Celastrol Supplementation Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Study Protocol
2.2. Diet and AngII Infusions
2.3. Blood Pressure Measurement
2.4. Measurement of Plasma Cholesterol
2.5. Quantification of AAAs by Ultrasound and Ex Vivo AAA Measurements
2.6. Quantification of Atherosclerosis
2.7. Histology and Immunohistochemistry
2.8. Gelatin Zymography and Western Blot Analyses
2.9. Statistical Analyses
3. Results
3.1. Celastrol Supplementation Increased AngII-Induced AAA in Male Mice
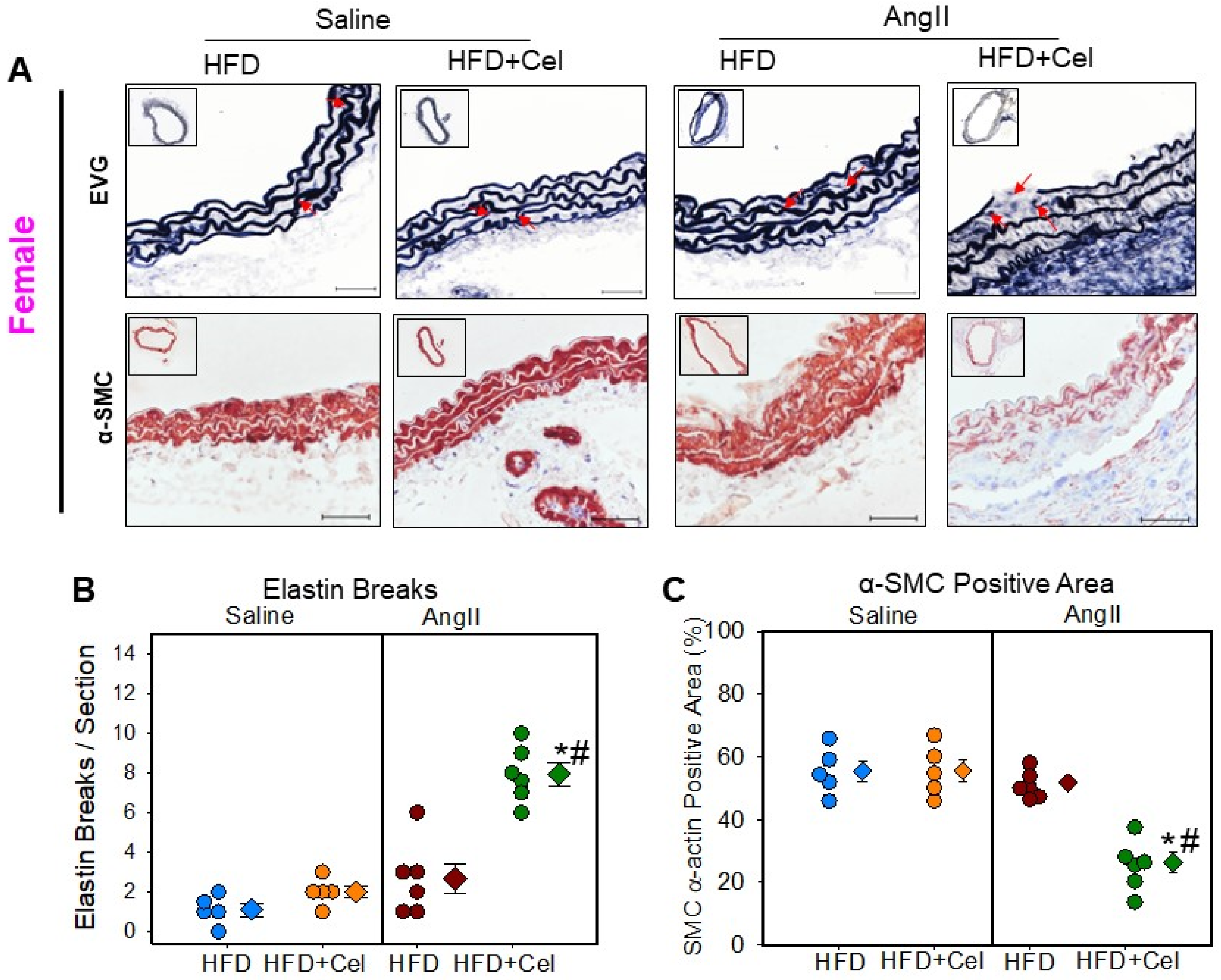
3.2. Celastrol Supplementation Increased AngII-Induced AAA in Female Mice
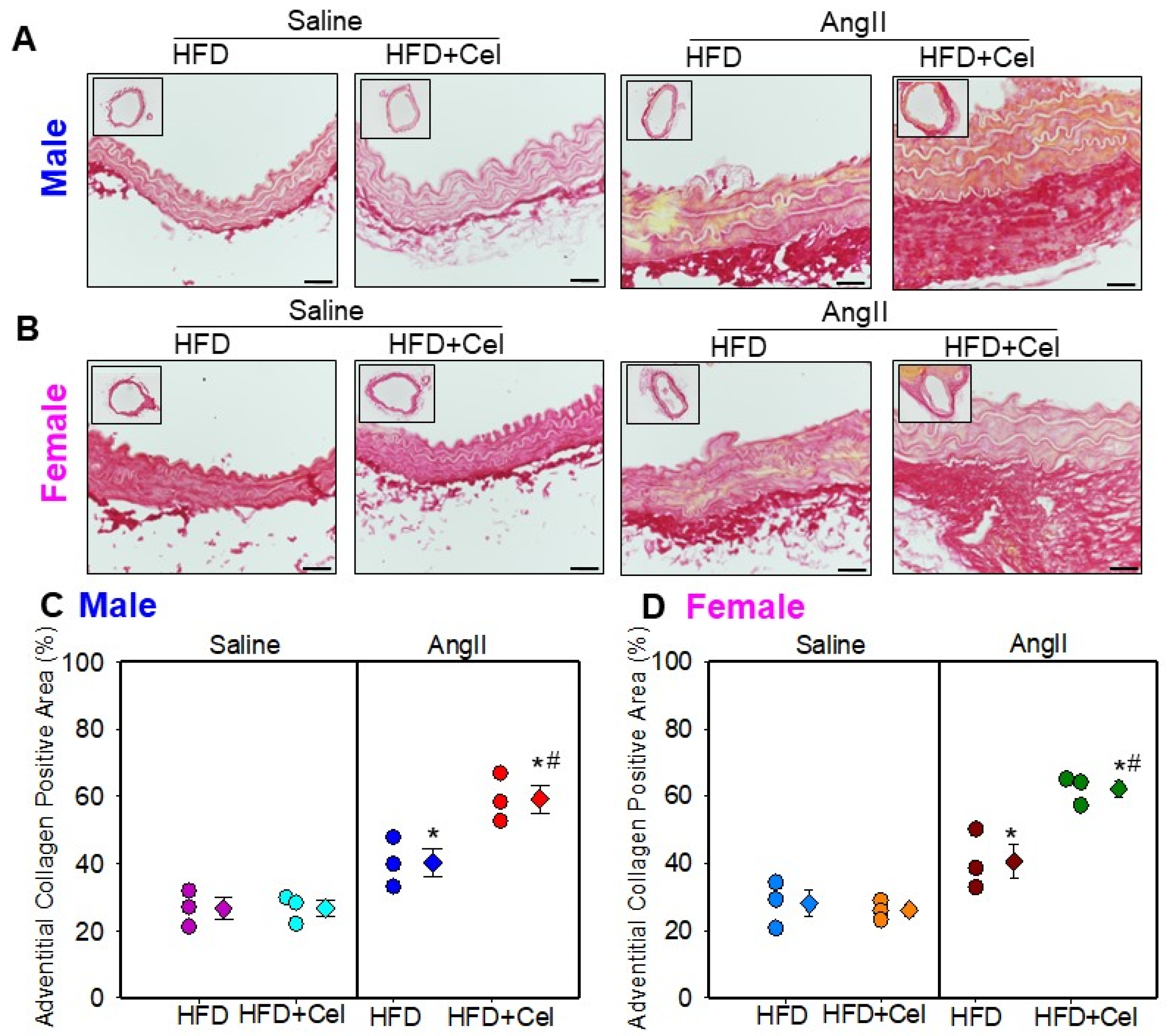
3.3. Celastrol Supplementation Increased Abdominal Aortic Medial Elastin Break and MMP9 Activity in Both Male and Female Mice Infused with AngII
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, F.M.; Rateri, D.L.; Daugherty, A. Mechanisms of aortic aneurysm formation: Translating preclinical studies into clinical therapies. Heart 2014, 100, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Alsiraj, Y.; Thatcher, S.; Charnigo, R.; Chen, K.; Blalock, E.; Daugherty, A.; Cassis, L.A. Female Mice With an XY Sex Chromosome Complement Develop Severe Angiotensin II-Induced Abdominal Aortic Aneurysms. Circulation 2017, 135, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.P.; Guo, W. Mechanisms of abdominal aortic aneurysm progression: A review. Vasc. Med. 2021, 27, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef]
- Subramanian, V.; Golledge, J.; Ijaz, T.; Bruemmer, D.; Daugherty, A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPARγ. Circ. Res. 2010, 107, 953–958. [Google Scholar] [CrossRef]
- Saraff, K.; Babamusta, F.; Cassis, L.A.; Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 2003, 23, 1621–1626. [Google Scholar] [CrossRef]
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef]
- Chen, S.R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium wilfordii Hook F. Front. Pharm. 2018, 9, 104. [Google Scholar] [CrossRef]
- Cascao, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef]
- Yu, X.; Tao, W.; Jiang, F.; Li, C.; Lin, J.; Liu, C. Celastrol attenuates hypertension-induced inflammation and oxidative stress in vascular smooth muscle cells via induction of heme oxygenase-1. Am. J. Hypertens. 2010, 23, 895–903. [Google Scholar] [CrossRef]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef]
- Muniappan, L.; Okuyama, M.; Javidan, A.; Thiagarajan, D.; Jiang, W.; Moorleghen, J.J.; Yang, L.; Balakrishnan, A.; Howatt, D.A.; Uchida, H.A.; et al. Inducible Depletion of Calpain-2 Mitigates Abdominal Aortic Aneurysm in Mice. Arter. Thromb. Vasc. Biol. 2021, 41, 1694–1709. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 15, 1291. [Google Scholar]
- Subramanian, V.; Moorleghen, J.J.; Balakrishnan, A.; Howatt, D.A.; Chishti, A.H.; Uchida, H.A. Calpain-2 compensation promotes angiotensin II-induced ascending and abdominal aortic aneurysms in calpain-1 deficient mice. PLoS ONE 2013, 8, e72214. [Google Scholar] [CrossRef]
- Cassis, L.A.; Rateri, D.L.; Lu, H.; Daugherty, A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arter. Thromb. Vasc. Biol. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Rateri, D.L.; Moorleghen, J.J.; Balakrishnan, A.; Owens, A.P., 3rd; Howatt, D.A.; Subramanian, V.; Poduri, A.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ. Res. 2011, 108, 574–581. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.L.; Lu, H.; Inagami, T.; Cassis, L.A. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation 2004, 110, 3849–3857. [Google Scholar] [CrossRef]
- Okuyama, M.; Jiang, W.; Javidan, A.; Chen, J.Z.; Howatt, D.A.; Yang, L.; Hamaguchi, M.; Yasugi, T.; Aono, J.; Vazquez-Padron, R.I.; et al. Lysyl Oxidase Inhibition Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice. Circulation 2020, 142, 1993–1995. [Google Scholar] [CrossRef]
- Subramanian, V.; Uchida, H.A.; Ijaz, T.; Moorleghen, J.J.; Howatt, D.A.; Balakrishnan, A. Calpain Inhibition Attenuates Angiotensin II-induced Abdominal Aortic Aneurysms and Atherosclerosis in Low-density Lipoprotein Receptor-deficient Mice. J. Cardiovasc. Pharm. 2012, 59, 66–76. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, G.; Song, Y.; Wang, L.; Tu, L.; Zhang, L.; Zhang, C. Celastrol-Induced Suppression of the MiR-21/ERK Signalling Pathway Attenuates Cardiac Fibrosis and Dysfunction. Cell. Physiol. Biochem. 2016, 38, 1928–1938. [Google Scholar] [CrossRef]
- Fashandi, A.Z.; Spinosa, M.; Salmon, M.; Su, G.; Montgomery, W.; Mast, A.; Lu, G.; Hawkins, R.B.; Cullen, J.M.; Sharma, A.K.; et al. Female Mice Exhibit Abdominal Aortic Aneurysm Protection in an Established Rupture Model. J. Surg. Res. 2019, 247, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guan, D.; Auen, T.; Choi, J.W.; Hernandez, M.A.S.; Lee, J.; Chun, H.; Faruk, F.; Kaplun, E.; Herbert, Z.; et al. IL1R1 is required for celastrol’s leptin-sensitization and antiobesity effects. Nat. Med. 2019, 25, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Zhao, M.; Su, Z.; Hu, Z.; Zhang, C.; Guo, B.; Liu, J.; Qin, L.; Zhang, W.; et al. Celastrol prevents high-fat diet-induced obesity by promoting white adipose tissue browning. Clin. Transl. Med. 2021, 11, e641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.T.; Zhang, R.; Fu, W.Y.; Wang, X.; Wang, F.; Gao, P.; Ding, Y.N.; Xie, Y.; Hao, D.L.; et al. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J. Exp. Med. 2016, 213, 2473–2488. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, C.; Liu, X.; Li, M.; Gao, M.; Liu, X.; Fang, F.; Chang, Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol. Metab. 2017, 6, 138–147. [Google Scholar] [CrossRef]
- Maedeker, J.A.; Stoka, K.V.; Bhayani, S.A.; Gardner, W.S.; Bennett, L.; Procknow, J.D.; Staiculescu, M.C.; Walji, T.A.; Craft, C.S.; Wagenseil, J.E. Hypertension and decreased aortic compliance due to reduced elastin amounts do not increase atherosclerotic plaque accumulation in Ldlr-/- mice. Atherosclerosis 2016, 249, 22–29. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Wang, N.; Bai, Y.; Shi, D. Celastrol Attenuates Intrahepatic Cholestasis of Pregnancy by Inhibiting Matrix Metalloproteinases-2 and 9. Ann. Hepatol. 2019, 18, 40–47. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, H.; Jang, S.W.; Ko, J. Celastrol inhibits breast cancer cell invasion via suppression of NF-ĸB-mediated matrix metalloproteinase-9 expression. Cell. Physiol. Biochem. 2011, 28, 175–184. [Google Scholar] [CrossRef]
- Mi, C.; Shi, H.; Ma, J.; Han, L.Z.; Lee, J.J.; Jin, X. Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9. Oncol. Rep. 2014, 32, 2527–2532. [Google Scholar] [CrossRef]
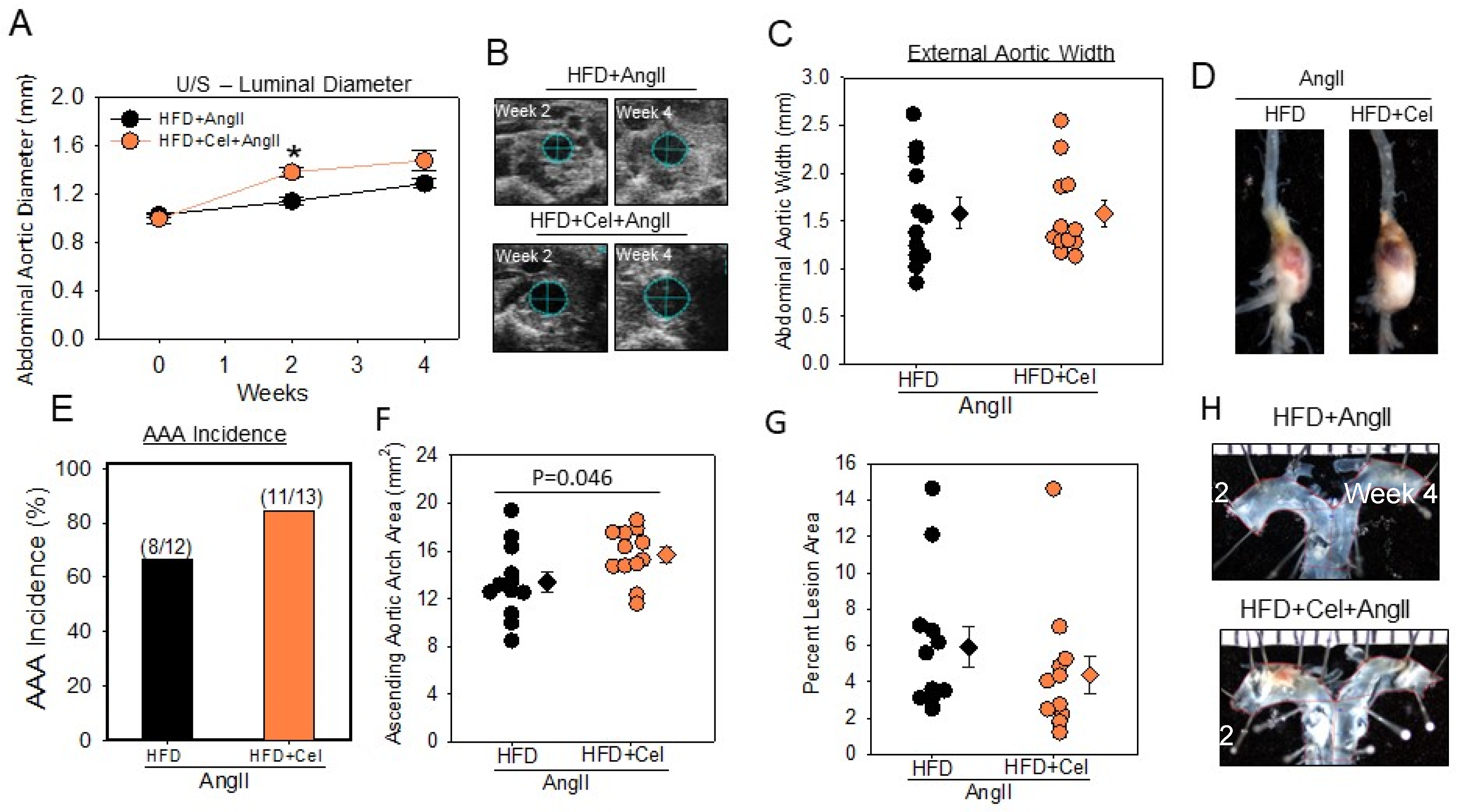
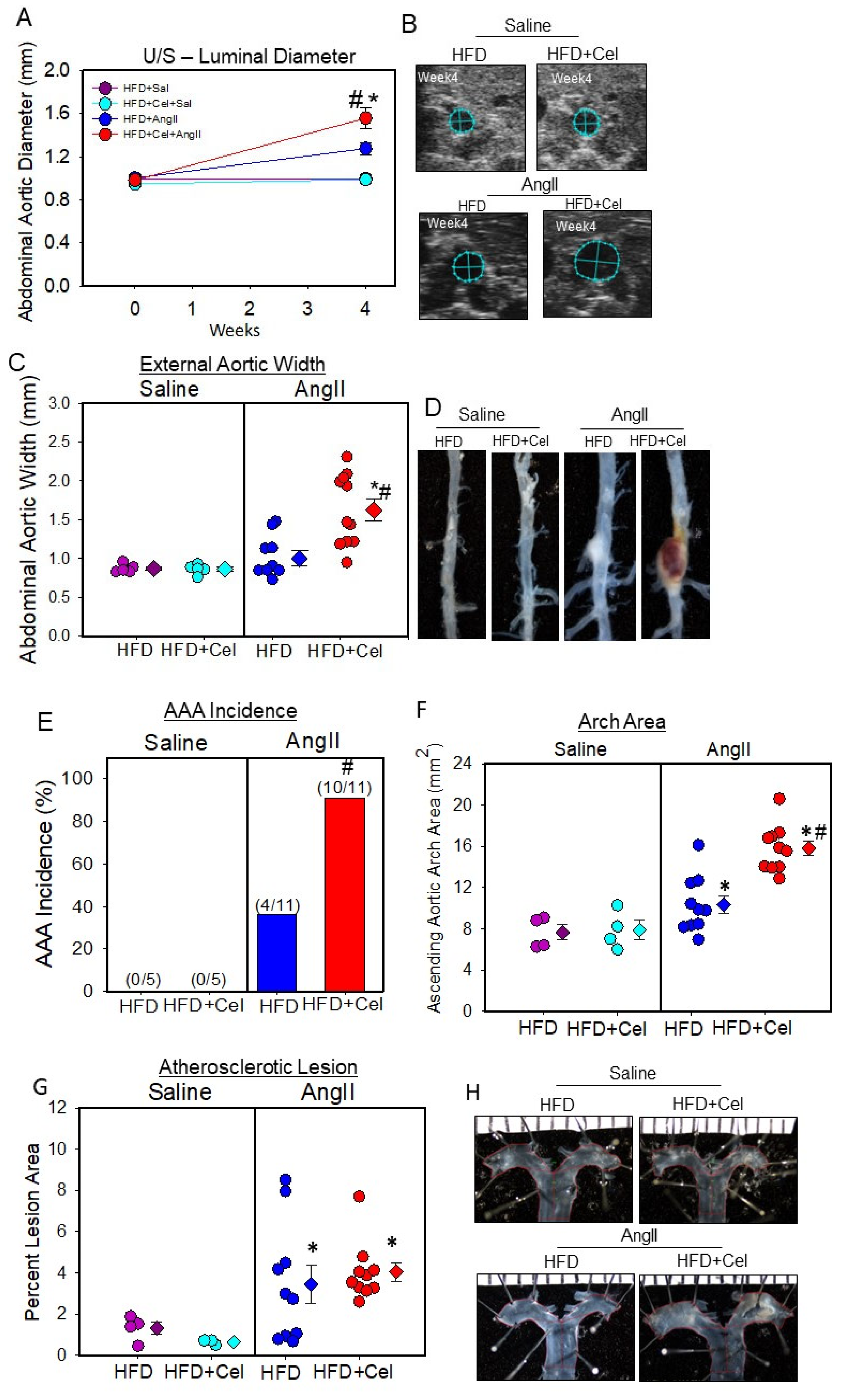
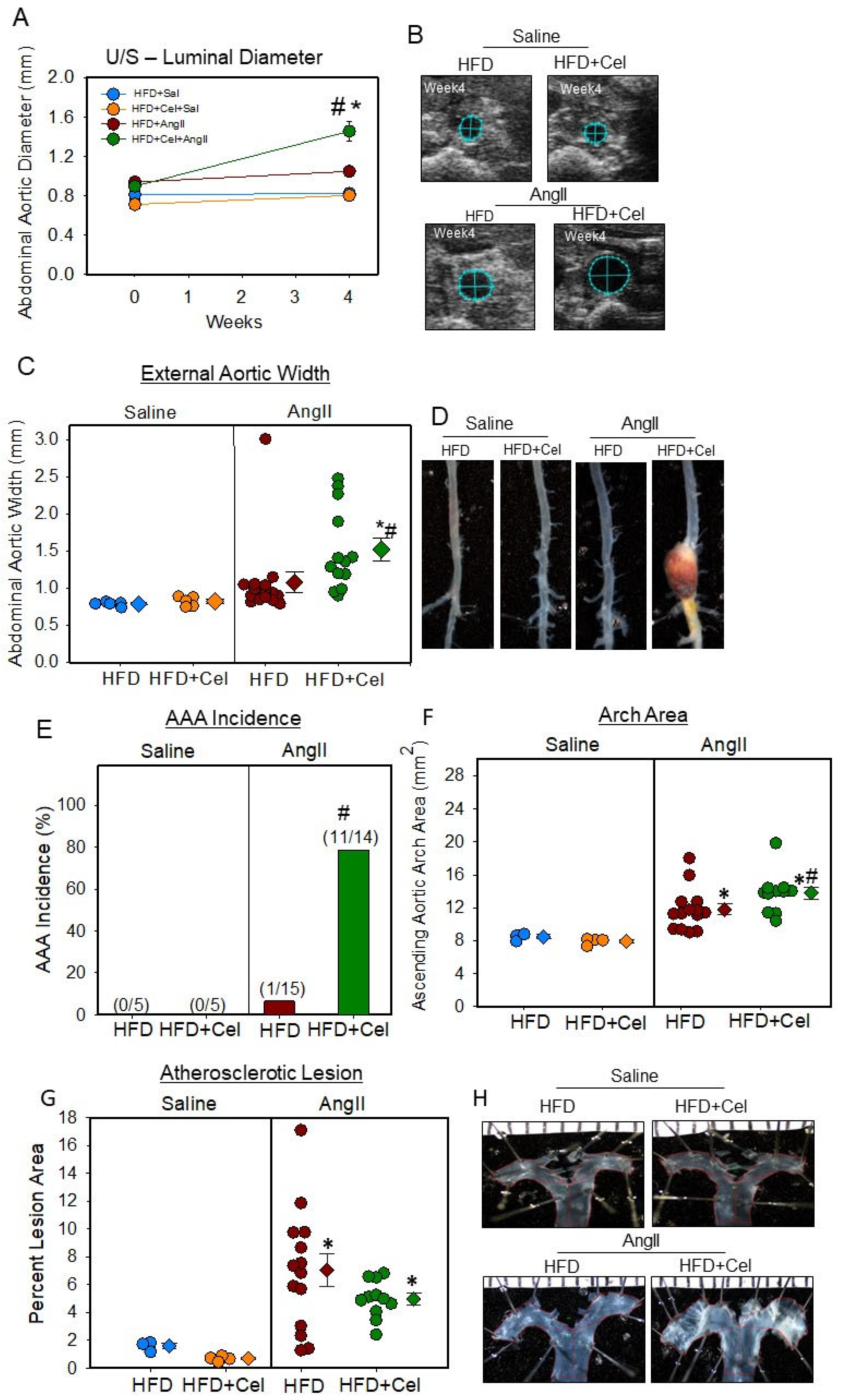
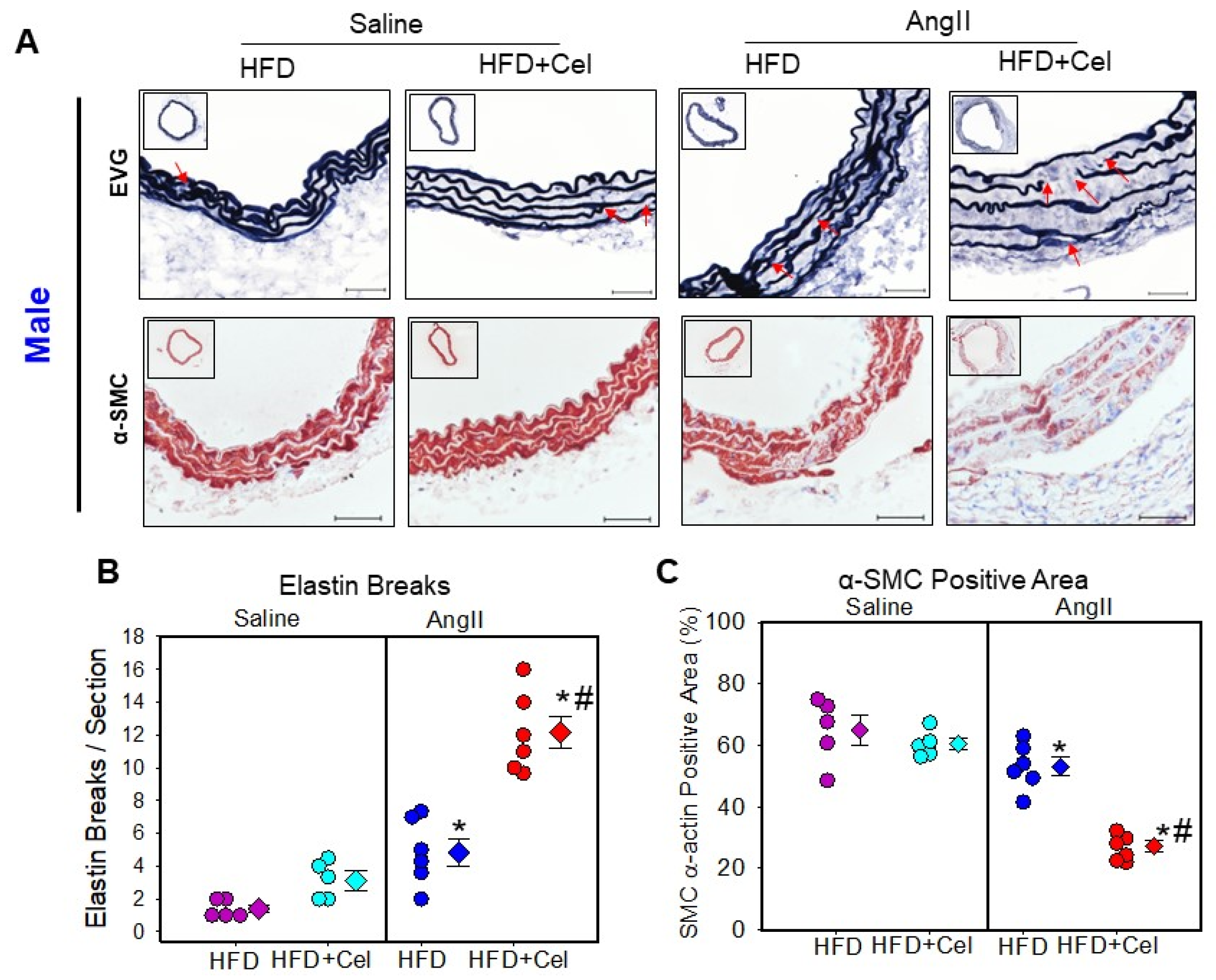

| Groups | HFD | HFD + Celastrol |
|---|---|---|
| Infusion | AngII | AngII |
| N | 12 | 13 |
| Body Weight Pre-Infusion (g) | 22.1 ± 0.7 | 21.9 ± 0.9 |
| Body Weight Post-Infusion (g) | 25.1 ± 0.7 | 18.1 ± 0.6 * |
| Plasma Cholesterol (mg/dL) | 951.5 ± 32.2 | 961 ± 56.5 |
| Systolic BP Pre-Infusion (mmHg) | 138 ± 4 | 133 ± 4 |
| Systolic BP Post-Infusion (mmHg) | 188 ± 5 ^ | 169 ± 8 ^* |
| Groups | HFD | HFD + Celastrol | ||
|---|---|---|---|---|
| Saline | AngII | Saline | AngII | |
| N | 5 | 10 | 5 | 11 |
| Body Weight Pre (g) | 27.1 ± 1.1 | 25.2 ± 1.0 | 25.3 ± 0.7 | 26.1 ± 0.3 |
| Body Weight Post (g) | 30.8 ± 1.4 | 29.6 ± 0.8 | 20.1 ± 1.2 *$ | 20.8 ± 0.4 *$ |
| TC (mg/dL) | 1192 ± 81 | 1208 ± 98 | 1351 ± 89 | 1437 ± 48 |
| SBP Pre (mmHg) | 136 ± 4 | 146 ± 3 | 145 ± 4 | 150 ± 3 |
| SBP Post (mmHg) | 154 ± 7 | 182 ± 6 # | 138 ± 4 | 181 ± 4 # |
| Groups | HFD | HFD + Celastrol | ||
|---|---|---|---|---|
| Saline | AngII | Saline | AngII | |
| N | 5 | 15 | 5 | 13 |
| Body Weight Pre (g) | 18.9 ± 0.7 | 19.6 ± 0.3 | 18.7 ± 0.6 | 19.2 ± 0.3 |
| Body Weight Post (g) | 21.9 ± 0.6 | 22.8 ± 0.5 | 16.6 ± 0.9 *$ | 16.7 ± 0.3 *$ |
| TC (mg/dL) | 982 ± 87 | 1079 ± 86 | 1032 ± 87 | 1147 ± 54 |
| SBP Pre (mmHg) | 150 ± 4 | 139 ± 3 | 147 ± 3 | 149 ± 3 |
| SBP Post (mmHg) | 169 ± 5 # | 166 ± 5 # | 133 ± 3 | 176 ± 4 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javidan, A.; Jiang, W.; Yang, L.; Frony, A.C.; Subramanian, V. Celastrol Supplementation Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice. Biomolecules 2023, 13, 603. https://doi.org/10.3390/biom13040603
Javidan A, Jiang W, Yang L, Frony AC, Subramanian V. Celastrol Supplementation Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice. Biomolecules. 2023; 13(4):603. https://doi.org/10.3390/biom13040603
Chicago/Turabian StyleJavidan, Aida, Weihua Jiang, Lihua Yang, Ana Clara Frony, and Venkateswaran Subramanian. 2023. "Celastrol Supplementation Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice" Biomolecules 13, no. 4: 603. https://doi.org/10.3390/biom13040603
APA StyleJavidan, A., Jiang, W., Yang, L., Frony, A. C., & Subramanian, V. (2023). Celastrol Supplementation Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice. Biomolecules, 13(4), 603. https://doi.org/10.3390/biom13040603






