The Acute Radiation Syndrome-Mitigator Romiplostim and Secreted Extracellular Vesicles Improved Survival in Mice Acutely Exposed to Myelosuppressive Doses of Ionizing Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Experimental Design
2.2. In Vivo TBI with X-rays
2.3. Administration of ARS Protective/Mitigative Agents
2.4. Serum Collection
2.5. EV Purification
2.6. Estimation of the Total Amount of Recovered EVs
2.7. Detection of Membrane Surface Proteins of EVs
2.8. Administration of EVs to Mice with Severe ARS
2.9. RNA Extraction from Purified EVs
2.10. Microarray Analysis
2.11. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.12. Functional Annotation of miRNA
2.13. Statistical Analysis
3. Results
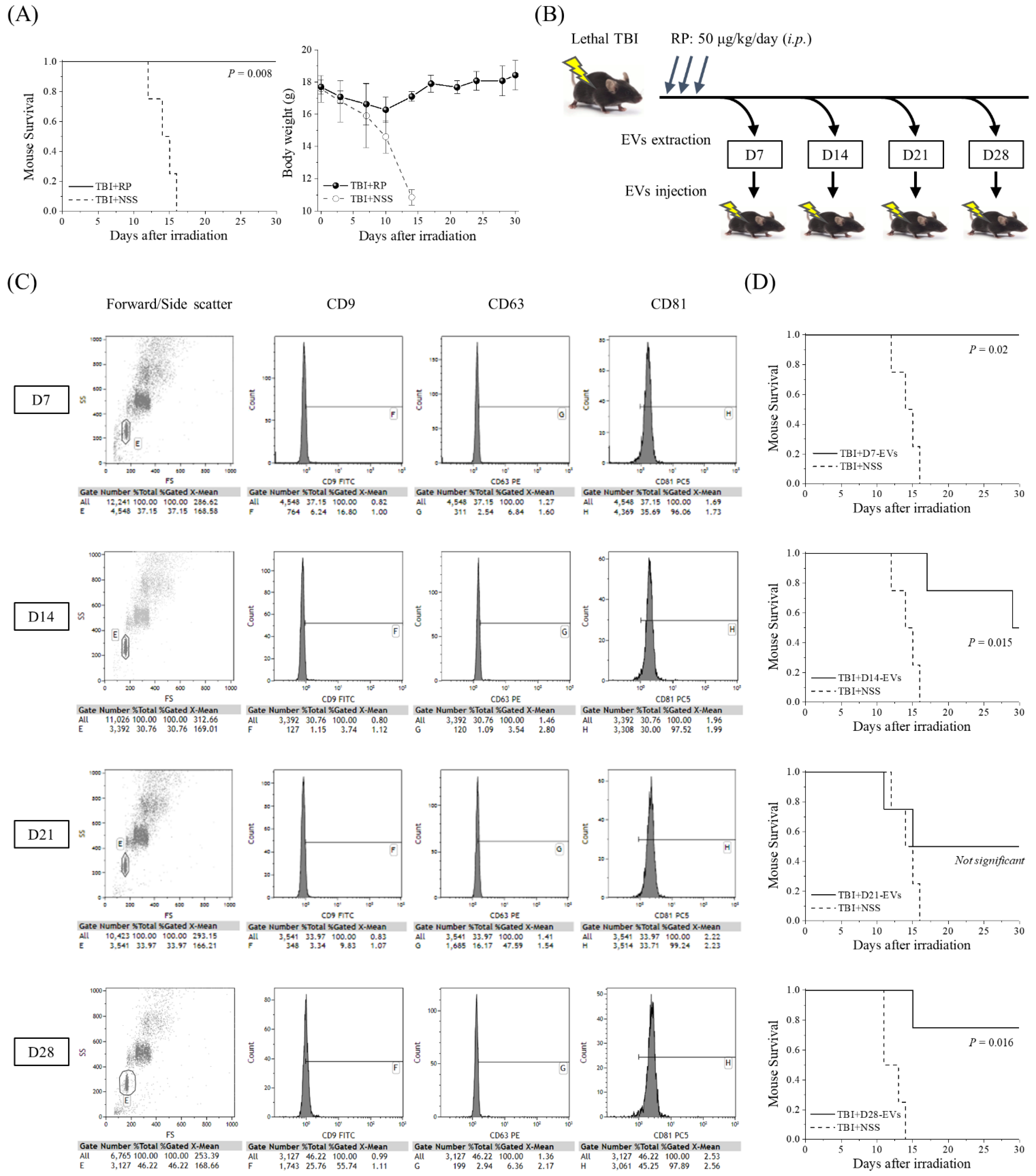
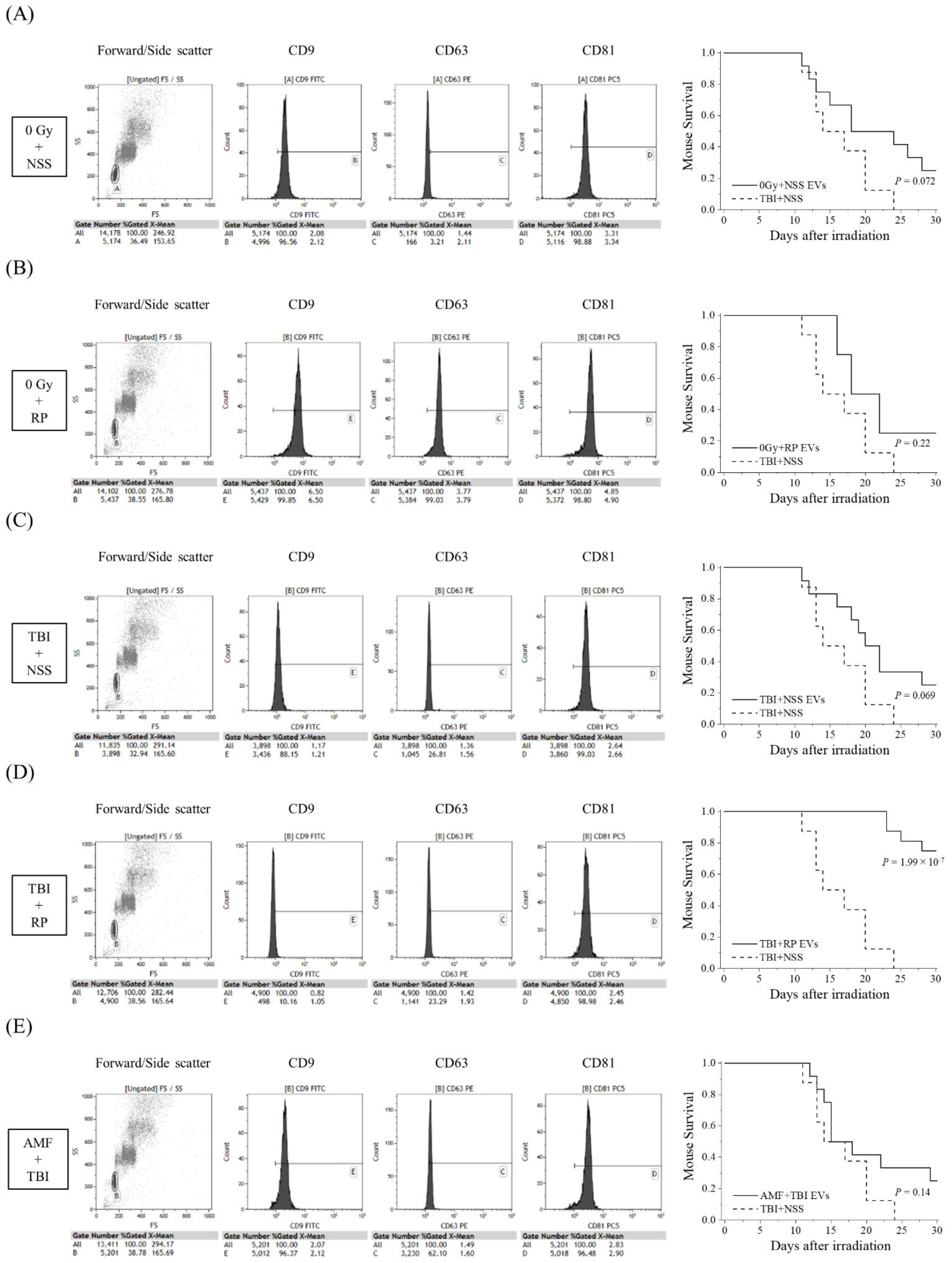
3.1. EVs Collected from Mice That Overcame ARS with RP Treatment Had Life-Saving Effects in Mice with Severe ARS
3.2. EVs That Contribute to the Survival of Mice with ARS Were Present in the Blood of Mice That Overcame ARS
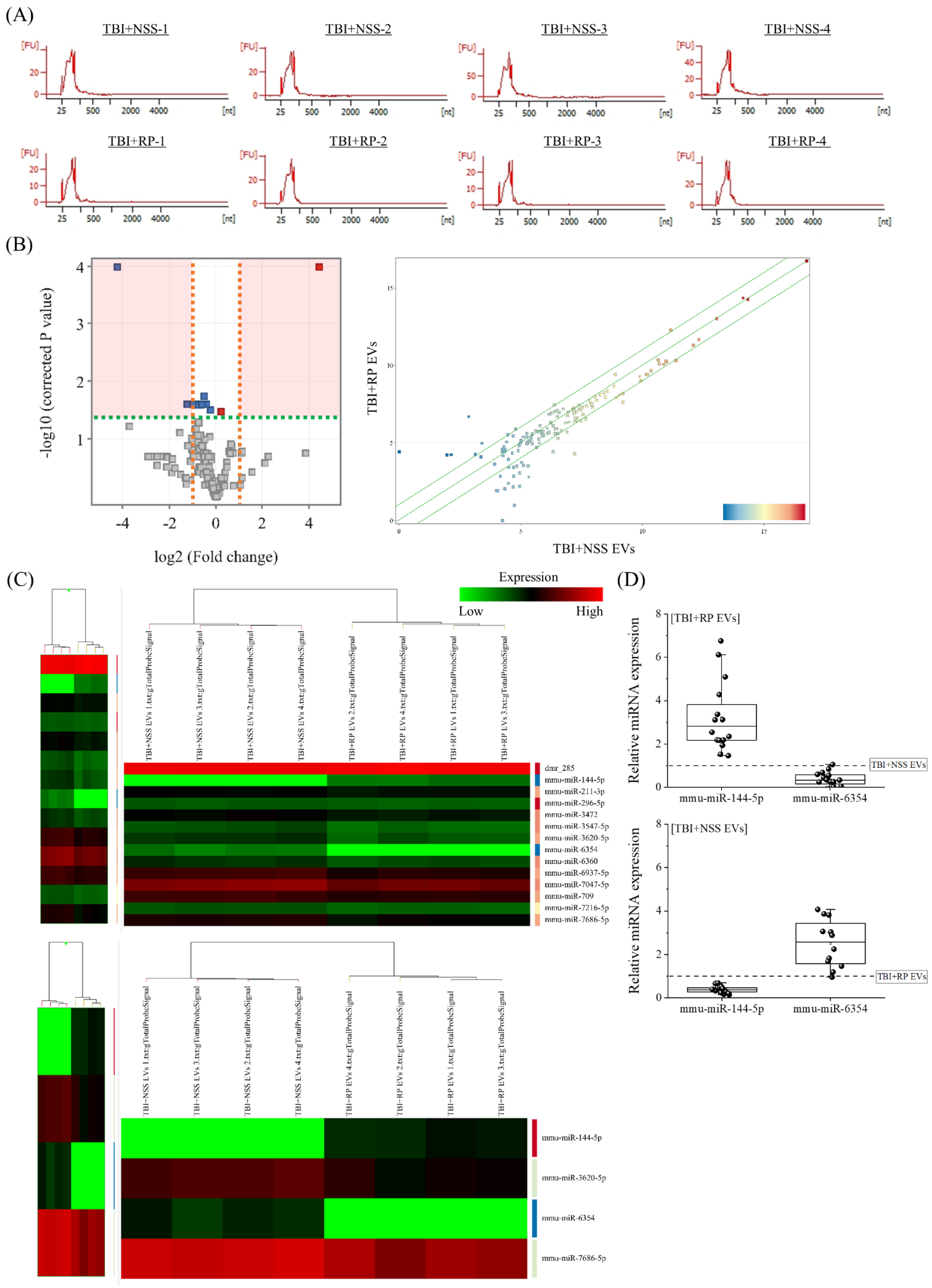
3.3. Specific miRNA Contained in EVs That Have Life-Saving Effects in Mice with Severe ARS
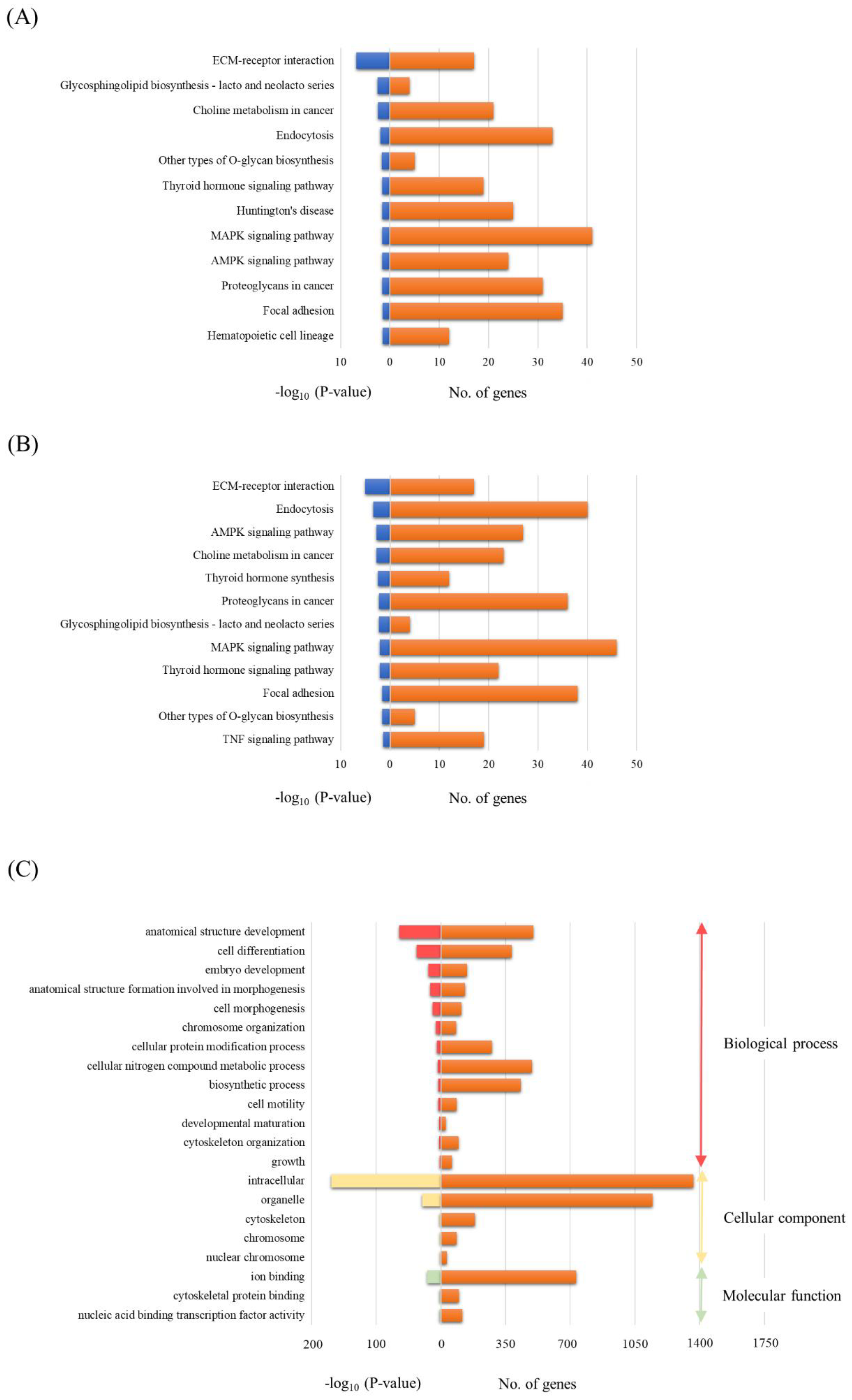
3.4. Functional Annotations of Differentially Expressed miRNAs in EVs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, K.G.; Mikkelsen, T.; Astrup, P.; Thykier-Nielsen, S.; Jacobsen, L.H.; Schou-Jensen, L.; Hoe, S.C.; Nielsen, S.P. Estimation of health hazards resulting from a radiological terrorist attack in a city. Radiat. Prot. Dosim. 2008, 131, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hagby, M.; Goldberg, A.; Becker, S.; Schwartz, D.; Bar-Dayan, Y. Health implications of radiological terrorism: Perspectives from Israel. J. Emerg. Trauma Shock 2009, 2, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Somosy, Z.; Horváth, G.; Telbisz, A.; Réz, G.; Pálfia, Z. Morphological aspects of ionizing radiation response of small intestine. Micron 2002, 33, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Seed, T.M. Radiation protectants: Current status and future prospects. Health Phys. 2005, 89, 531–545. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef]
- Singh, V.K.; Garcia, M.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int. J. Radiat. Biol. 2017, 93, 870–884. [Google Scholar] [CrossRef]
- Singh, V.K.; Hanlon, B.K.; Santiago, P.T.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int. J. Radiat. Biol. 2017, 93, 885–906. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. Radiation countermeasures for hematopoietic acute radiation syndrome: Growth factors, cytokines and beyond. Int. J. Radiat. Biol. 2021, 97, 1526–1547. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today 2022, 58, 133–145. [Google Scholar] [CrossRef]
- Bunin, D.I.; Bakke, J.; Green, C.E.; Javitz, H.S.; Fielden, M.; Chang, P.Y. Romiplostim (Nplate®) as an effective radiation countermeasure to improve survival and platelet recovery in mice. Int. J. Radiat. Biol. 2022, 96, 145–154. [Google Scholar] [CrossRef]
- Wong, K.; Chang, P.Y.; Fielden, M.; Downey, A.M.; Bunin, D.; Bakke, J.; Gahagen, J.; Iyer, L.; Doshi, S.; Wierzbicki, W.; et al. Pharmacodynamics of romiplostim alone and in combination with pegfilgrastim on acute radiation-induced thrombocytopenia and neutropenia in non-human primates. Int. J. Radiat. Biol. 2020, 96, 155–166. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirouchi, T.; Yokoyama, K.; Nishiyama, A.; Murakami, S.; Kashiwakura, I. The thrombopoietin mimetic romiplostim leads to the complete rescue of mice exposed to lethal ionizing radiation. Sci. Rep. 2018, 8, 10659. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirouchi, T.; Yoshioka, H.; Watanabe, J.; Kashiwakura, I. Diverse functions of the thrombopoietin receptor agonist romiplostim rescue individuals exposed to lethal radiation. Free Radic. Biol. Med. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- Nishida, T.; Yamaguchi, M.; Tatara, Y.; Kashiwakura, I. Proteomic changes by radio-mitigative thrombopoietin receptor agonist romiplostim in the blood of mice exposed to lethal total-body irradiation. Int. J. Radiat. Biol. 2020, 96, 1125–1134. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, M.; Funaba, M.; Chiba, A.; Kashiwakura, I. Mitigative efficacy of the clinical dosage administration of granulocyte colony-stimulating factor and romiplostim in mice with severe acute radiation syndrome. Stem Cell Res. Ther. 2020, 11, 339. [Google Scholar] [CrossRef]
- Chiba, A.; Kawabata, N.; Yamaguchi, M.; Tokonami, S.; Kashiwakura, I. Regulation of Antioxidant Stress-Responsive Transcription Factor Nrf2 Target Gene in the Reduction of Radiation Damage by the Thrombocytopenia Drug Romiplostim. Biol. Pharm. Bull. 2020, 43, 1876–1883. [Google Scholar] [CrossRef]
- Sato, Y.; Yamaguchi, M.; Kashiwakura, I. An Analysis of the Serum Metabolomic Profile for the Radiomitigative Effect of the Thrombopoietin Receptor Agonist Romiplostim in Lethally Whole-Body-Irradiated Mice. Metabolites 2022, 12, 161. [Google Scholar] [CrossRef]
- Hurley, J.H.; Boura, E.; Carlson, L.A.; Różycki, B. Membrane budding. Cell 2010, 143, 875–887. [Google Scholar] [CrossRef]
- Johansson, S.M.; Admyre, C.; Scheynius, A.; Gabrielsson, S. Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes. Immunology 2008, 123, 491–499. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.S.; Fendler, W.; Watson, J.; Hamilton, A.; Pan, Y.; Gaudiano, E.; Moskwa, P.; Bhanja, P.; Saha, S.; Guha, C.; et al. Serum microRNAs are early indicators of survival after radiation-induced hematopoietic injury. Sci. Transl. Med. 2015, 7, 287ra69. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Tatto, M.D.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- Schoefinius, J.S.; Spickenheier, B.B.; Speiseder, T.; Krebs, S.; Just, U.; Lange, C. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Provide Long-Term Survival after Total Body Irradiation Without Additional Hematopoietic Stem Cell Support. Stem Cells 2017, 35, 2379–2389. [Google Scholar] [CrossRef]
- Kink, J.A.; Forsberg, M.H.; Reshetylo, S.; Besharat, S.; Childs, C.J.; Pederson, J.D.; Gendron-Fitzpatrick, A.; Graham, M.; Bates, P.D.; Schmuck, E.G.; et al. Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol. Blood Marrow Transpl. 2019, 25, 2124–2133. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Zhao, Y.; Xue, X.; Fan, S. Mouse serum protects against total body irradiation-induced hematopoietic system injury by improving the systemic environment after radiation. Free Radic. Biol. Med. 2019, 131, 382–392. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Kink, J.A.; Thickens, A.S.; Lewis, B.M.; Childs, C.J.; Hematti, P.; Capitini, C.M. Exosomes from primed MSCs can educate monocytes as a cellular therapy for hematopoietic acute radiation syndrome. Stem Cell Res. Ther. 2021, 12, 459. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; He, L.; Chen, M.; Li, W.; Xiao, T.; Guan, J.; Qi, Z.; Wang, Q.; Li, S.; et al. Haptoglobin is an early indicator of survival after radiation-induced severe injury and bone marrow transplantation in mice. Stem Cell Res. Ther. 2022, 13, 461. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef]
- Jabbari, N.; Nawaz, M.; Rezaie, J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3649. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Piryani, S.O.; Jiao, Y.; Kam, A.Y.F.; Liu, Y.; Vo-Dinh, T.; Chen, B.J.; Chao, N.J.; Doan, P.L. Endothelial Cell-Derived Extracellular Vesicles Mitigate Radiation-Induced Hematopoietic Injury. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 291–301. [Google Scholar] [CrossRef]
- Heldring, N.; Mäger, I.; Wood, M.J.A.; Blanc, K.L.; Andaloussi, S.E.L. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Yamada, Y.; Arai, T.; Kojima, S.; Sugawara, S.; Kato, M.; Okato, A.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 2018, 109, 2919–2936. [Google Scholar] [CrossRef]
- Matsushita, R.; Seki, N.; Chiyomaru, T.; Inoguchi, S.; Ishihara, T.; Goto, Y.; Nishikawa, R.; Mataki, H.; Tatarano, S.; Itesako, T.; et al. Tumour-suppressive microRNA-144-5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br. J. Cancer 2015, 113, 282–289. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Wei, Q.; Zou, L.; Zhou, L.; Yu, Y.; Wang, D. Arginine attenuates chronic mountain sickness in rats via microRNA-144-5p. Mamm. Genome 2023, 34, 76–89. [Google Scholar] [CrossRef]
- Liu, L.; Sun, S.; Li, X. LncRNA ZFAS1 ameliorates injury led by non-alcoholic fatty liver disease via suppressing lipid peroxidation and inflammation. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102067. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ruan, H.J.; Chen, S.Y.; Wang, X.Q.; Wen, J.M.; Wang, Z.X. MiR-144-5p/CCL12 Signaling Axis Modulates Ischemic Preconditioning-Mediated Cardio-protection by Reducing Cell Viability, Enhancing Cell Apoptosis, Fibrosis, and Pyroptosis. Appl. Biochem. Biotechnol. 2022, 195, 1999–2014. [Google Scholar] [CrossRef]
- Li, T.; Qing, B.L.; Deng, Y.; Que, X.T.; Wang, C.Z.; Lu, H.W.; Wang, S.H.; Wang, Z.J. Inhibition of Long non-coding RNA zinc finger antisense 1 improves functional recovery and angiogenesis after focal cerebral ischemia via microRNA-144-5p/fibroblast growth factor 7 axis. Bioengineered 2022, 13, 1702–1716. [Google Scholar] [CrossRef]
- Yang, G.; Qin, H.; Liu, B.; Zhao, X.; Yin, H. Mesenchymal stem cells-derived exosomes modulate vascular endothelial injury via miR-144-5p/PTEN in intracranial aneurysm. Hum. Cell 2021, 34, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investg. 2020, 100, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Childers, G.M.; Perry, C.A.; Blachut, B.; Martin, N.; Bortner, C.D.; Sieber, S.; Li, J.L.; Fessler, M.B.; Harry, G.J. Assessing the Association of Mitochondrial Function and Inflammasome Activation in Murine Macrophages Exposed to Select Mitotoxic Tri-Organotin Compounds. Environ. Health Perspect. 2021, 129, 47015. [Google Scholar] [CrossRef]
- Zhong, J.Y.; Cui, X.J.; Zhan, J.K.; Wang, Y.J.; Li, S.; Lin, X.; Xiang, Q.Y.; Ni, Q.Y.; Liu, L.; Liu, Y.S. LncRNA-ES3 inhibition by Bhlhe40 is involved in high glucose-induced calcification/senescence of vascular smooth muscle cells. Ann. NY Acad. Sci. 2020, 1474, 61–72. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, W.; Xing, Y.; Wang, T.; Xu, X.; Wang, J. NF-κB target microRNAs and their target genes in TNFα-stimulated HeLa cells. Biochim. Biophys. Acta 2014, 1839, 344–354. [Google Scholar] [CrossRef]
| Systematic Name | Accession Number | Active Sequence |
|---|---|---|
| mmu-miR-144-5p | MIMAT0016988 | GGAUAUCAUCAUAUACUGUAAGU |
| mmu-miR-211-3p | MIMAT0017059 | GCAAGGACAGCAAAGGGGGGC |
| mmu-miR-296-5p | MIMAT0000374 | AGGGCCCCCCCUCAAUCCUGU |
| mmu-miR-3472 | MIMAT0015643 | UAAUAGCCAGAAGCUGGAAGGAACC |
| mmu-miR-3547-5p | MIMAT0027832 | GUGGGAAGAGGGGUGGGGCCCGGGA |
| mmu-miR-3620-5p | MIMAT0029878 | CUGUGGGCUGGGCUGGGAAGCA |
| mmu-miR-6354 | MIMAT0025097 | UGCCCUGGGGAUCAGGUCUCU |
| mmu-miR-6360 | MIMAT0025103 | UAGUGUUGCUCAGGCAGCAGGA |
| mmu-miR-6937-5p | MIMAT0027774 | UAGCUGUAAGGGCUGGGUCUGUGU |
| mmu-miR-7047-5p | MIMAT0027998 | UGAGGGAGGAGGGCUGGGUCUGA |
| mmu-miR-709 | MIMAT0003499 | GGAGGCAGAGGCAGGAGGA |
| mmu-miR-7216-5p | MIMAT0028400 | UGGAGAGCUGGCAGAGGACCCAGA |
| mmu-miR-7686-5p | MIMAT0029898 | CCUUCCACUGGACCUGGGGCUGGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M.; Kashiwakura, I. The Acute Radiation Syndrome-Mitigator Romiplostim and Secreted Extracellular Vesicles Improved Survival in Mice Acutely Exposed to Myelosuppressive Doses of Ionizing Radiation. Biomolecules 2023, 13, 837. https://doi.org/10.3390/biom13050837
Yamaguchi M, Kashiwakura I. The Acute Radiation Syndrome-Mitigator Romiplostim and Secreted Extracellular Vesicles Improved Survival in Mice Acutely Exposed to Myelosuppressive Doses of Ionizing Radiation. Biomolecules. 2023; 13(5):837. https://doi.org/10.3390/biom13050837
Chicago/Turabian StyleYamaguchi, Masaru, and Ikuo Kashiwakura. 2023. "The Acute Radiation Syndrome-Mitigator Romiplostim and Secreted Extracellular Vesicles Improved Survival in Mice Acutely Exposed to Myelosuppressive Doses of Ionizing Radiation" Biomolecules 13, no. 5: 837. https://doi.org/10.3390/biom13050837
APA StyleYamaguchi, M., & Kashiwakura, I. (2023). The Acute Radiation Syndrome-Mitigator Romiplostim and Secreted Extracellular Vesicles Improved Survival in Mice Acutely Exposed to Myelosuppressive Doses of Ionizing Radiation. Biomolecules, 13(5), 837. https://doi.org/10.3390/biom13050837






