β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for β3 Agonists as Tocolytics
Abstract
1. Introduction
Current Tocolytic Management
2. Dysregulated Pathways in Myometrium
2.1. Calcium
2.2. NO Signaling Exception
2.3. S-Nitrosation
2.4. Nitric Oxide Synthase
3. β3 Adrenergic Receptors
3.1. β3 Adrenergic Receptor Pharmacology
3.2. β3 Adrenergic Receptor Signaling
4. Src Kinase
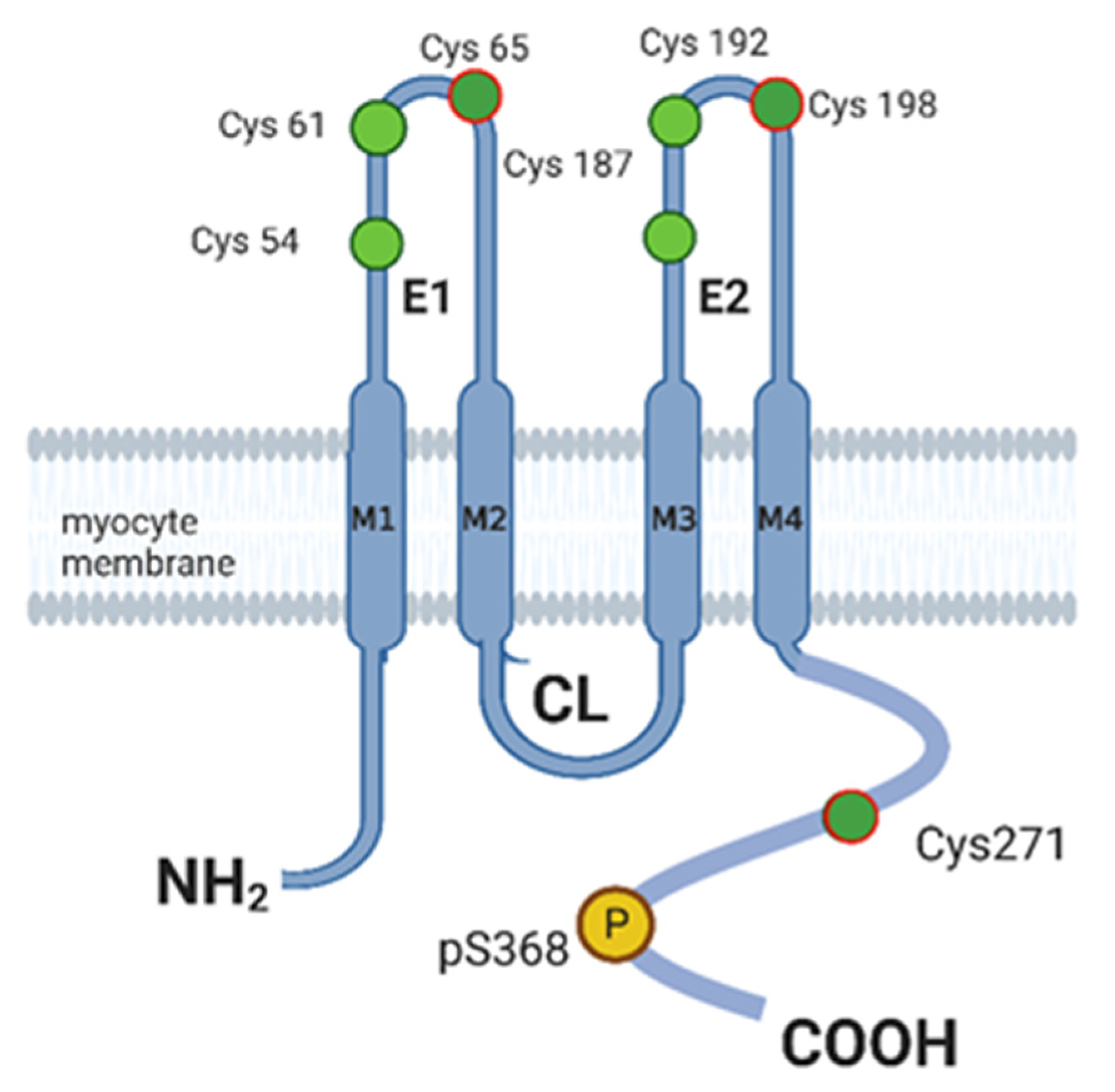
5. Connexin 43
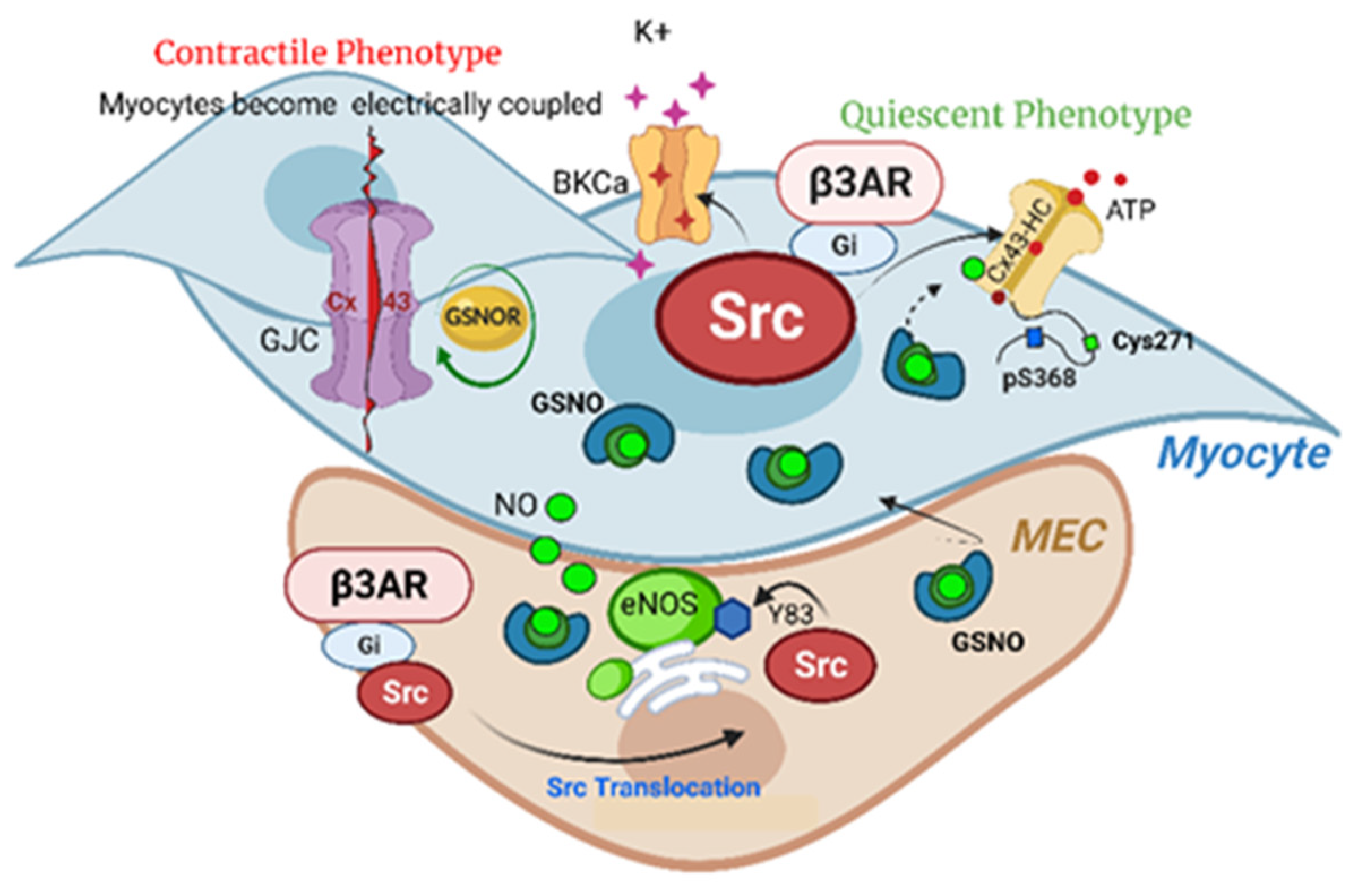
6. Conclusions
Funding
Conflicts of Interest
References
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F.A. The Worldwide Incidence of Preterm Birth: A Systematic Review of Maternal Mortality and Morbidity. Bull. World Health Organ. 2010, 88, 31. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and Causes of Preterm Birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, M.H.; Carpenter, M.; Bourjeily, G. Preterm Birth Disparities between States in the United States: An Opportunity for Public Health Interventions. J. Psychosom. Obstet. Gynecol. 2019, 41, 38–46. [Google Scholar] [CrossRef]
- Korinek, K.; Ahmmad, Z. The Racial Configuration of Parent Couples and Premature Birth: An Analysis of the Utah Population Database. J. Racial Ethn. Health Disparities 2021, 9, 655–669. [Google Scholar] [CrossRef]
- Manuck, T.A. Racial and Ethnic Differences in Preterm Birth: A Complex, Multifactorial Problem. Semin. Perinatol. 2017, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Åkerlund, M.; Carlsson, A.M.; Melin, P.; Trojnar, J. The Effect on the Human Uterus of Two Newly Developed Competitive Inhibitors of Oxytocin and Vasopressin. Acta Obstet. Gynecol. Scand. 1985, 64, 499–504. [Google Scholar] [CrossRef]
- Couceiro Naveira, E.; López Ramón y Cajal, C. Atosiban versus Ritodrine as Tocolytics in External Cephalic Version. J. Matern. Fetal. Neonatal Med. 2022, 35, 80–85. [Google Scholar] [CrossRef]
- Chang, C.Y.; Nguyen, C.P.; Wesley, B.; Guo, J.; Johnson, L.L.; Joffe, H.V. Withdrawing Approval of Makena—A Proposal from the FDA Center for Drug Evaluation and Research. N. Engl. J. Med. 2020, 383, e131. [Google Scholar] [CrossRef] [PubMed]
- Giouleka, S.; Tsakiridis, I.; Kostakis, N.; Koutsouki, G.; Kalogiannidis, I.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Preterm Labor: A Comprehensive Review of Guidelines on Diagnosis, Management, Prediction and Prevention. Obs. Gynecol. Surv. 2022, 77, 302–317. [Google Scholar] [CrossRef]
- Brookfield, K.; Vinson, A. Magnesium Sulfate Use for Fetal Neuroprotection. Curr. Opin. Obs. Gynecol. 2019, 31, 110–115. [Google Scholar] [CrossRef]
- Lamont, R.F.; Jørgensen, J.S. Safety and Efficacy of Tocolytics for the Treatment of Spontaneous Preterm Labour. Curr. Pharm. Des. 2019, 25, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Amberg, G.C.; Navedo, M.F. Calcium Dynamics in Vascular Smooth Muscle. Microcirculation 2013, 20, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.; Jones, K.; Kupittayanant, S.; Li, Y.; Matthew, A.; Monir-Bishty, E.; Noble, K.; Pierce, S.J.; Quenby, S.; Shmygol, A. V Calcium Signaling and Uterine Contractility. J. Soc. Gynecol. Investig. 2003, 10, 252–264. [Google Scholar] [CrossRef]
- van Vliet, E.; Dijkema, G.; Schuit, E.; Heida, K.; Roos, C.; van der Post, J.; Parry, E.; McCowan, L.; Lyell, D.; El-Sayed, Y.; et al. Nifedipine Maintenance Tocolysis and Perinatal Outcome: An Individual Participant Data Meta-Analysis. BJOG An Int. J. Obstet. Gynaecol. 2016, 123, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Songthamwat, S.; Na Nan, C.; Songthamwat, M. Effectiveness of Nifedipine in Threatened Preterm Labor: A Randomized Trial. Int. J. Womens Health 2018, 10, 317–323. [Google Scholar] [CrossRef]
- Nijman, T.A.J.; van Vliet, E.O.G.; Naaktgeboren, C.A.; Oude Rengerink, K.; de Lange, T.S.; Bax, C.J.; Bloemenkamp, K.W.M.; van Eyck, J.; Kok, M.; Scheepers, H.C.J.; et al. Nifedipine versus Placebo in the Treatment of Preterm Prelabor Rupture of Membranes: A Randomized Controlled Trial: Assessment of Perinatal Outcome by Use of Tocolysis in Early Labor—APOSTEL IV Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Buxton, I.L.O.L.O.; Brunton, L.L.L. Compartments of Cyclic AMP and Protein Kinase in Mammalian Cardiomyocytes. J. Biol. Chem. 1983, 258, 10233–10239. [Google Scholar] [CrossRef]
- Barnett, S.D.; Asif, H.; Buxton, I.L.O. Novel Identification and Modulation of the Mechanosensitive Piezo1 Channel in Human Myometrium. J. Physiol. 2023, 601, 1675–1690. [Google Scholar] [CrossRef]
- Chan, Y.-W.; van den Berg, H.A.; Moore, J.D.; Quenby, S.; Blanks, A.M. Assessment of Myometrial Transcriptome Changes Associated with Spontaneous Human Labour by High-Throughput RNA-Seq. Exp. Physiol. 2014, 99, 510–524. [Google Scholar] [CrossRef]
- Cowles, C.L.; Wu, Y.-Y.; Barnett, S.D.; Lee, M.T.; Burkin, H.R.; Buxton, I.L.O. Alternatively Spliced Human TREK-1 Variants Alter TREK-1 Channel Function and Localization. Biol. Reprod. 2015, 93, 122. [Google Scholar] [CrossRef]
- Lorca, R.A.; Prabagaran, M.; England, S.K. Functional Insights into Modulation of BKCa Channel Activity to Alter Myometrial Contractility. Front. Physiol. 2014, 5, 289. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Oberti, C.; Perez, G.J.; Perez-Reyes, N.; McDougall, J.K.; Monga, M.; Sanborn, B.M.; Stefani, E.; Toro, L. Calcium-Activated K+ Channels as Modulators of Human Myometrial Contractile Activity. Am. J. Physiol. Physiol. 1993, 265, C976–C985. [Google Scholar] [CrossRef]
- Choudhury, S.; Garg, S.K.; Singh, T.U.; Mishra, S.K. Functional and Molecular Characterization of Maxi K+-Channels (BKCa) in Buffalo Myometrium. Anim. Reprod. Sci. 2011, 126, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.-P. Selective Activation of Ca2+-Dependent K+ Channels by Novel Benzimidazolone. Eur. J. Pharmacol. 1994, 4, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, A.; Augustynek, B.; Zochowska, M.; Szewczyk, A. Mitochondrial Potassium Channels as Druggable Targets. Biomokecules 2020, 10, 1200. [Google Scholar] [CrossRef]
- Barnett, S.D.; Smith, C.R.; Ulrich, C.C.; Baker, J.E.; Buxton, I.L.O. S-Nitrosoglutathione Reductase Underlies the Dysfunctional Relaxation to Nitric Oxide in Preterm Labor. Sci. Rep. 2018, 8, 5614. [Google Scholar] [CrossRef]
- Tichenor, S.D.; Malmquist, N.A.; Buxton, I.L. Dissociation of CGMP Accumulation and Relaxation in Myometrial Smooth Muscle: Effects of S-Nitroso-N-Acetylpenicillamine and 3-Morpholinosyndonimine. Cell. Signal. 2003, 15, 763–772. [Google Scholar] [CrossRef]
- Bradley, K.K.; Buxton, I.L.; Barber, J.E.; McGaw, T.; Bradley, M.E. Nitric Oxide Relaxes Human Myometrium by a CGMP-Independent Mechanism. Am. J. Physiol. 1998, 275, C1668–C1673. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.D.; Buxton, I.L.O. Hiding in Plain Sight: Nebivolol Exhibits Compelling Tocolytic Properties. J. Cell. Mol. Med. 2018, 22, 6391–6395. [Google Scholar] [CrossRef]
- Buxton, I.L.; Kaiser, R.A.; Malmquist, N.A.; Tichenor, S. NO-Induced Relaxation of Labouring and Non-Labouring Human Myometrium Is Not Mediated by Cyclic GMP. Br. J. Pharamcology 2001, 134, 206–214. [Google Scholar] [CrossRef]
- Buxton, I.L.O. Regulation of Uterine Function: A Biochemical Conundrum in the Regulation of Smooth Muscle Relaxation. Mol. Pharmacol. 2004, 65, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Buxton, I.L.; Milton, D.; Barnett, S.D.; Tichenor, S.D. Agonist-Specific Compartmentation of CGMP Action in Myometrium. J. Pharmacol. Exp. Ther. 2010, 335, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-Nitrosothiol Formation and Selectivity in Nitric Oxide Signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wu, J.; Liang, Z.; Zhang, Y.; Huang, Z. Protein S-Nitrosation: Biochemistry, Identification, Molecular Mechanisms, and Therapeutic Applications. J. Med. Chem. 2022, 65, 5902–5925. [Google Scholar] [CrossRef]
- Bartlett, S.R.; Bennett, P.R.; Campa, J.S.; Dennes, W.J.; Slater, D.M.; Mann, G.E.; Poston, L.; Poston, R. Expression of Nitric Oxide Synthase Isoforms in Pregnant Human Myometrium. J. Physiol. 1999, 521 Pt 3, 705–716. [Google Scholar] [CrossRef]
- Everett, T.R.; Wilkinson, I.B.; Lees, C.C. Pre-Eclampsia: The Potential of GSNO Reductase Inhibitors. Curr. Hypertens. Rep. 2017, 19, 20. [Google Scholar] [CrossRef]
- Valdes, G.; Corthorn, J. Review: The Angiogenic and Vasodilatory Utero-Placental Network. Placenta 2011, 32 (Suppl. S2), S170–S175. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Lye, S.J. Myometrial Immune Cells Contribute to Term Parturition, Preterm Labour and Post-Partum Involution in Mice. J. Cell. Mol. Med. 2012, 17, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Antonakopoulos, N.; Sifakis, S.; Tsikouras, P.; Daniilidis, A.; Dafopoulos, K.; Botsis, D.; Vrachnis, N. Endocrine, Paracrine, and Autocrine Placental Mediators in Labor. Hormones 2012, 11, 397–409. [Google Scholar] [CrossRef]
- Toda, N.; Toda, H.; Okamura, T. Regulation of Myometrial Circulation and Uterine Vascular Tone by Constitutive Nitric Oxide. Eur. J. Pharmacol. 2013, 714, 414–423. [Google Scholar] [CrossRef]
- Ulrich, C.; Quilici, D.R.; Schlauch, K.A.; Buxton, I.L.O. The Human Uterine Smooth Muscle S-Nitrosoproteome Fingerprint in Pregnancy, Labor, and Preterm Labor. AJP Cell Physiol. 2013, 305, C803–C816. [Google Scholar] [CrossRef]
- Norman, J.E.; Thompson, A.J.; Telfer, J.F.; Young, A.; Greer, I.A.; Cameron, I.T. Myometrial Constitutive Nitric Oxide Synthase Expression Is Increased during Human Pregnancy. Mol. Hum. Reprod. 1999, 5, 175–181. [Google Scholar] [CrossRef]
- Ogando, D.; Farina, M.; Ribeiro, M.L.; Perez Martinez, S.; Cella, M.; Rettori, V.; Franchi, A. Steroid Hormones Augment Nitric Oxide Synthase Activity and Expression in Rat Uterus. Reprod. Fertil. Dev. 2003, 15, 269. [Google Scholar] [CrossRef]
- Asif, H.; Barnett, S.D.; Buxton, I.L.O. Β3 Adrenergic Receptor Signaling in the Human Myometrium. Reprod. Sci. 2023, 30, 124–134. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of Nitric Oxide Synthase in Endothelial Cells by Akt-Dependent Phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.A.; Lin, A.J.; Golan, D.E.; Michel, T. Receptor-Regulated Dynamic S-Nitrosylation of Endothelial Nitric-Oxide Synthase in Vascular Endothelial Cells. J. Biol. Chem. 2005, 280, 19888–19894. [Google Scholar] [CrossRef] [PubMed]
- Feron, O.; Balligand, J.L. Caveolins and the Regulation of Endothelial Nitric Oxide Synthase in the Heart. Cardiovasc. Res. 2006, 69, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Church, J.E.; Ruan, L.; Li, C.; Sood, S.G.; Kemp, B.E.; Jennings, I.G.; Venema, R.C. Src Kinase Activates Endothelial Nitric-Oxide Synthase by Phosphorylating Tyr-83. J. Biol. Chem. 2005, 280, 35943–35952. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Belcastro, E.; Wu, W.; Fries-Raeth, I.; Corti, A.; Pompella, A.; Leroy, P.; Lartaud, I.; Gaucher, C. Oxidative Stress Enhances and Modulates Protein S-Nitrosation in Smooth Muscle Cells Exposed to S-Nitrosoglutathione. Nitric Oxide 2017, 15, 114–124. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular Characterization of the Human Β3-Adrenergic Receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Breuiller-Fouché, M.; Mercier, F.J.; Leroy, M.J.; Loustalot, C.; Naline, E.; Frydman, R.; Croci, T.; Morcillo, E.J.; Advenier, C.; et al. The Human Near-Term Myometrial β 3 -Adrenoceptor but Not the β 2 -Adrenoceptor Is Resistant to Desensitisation after Sustained Agonist Stimulation. Br. J. Pharmacol. 2004, 141, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Simard, P.-M.; Atgié, C.; Mauriège, P.; D’Allaire, F.; Bukowiecki, L.J. Comparison of the Lipolytic Effects of Norepinephrine and BRL 37344 in Rat Brown and White Adipocytes. Obes. Res. 1994, 2, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, D.S.; Chernogubova, E.; Dallner, O.S.; Cannon, B.; Bengtsson, T. β-Adrenoceptors, but Not α-Adrenoceptors, Stimulate AMP-Activated Protein Kinase in Brown Adipocytes Independently of Uncoupling Protein-1. Diabetologia 2005, 48, 2386–2395. [Google Scholar] [CrossRef]
- Deeks, E.D. Mirabegron: A Review in Overactive Bladder Syndrome. Drugs 2018, 78, 833–844. [Google Scholar] [CrossRef]
- Rouget, C.; Bardou, M.; Breuiller-Fouché, M.; Loustalot, C.; Qi, H.; Naline, E.; Croci, T.; Cabrol, D.; Advenier, C.; Leroy, M.J. β 3 -Adrenoceptor Is the Predominant β-Adrenoceptor Subtype in Human Myometrium and Its Expression Is Up-Regulated in Pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Doheny, H.C.; Lynch, C.M.; Smith, T.J.; Morrison, J.J. Functional Coupling of Β3-Adrenoceptors and Large Conductance Calcium-Activated Potassium Channels in Human Uterine Myocytes. J. Clin. Endocrinol. Metab. 2005, 90, 5786–5796. [Google Scholar] [CrossRef]
- Croci, T.; Cecchi, R.; Marini, P.; Viviani, N.; Germain, G.; Guagnini, F.; Fradin, Y.; Descamps, L.; Pascal, M.; Advenier, C.; et al. In Vitro and in Vivo Pharmacological Characterization of Ethyl-4-{trans-4-[((2S)-2-Hydroxy-3-{4-Hydroxy-3[(Methylsulfonyl)Amino]-Phenoxy}propyl) Amino]Cyclohexyl}benzoate Hydrochloride (SAR150640), a New Potent and Selective Human 3-Adrenoceptor Agonist F. Experiment 2007, 321, 1118–1126. [Google Scholar] [CrossRef]
- Cha, K.-S.; Lee, W.-C.; Rudzik, A.; Miller, J.W. A Comparison of the Catecholamine Concentrations of Uteri from Several Species and the Alterations Which Occur during Pregnancy. J. Pharmacol. Exp. Ther. 1965, 148, 9–13. [Google Scholar] [PubMed]
- Wikland, M.; Lindblom, B.; Dahlstrom, A.; Haglid, K.G. Structural and Functional Evidence for the Denervation of Human Myometrium during Preganancy. Obstet. Gynecol. 1984, 64, 503–509. [Google Scholar]
- Buxton, I.L.O. Pharmacokinetics and Pharmacodynamics. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Brunton, L., Chabner, B., Knollman, B., Eds.; Mcgraw-Hill: New York, NY, USA, 2006; pp. 1–39. ISBN 0-07-142280-3. [Google Scholar]
- Tagaya, E.; Tamaoki, J.; Takemura, H.; Isono, K.; Nagai, A. Atypical Adrenoceptor-Mediated Relaxation of Canine Pulmonary Artery Through a Cyclic Adenosine Monophosphate-Dependent Pathway. Lung 1999, 177, 321–332. [Google Scholar] [CrossRef]
- Dessy, C.; Balligand, J.L. Beta3-Adrenergic Receptors in Cardiac and Vascular Tissues. Emerging Concepts and Therapeutic Perspectives. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2010; Volume 59, pp. 135–163. [Google Scholar]
- Fincham, V. Functions of the V-SRC Protein Tyrosine Kinase. Cell Biol. Int. 1994, 18, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Src Protein-Tyrosine Kinase Structure and Regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Cooper, J.A. Regulation, Substrates and Functions of Src. Biochim. Biophys. Acta 1996, 1287, 121–149. [Google Scholar] [CrossRef]
- Anguita, E.; Villalobo, A. Src-Family Tyrosine Kinases and the Ca2+ Signal. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 915–932. [Google Scholar] [CrossRef]
- Anguita, E.; Villalobo, A. Ca2+ Signaling and Src-Kinases-Controlled Cellular Functions. Arch. Biochem. Biophys. 2018, 650, 59–74. [Google Scholar] [CrossRef]
- Szal, S.E.; Repke, J.T.; Seely, E.W.; Graves, S.W.; Parker, C.A.; Morgan, K.G. [Ca2+]i Signaling in Pregnant Human Myometrium. Am. J. Physiol. Metab. 1994, 267, E77–E87. [Google Scholar] [CrossRef]
- Alioua, A.; Mahajan, A.; Nishimaru, K.; Zarei, M.M.; Stefani, E.; Toro, L. Coupling of C-Src to Large Conductance Voltage-and Ca 2-Activated K Channels as a New Mechanism of Agonist-Induced Vasoconstriction. Proc. Natl. Acad. Sci. USA 2002, 99, 14560–14565. [Google Scholar] [CrossRef]
- Gui, P.; Chao, J.T.; Wu, X.; Yang, Y.; Davis, G.E.; Davis, M.J. Coordinated Regulation of Vascular Ca2+ and K+ Channels by Integrin Signaling. Adv. Exp. Med. Biol. 2010, 674, 69–79. [Google Scholar]
- Cao, W.; Luttrell, L.M.; Medvedev, A.V.; Pierce, K.L.; Daniel, K.W.; Dixon, T.M.; Lefkowitz, R.J.; Collins, S. Direct Binding of Activated C-Src to the Β3-Adrenergic Receptor Is Required for MAP Kinase Activation. J. Biol. Chem. 2000, 275, 38131–38134. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, Connexons, and Intercellular Communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef]
- Contreras, J.E.; Sáez, J.C.; Bukauskas, F.F.; Bennett, M.V.L. Gating and Regulation of Connexin 43 (Cx43) Hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef]
- Pohl, U. Connexins: Key Players in the Control of Vascular Plasticity and Function. Physiol. Rev. 2020, 100, 525–572. [Google Scholar] [CrossRef]
- Straub, A.C.; Billaud, M.; Johnstone, S.R.; Best, A.K.; Yemen, S.; Dwyer, S.T.; Looft-Wilson, R.; Lysiak, J.J.; Gaston, B.; Palmer, L.; et al. Compartmentalized Connexin 43 S-Nitrosylation/Denitrosylation Regulates Heterocellular Communication in the Vessel Wall. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chang, H.; Wu, Y.; Zhou, L.; Wang, Y.; Wang, M.; Wu, P.; Qi, Z.; Zou, J. Up-Regulated Cx43 Phosphorylation at Ser368 Prolongs QRS Duration in Myocarditis. J. Cell. Mol. Med. 2018, 22, 3537–3547. [Google Scholar] [CrossRef] [PubMed]
- Lillo, M.A.; Himelman, E.; Shirokova, N.; Xie, L.-H.; Fraidenraich, D.; Contreras, J.E. S-Nitrosylation of Connexin43 Hemichannels Elicits Cardiac Stress–Induced Arrhythmias in Duchenne Muscular Dystrophy Mice. JCI Insight 2019, 4, e130091. [Google Scholar] [CrossRef]
- Miyoshi, H.; Boyle, M.B.; MacKay, L.B.; Garfield, R.E. Voltage-Clamp Studies of Gap Junctions between Uterine Muscle Cells during Term and Preterm Labor. Biophys. J. 1996, 71, 1324–1334. [Google Scholar] [CrossRef]
- Young, R.C. Myocytes, Myometrium, and Uterine Contractions. Ann. N. Y. Acad. Sci. 2007, 1101, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.D.; Asif, H.; Anderson, M.; Buxton, I.L.O. Novel Tocolytic Strategy: Modulating Cx43 Activity by S-Nitrosation. J. Pharmacol. Exp. Ther. 2021, 376, 444–453. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Pedroni, S.M.A.; Girardi, G. Statins Prevent Cervical Remodeling, Myometrial Contractions and Preterm Labor through a Mechanism That Involves Hemoxygenase-1 and Complement Inhibition. Mol. Hum. Reprod. 2014, 20, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ji, H.; Liu, H.; Liu, J.; Gu, W.; Peng, T.; Li, X. Pro-Inflammatory Cytokine-Induced MicroRNA-212-3p Expression Promotes Myocyte Contraction via Methyl-CpG-Binding Protein 2: A Novel Mechanism for Infection-Related Preterm Parturition. MHR Basic Sci. Reprod. Med. 2019, 25, 274–282. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Mesiano, S.; Lye, S. Progesterone Via Its Type-A Receptor Promotes Myometrial Gap Junction Coupling. Sci. Rep. 2017, 7, 13357. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Specific Cx43 Phosphorylation Events Regulate Gap Junction Turnover in Vivo. FEBS Lett. 2014, 588, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin43 Phosphorylation: Structural Changes and Biological Effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef]
- Solan, J.L.; Marquez-Rosado, L.; Sorgen, P.L.; Thornton, P.J.; Gafken, P.R.; Lampe, P.D. Phosphorylation at S365 Is a Gatekeeper Event That Changes the Structure of Cx43 and Prevents Down-Regulation by PKC. J. Cell Biol. 2007, 179, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Musil, L.S.; Cunningham, B.A.; Edelman, G.M.; Goodenough, D.A. Differential Phosphorylation of the Gap Junction Protein Connexin43 in Junctional Communication-Competent and -Deficient Cell Lines. J. Cell Biol. 1990, 111, 2077–2088. [Google Scholar] [CrossRef]
- Chevallier, D.; Carette, D.; Segretain, D.; Gilleron, J.; Pointis, G. Connexin 43 a Check-Point Component of Cell Proliferation Implicated in a Wide Range of Human Testis Diseases. Cell. Mol. Life Sci. 2013, 70, 1207–1220. [Google Scholar] [CrossRef]
- Kandouz, M.; Batist, G. Gap Junctions and Connexins as Therapeutic Targets in Cancer. Expert Opin. Ther. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef]
- Asif, H.; Barnett, S.D.; Saxon, D.; Younis, H.; Buxton, I.L.O. Β3 Adrenergic Receptor Activation Modulates Connexin 43 Activity to Relax Human Myometrium. Cell. Signal. 2023, 106, 110640. [Google Scholar] [CrossRef] [PubMed]
- Navathe, R.; Berghella, V. Tocolysis for Acute Preterm Labor: Where Have We Been, Where Are We Now, and Where Are We Going? Am. J. Perinatol. 2016, 33, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Behrman, R.E.; Butler, A.S. (Eds.) Preterm Birth: Causes, Consequences, and Prevention; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Scaffidi, J.; Mol, B.; Keelan, J. The Pregnant Women as a Drug Orphan: A Global Survey of Registered Clinical Trials of Pharmacological Interventions in Pregnancy. BJOG An Int. J. Obstet. Gynaecol. 2016, 124, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Whitaker, M.; O’Halloran, A.; Chai, S.J.; Kirley, P.D.; Alden, N.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; Anderson, E.J.; et al. Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19—COVID-NET, 13 States, March 1–August 22, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1347–1354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buxton, I.L.O.; Asif, H.; Barnett, S.D. β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for β3 Agonists as Tocolytics. Biomolecules 2023, 13, 1005. https://doi.org/10.3390/biom13061005
Buxton ILO, Asif H, Barnett SD. β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for β3 Agonists as Tocolytics. Biomolecules. 2023; 13(6):1005. https://doi.org/10.3390/biom13061005
Chicago/Turabian StyleBuxton, Iain L. O., Hazik Asif, and Scott D. Barnett. 2023. "β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for β3 Agonists as Tocolytics" Biomolecules 13, no. 6: 1005. https://doi.org/10.3390/biom13061005
APA StyleBuxton, I. L. O., Asif, H., & Barnett, S. D. (2023). β3 Receptor Signaling in Pregnant Human Myometrium Suggests a Role for β3 Agonists as Tocolytics. Biomolecules, 13(6), 1005. https://doi.org/10.3390/biom13061005





