7 |
| Individual Animal | Baseline IOP | M1 IOP | M2 IOP | M3 IOP | M4 IOP | M5 IOP | M6 IOP | M7 IOP | M8 IOP | M9 IOP | M10 IOP | Max IOP | Mean IOP M2–10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primate 1 OD (Ngb) | 13 | 15 | 45 | 30 | 33 | 42 | 29 | 24 | 27 | 39 | 30 | 45 | 33 |
| Primate 1 OS | 15 | 13 | 20 | 25 | 28 | 25 | 23 | 36 | 47 | 40 | 32 | 47 | 31 |
| Primate 2 OD (Ngb) | 16 | 13 | 23 | 28 | 38 | 43 | 50 | 32 | 50 | 57 | 20 | 57 | 38 |
| Primate 2 OS | 12 | 15 | 66 | 56 | 35 | 27 | 27 | 36 | 46 | 56 | 31 | 66 | 42 |
| Primate 3 OD (Ngb) | 14 | 23 | 41 | 38 | 43 | 38 | 15 | 50 | 12 | 20 | 18 | 50 | 31 |
| Primate 3 OS | 14 | 15 | 25 | 44 | 43 | 28 | 21 | 26 | 22 | 33 | 23 | 44 | 29 |
| Primate 4 OD (Ngb) | 15 | 15 | 23 | 49 | 40 | 33 | 27 | 19 | 42 | 56 | 21 | 56 | 34 |
| Primate 4 OS | 17 | 15 | 54 | 24 | 40 | 77 | 56 | 34 | 33 | 45 | 23 | 77 | 43 |
| Primate 5 OD (Ngb) | 16 | 16 | 56 | 25 | 56 | 28 | 26 | 23 | 54 | 70 | 24 | 70 | 40 |
| Primate 5 OS | 15 | 11 | 41 | 26 | 28 | 29 | 23 | 26 | 27 | 50 | 30 | 50 | 31 |
| Primate 6 OD (Ngb) | 12 | 12 | 32 | 50 | 26 | 45 | 20 | 23 | 21 | 24 | 19 | 50 | 29 |
| Primate 6 OS | 16 | 18 | 27 | 31 | 30 | 32 | 22 | 29 | 21 | 33 | 25 | 33 | 28 |
| All animals | Monthly mean intraocular pressure in all six animals | Mean Max IOP | Change in IOP M2–10 | ||||||||||
| Baseline | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | |||
| Left eye, OS | 14.6 ± 5.1 | 14.4 ± 5.1 | 33.1 ± 5.1 | 26.7 ± 5.1 | 28.8 ± 5.1 | 39 ± 5.1 | 20.6 ± 5.4 | 29.5 ± 5.1 | 25.5 ± 5.1 | 22.1 ± 5.1 | 23 ± 5.1 | 53 ± 15.9 | 10.1 ± 6.6 |
| Right eye, OD (Ngb) | 15.7 ± 5.1 | 15.8 ± 5.1 | 33.8 ± 5.1 | 26.3 ± 5.1 | 42.9 ± 5.1 | 32.4 ± 5.1 | 27.3 ± 5.4 | 28.4 ± 5.1 | 26.6 ± 5.1 | 21.8 ± 5.1 | 19.2 ± 5.1 | 55 ± 8.7 | 14.6 ± 6.6 |
| p-value (OD between OS) | 0.611 | 0.543 | 0.818 | 0.742 | 0.301 | 0.836 | 0.912 | 0.607 | 0.901 | 0.871 | 0.056 | 0.810 | 0.127 |
| Time-Point | RNFL, µm | LCD, µm | MRW, µm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | OD IVT | Difference (95%CI) | p-Value | OS | OD IVT | Difference (95%CI) | p-Value | OS | OD IVT | Difference (95%CI) | p-Value | |
| Baseline | 115.93 (2.78) | 113.24 (2.78) | 2.69 (−5.12, 10.5) | 0.4961 | 216.94 (9.00) | 214.65 (9.00) | 2.29 (−22.97, 27.55) | 0.8576 | 352.38 (20.94) | 360.25 (20.94) | −7.87 (−66.63, 50.88) | 0.7909 |
| M1 | 117.79 (4.5) | 117.47 (4.5) | 0.32 (−12.32, 12.95) | 0.9604 | 225.07 (11.98) | 221.22 (11.98) | 3.86 (−29.75, 37.46) | 0.8204 | 320.07 (15.49) | 326.27 (15.49) | −6.20 (−49.67, 37.27) | 0.7778 |
| M2 | 114.69 (5.76) | 116.91 (5.76) | −2.22 (−18.39, 13.96) | 0.7864 | 257.18 (18.70) | 274.78 (18.7) | −17.61 (−70.08, 34.87) | 0.5071 | 290.92 (13.78) | 293.6 (13.78) | −2.68 (−41.35, 35.98) | 0.8907 |
| M3 | 96.17 (6.31) | 93.52 (6.31) | 2.65 (−15.06, 20.35) | 0.7675 | 291.11 (15.28) | 297.88 (15.28) | −6.78 (−49.67, 36.11) | 0.7546 | 272.86 (12.75) | 290.98 (12.75) | −18.13 (−53.9, 17.64) | 0.3171 |
| M4 | 94.91 (3.03) | 103.75 (3.03) | −8.84 (−17.34, −0.34) | 0.0417 | 313.64 (7.47) | 288.53 (7.47) | 25.11 (4.15, 46.07) | 0.0194 | 249.99 (10.18) | 282.84 (10.18) | −32.84 (−61.4, −4.28) | 0.0246 |
| M5 | 94.06 (2.33) | 103.65 (2.33) | −9.59 (−16.12, −3.06) | 0.0044 | 323.79 (7.83) | 278.12 (7.83) | 45.67 (23.7, 67.64) | <0.0001 | 232.3 (11.15) | 276.63 (11.15) | −44.33 (−75.61, −13.04) | 0.0059 |
| M6 | 93.54 (1.98) | 103.55 (1.98) | −10.01 (−15.57, −4.46) | 0.0005 | 327.92 (7.99) | 282.57 (7.99) | 45.35 (22.94, 67.75) | 0.0001 | 209.64 (10.12) | 262.86 (10.12) | −53.22 (−81.62, −24.81) | 0.0003 |
| M7 | 92.09 (2.14) | 103.91 (2.14) | −11.82 (−17.83, −5.81) | 0.0002 | 333.87 (6.39) | 287.1 (6.39) | 46.76 (28.82, 64.71) | <0.0001 | 199.3 (10.11) | 248.01 (10.11) | −48.71 (−77.09, −20.33) | 0.0010 |
| M8 | 92.04 (2.67) | 104.07 (2.67) | −12.02 (−19.51, −4.53) | 0.0019 | 338.94 (6.86) | 293.4 (6.86) | 45.54 (26.3, 64.79) | <0.0001 | 184.48 (9.65) | 238.87 (9.65) | −54.40 (−81.47, −27.33) | 0.0001 |
| M9 | 93.37 (3.07) | 105.89 (3.07) | −12.53 (−21.13, −3.92) | 0.0048 | 344.55 (7.25) | 302.16 (7.25) | 42.39 (22.04, 62.74) | <0.0001 | 177.66 (8.87) | 224.25 (8.87) | −46.59 (−71.49, −21.69) | 0.0003 |
| M10 | 89.07 (3.04) | 106.17 (3.04) | −17.09 (−25.63, −8.56) | 0.0001 | 358.27 (7.45) | 307.78 (7.45) | 50.49 (29.59, 71.38) | <0.0001 | 165.16 (11.89) | 212.27 (11.89) | −47.11 (−80.49, −13.74) | 0.0061 |
| Percentage change at M10 from M2 | −23.04 (9.22) | −6.10 (5.85) | −16.94 (−27.12, −6.76) | 0.0047 | 65.31 (11.59) | 43.85 (9.85) | 21.46 (7.57, 35.35) | 0.0064 | −52.84 (11.18) | −40.81 (9.78) | −12.03 (−25.58, 1.51) | 0.0759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, A.S.Y.; Tun, S.B.B.; Lynn, M.N.; Ho, C.; Tun, T.A.; Girard, M.J.A.; Sultana, R.; Barathi, V.A.; Aung, T.; Aihara, M. Intravitreal Neuroglobin Mitigates Primate Experimental Glaucomatous Structural Damage in Association with Reduced Optic Nerve Microglial and Complement 3-Astrocyte Activation. Biomolecules 2023, 13, 961. https://doi.org/10.3390/biom13060961
Chan ASY, Tun SBB, Lynn MN, Ho C, Tun TA, Girard MJA, Sultana R, Barathi VA, Aung T, Aihara M. Intravitreal Neuroglobin Mitigates Primate Experimental Glaucomatous Structural Damage in Association with Reduced Optic Nerve Microglial and Complement 3-Astrocyte Activation. Biomolecules. 2023; 13(6):961. https://doi.org/10.3390/biom13060961
Chicago/Turabian StyleChan, Anita S. Y., Sai B. B. Tun, Myoe N. Lynn, Candice Ho, Tin A. Tun, Michaël J. A. Girard, Rehena Sultana, Veluchamy A. Barathi, Tin Aung, and Makoto Aihara. 2023. "Intravitreal Neuroglobin Mitigates Primate Experimental Glaucomatous Structural Damage in Association with Reduced Optic Nerve Microglial and Complement 3-Astrocyte Activation" Biomolecules 13, no. 6: 961. https://doi.org/10.3390/biom13060961
APA StyleChan, A. S. Y., Tun, S. B. B., Lynn, M. N., Ho, C., Tun, T. A., Girard, M. J. A., Sultana, R., Barathi, V. A., Aung, T., & Aihara, M. (2023). Intravitreal Neuroglobin Mitigates Primate Experimental Glaucomatous Structural Damage in Association with Reduced Optic Nerve Microglial and Complement 3-Astrocyte Activation. Biomolecules, 13(6), 961. https://doi.org/10.3390/biom13060961







